ABSTRACT
The present study is designed to evaluate the phytochemical profile of Elaeocarpus ganitrus and Desmostachya bipinnata. Extraction was performed using ethanol as a solvent for both the plants. Preliminary phytochemical screening was performed to determine presence of secondary metabolites present in the plants. Total phenolic and flavonoid contents were determined using recommended methods. Results showed that the yield of the E. ganitrus extract was 25.14%; however, it was 11.25% for D. bipinnata extract. The ethanolic extract of E. ganitrus showed presence of alkaloids, flavonoids, saponins, phenol, glycosides, steroids, terpenoids, and fixed oils. On the other hand, D. bipinnata extracts showed presence of alkaloids, flavonoids, saponins, phenol, carbohydrates, glycosides, steroids and terpenoids. Proteins and amino acids were not present in both the plant extracts. It was revealed from the results that total phenolic content was 214.5 mg/g in E. ganitrus and 134.6 mg/g in D. bipinnata. However, flavonoid content was 83.56 mg/g and 67.90 mg/g in E. ganitrus and D. bipinnata ethanolic extracts, respectively. The present study revealed that E. ganitrus and D. bipinnata extracts were rich in phenolic and flavonoid compounds. The plants extract also possess several phytoconstituents that may be responsible for their therapeutic activity. The results of these investigations are expected to help in correct identification, characterization and other relevant findings.
INTRODUCTION
The Rudraksha tree is botanically known as Elaeocarpus ganitrus roxb. Its english name is Utrasum bead tree.1,2 From ancient times, the power of Holy Rudraksha beads have been scripted in various religious texts like Shivmaha Purana, Shreemad devi bhagwat, Padma Purana, in Vedas etc. Elaeocarpus is a medium sized evergreen broad-leaved tree, which grow up to the height of 200 ft and about 4 feet in girth. Fruits start appearing in June and ripen near October. Ripe fruit is fleshy and has a seed with blue shell. Inner part or bead lying in the seed is called Rudraksha. The Elaeocarpus ganitrus fruit have many phytoconstituents such as alkaloids, flavonoids, tannins, steroids, triterpenoids, carbohydrates and cardiac glycosides.3 It has reported that, fruit possess significant amount of phytocomponents such as isoelaeocarpicine, elaeocapine cough, the paste of ten-faced.4 Elaeocarpus sphaericus with milk relieves prolonged cough. It can be used as a cure for skin diseases, sores, ringworm, pimples, boils and burns also. This also helps in increasing memory.5
Rudraksha have many Ayurvedic properties that refer to this wonderful bead and gives details of rudraksha for increase body constitutions. The beads, bark and leaves of Rudraksha all are used to cure various ailments like mental disorders, headache, fever, skin diseases etc.6 Rudraksha may be worn either on arm, wrist or other parts of the body. For improving memory power Rudraksha can be used for improving memory power when taken with milk. For all brain diseases Rudraksha can be used for treating all brain diseases like brain fever etc. As a blood purifier Rudraksha shall be used for treating the blood impurities and strengthens the body substance. Rudraksha fruit or bark, can be used for controlling epilepsy.7 For curing liver related problems, jaundice, and stomachache Rudraksha can be used for treating stomach pain and liver problems. For blood pressure Rudraksha can be used to treat high blood pressure, heart diseases etc.
The halfa grass, Desmostachya bipinnata (L.) Stapf. (Fam. Poaceae) is also called as Big cord grass. It is culturally important grass. In India, halfa grass, Desmostachya bipinnata (L.) is called as Kusha in Sanskrit language.8 Halfa grass is with three species under its genus, D. bipinnata, D. cynosuroides Stapf ex Massey, D. pingalaiae Raole & R. J. Desai. In Sanskrit language, it is recognized as Dharbha. It has nine species under its genus. The nine species of cogon grass include I. conferta; I. cylindrica; I. contracta; I. brevifolia; I. brasiliensis; I. tenius; I. cheesemanii; I. condensate; and I. minutiflora.9
The halfa grass and cogon grass both are widely used in various religious sacrifices and rituals in India. It has been mentioned in Rigveda and Atharvaveda. It is perennial green grasses with dense foliage and are always seen spread over vast areas. There is major problem with large scale production of any natural products. The major significance of the described grasses is that they are abundantly available and are therefore sustainable sources. Even though, both the mentioned plants have various biologically active principles and medicinal value, they have been utilized barely for commercial therapeutic solutions. Thus, this article aims to evaluate the phytochemical profile of the plants.
MATERIALS AND METHODS
Collection and extraction of plants parts
E. ganitrus and D. bipinnata grass were collected by researchers from Raigarh Pharmacy College in Raigarh, India, in April 2021. The taxonomic validity of the plant was confirmed by Mr. R.S. Jayasomu, a Senior Principal Scientist in New Delhi. A voucher specimen can be found in the herbarium of the same department (NISCAIR/RHMD/Consult/2020/3710-11-2). Twenty days of room temperature air drying in the shadow was required for drying the collected plant parts. Grinding and sieving plant parts in a mechanical grinder produced a range of 50 to 150 mm particles. Dried powder was used in the Soxhlet device and extracted with 90 percent ethanol for 48 hours. The extract was concentrated in a vacuum evaporator at 40°C and reduced pressure, and its solvent was removed. Glass jars with the concentrated extract were then stored at 4°C until needed. Yield of the extract obtained was calculated by formula as mentioned below:
Preliminary Phytochemical Screening
The extracts of E. ganitrus and D. bipinnata were subjected to preliminary qualitative phytochemical screening for the detection of plant constituents. Various colour reactions were used to identify the nature of the components.10 The crude fruit extract was tested for the presence or absence of several phytoconstituents such as carbohydrate, alkaloids, the phenolic compound, tannins, glycoside, flavonoids, saponins and fixed oils.
Detection of alkaloids
Alkaloids are detected by a number of tests. Firstly the extracts are solubilized in dilute hydrochloric acid and then clarified by filtration to remove impurities.
- Dragendroff’s Test: In this test the filtrates are reacted with Dragendroff’s reagent. If alkaloids are present, a red precipitate is obtained.
- Hager’s Test: In this test the filtrate is reacted with Hager’s reagent which is a concentrated solution of picric acid. If yellow coloured precipitate is obtained, it indicates the presence of alkaloids.
- Mayer’s Test: In this test the filtrate is reacted with Mayer’s reagent which is potassiomercuric iodide solution and obtained by dissolving mercuric chloride and potassium iodide. Development of precipitate having yellow colour confirms the existence of alkaloids.
- Wagner’s Test: In this test the filtrate is reacted with Wagner’s reagent. The development of precipitate having brown/red colour confirms the existence of alkaloids.
Detection of carbohydrates
For performing this test the extract is solubilized individually in 5 ml of purified water and then filtered. This filtrate is utilized for the detection of carbohydrates in the sample. A number of tests can be used for the detection of the carbohydrates as follows:
- Benedict’s test: The filtrate was reacted with Benedict’s reagent and then heated delicately. Development of precipitate having red colour confirms the existence of reducing sugars such as glucose, galactose, fructose, etc.
- Fehling’s test: In this method the filtrate is reacted with dilute hydrochloric acid to make it hydrolysed, neutralized with an alkali and then warmed with Fehling’s solutions A & B. Appearance of precipitate having red color specify the existence of reducing sugars.
Detection of flavonoids
- Alkaline reagent test: The extract is reacted with few drops of solution having sodium hydroxide. Development of extreme yellow shading demonstrates the existence of flavonoids.
- Lead acetate Test: The extract is reacted with some drops of solution of lead acetate. Development of precipitate having yellow colour demonstrates the existence of flavonoids.
Detection of glycosides
Firstly the extract is reacted with dilute HCl to hydrolyse it and afterward subjected to test for glycosides as follows:
- Legal test: In this test the extract is reacted with sodium nitroprusside (present in a mixture of pyridine and sodium hydroxide). Development of pink to red colour demonstrates the existence of cardiac glycoside.
- Modified Borntrager’s test: The extract is reacted with a solution containing ferric chloride and then drenched in boiled water for around 5 minutes. The blend is cooled and removed with equivalent volumes of benzene. The benzene layer was isolated and reacted with ammonia solution. Development of rose-pink shading in the ammonical layer demonstrates the existence of anthranol glycosides.
Detection of phenols
Ferric Chloride Test: The extract is reacted with few drops of solution containing ferric chloride. Development of bluish black colour demonstrates the existence of phenol.
Detection of phytosterols
- Libermann Burchard’s test: Extract is made to react with chloroform and then filtered. Filtrate is made to react with some drops of acetic anhydride and then after boiling it is cooled. Concentrated sulphuric acid is included to it. Development of brown ring at the intersection demonstrates the existence of phytosterols.
- Salkowski’s Test: The extract is reacted with chloroform and filtered. Filtrate is then made to react with some drops of conc. Sulphuric acid, stirred and permitted to stand. Development of brilliant yellow colour demonstrates the existence of triterpenes.
Detection of proteins and amino acids
- Ninhydrin Test: 0.25% w/v ninhydrin reagent is made to react with the extract and then boiled for some minutes. Development of blue colour demonstrates the existence of amino acid.
- Xanthoproteic Test: The extract is reacted with some drops of concentrated nitric acid. Development of yellow colour demonstrates the existence of proteins.
Detection of saponins
- Foam Test: The extract (0.5 gm) is taken with about 2 ml of water. In case the foam appears on shaking and remains for ten minutes, it demonstrates the existence of saponins.
- Froth Test: In this method the extract is diluted to small quantity of water (20 ml) and then it is stirred in a graduated cylinder for about 15 minutes. Development of foam of 1 cm, demonstrate the existence of saponins.
Detection of tannins
Gelatin Test: 1% solution of gelatin was made to react with the extract. Development of a precipitate having white color demonstrates the existence of tannins.
Detection of diterpenes
Copper acetate Test: The extract is solubilized in water and then made to react with some drops of copper acetate solution. Development of emerald green colour demonstrates the existence of diterpenes.
Estimation of total Phenolic content
The amount of total phenolic content in the fruit extracts of E. ganitrus was determined by the spectrophotometric methods of Madaan et al., 2011 with slight modification.11 An aliquot of extract or standard gallic acid was used in this analysis. The reaction mixture was prepared into a 25 ml of the volumetric flask. 1 ml of diluted extract in ethanol (100μg/ml) was mixed with 9 ml of distilled water and 1 ml of Folin-Ciocalteu phenol reagent was also added to the mixture and shaken. The solution was allowed to stand at 25˚C for 5-8 min before adding 10 ml of 7% sodium carbonate solution which was made in distilled water. The test sample was diluted to 25 ml with distilled water and mixed thoroughly. The reaction mixture was allowed to stand for 2 h at room temperature. The absorbance was determined by UV-Spectrophotometer at 765 nm. A calibration curve was plotted by using different concentration of standard Gallic acid. Total phenolic contents were calculated as mg of Gallic acid equivalent per gram of the dried sample (mg/g).
Estimation of total flavonoid content
In a test tube, plant extract (0.5 ml) was taken from pre prepared stock solution of extract was mixed with 1.5 ml of ethanol, 0.1 ml of 10% aluminum chloride, 0.1 ml of 1M potassium acetate and diluted with 2.8 ml distilled water. Then the mixtures were tolerable to stand for incubation at room temperature for 30 min and absorbance of the reaction mixtures were measured at 510 nm against the prepared blank solution using spectrophotometer. Blank solution was prepared by addition of all the other reagents except the plant extracts. Total flavonoids content was expressed in mg of quercetin equivalent per gram of the dry plant extract (mg/g).11
RESULTS AND DISCUSSION
Percentage of yield of the extracts
The percentage yield of both the extracts are shown in Table 1. It was found that the yield of the E. ganitrus extract was 25.14%; however, it was 11.25% for D. bipinnata extract using ethanol as a solvent.
| Solvent | Weight of powder | Volume of solvent | Weight of extract | % of yield extraction |
|---|---|---|---|---|
| E. ganitrus | 50 g | 200 ml | 12.57 g | 25.14% |
| D. bipinnata | 40 g | 200 ml | 4.5 g | 11.25% |
Percentage yield of crude extract of
E. ganitrus
prepared by soxhlet extraction techniques.
Phytochemical analysis of E. ganitrus and D. bipinnata extracts
The phytochemical analysis of both the plant extracts are shown in Table 2. The ethanolic extract of E. ganitrus showed presence of alkaloids, flavonoids, saponins, phenol, glycosides, steroids, terpenoids, and fixed oils. On the other hand, D. bipinnata extracts showed presence of alkaloids, flavonoids, saponins, phenol, carbohydrates, glycosides, steroids and terpenoids. Proteins and amino acids were not present in both the plant extracts. Sakat et al., (2009) were also studies on phytochemical analysis of bioactive compounds by using aqueous seed extract of E. ganitrus and evaluated for antihypertensive activity in renal artery occluded hypertensive rats.12 Similarly, Bharti, (2013) revealed that Kaempferol and Quercetin were found to dominate in seeds of E. ganitrus.13 The content of C-glycosides derivatives of apigenin and quercetin in all seed samples was in a standard quantity than flavonol type compounds. Singh et al., (2013) reported that ethanolic extract of E. ganitrus fruits exhibited significant anti anxiety activity.14 An anxiolytic constituent of quercetin derivative was isolated from ethanol extract of E. ganitrus beads and it was also used as the marker to standardize the plant material. This research work provides a scientific validation for utilization of ecofriendly fruit of medicinal plant E. ganitrus, in having the potential to be a good drug.
| Chemical constituents | E. ganitrus extract | D. bipinnata extract |
|---|---|---|
| Alkaloids | +ve | +ve |
| Flavonoid | +ve | +ve |
| Saponins | +ve | +ve |
| Phenol | +ve | +ve |
| Carbohydrates | -ve | +ve |
| Glycosides | +ve | +ve |
| Protein and Amino Acid | -ve | -ve |
| Steroids and Terpenoids | +ve | +ve |
| Fixed Oils and fat test | +ve | -ve |
Qualitative phytochemical analysis of ethanolic extract of
E. ganitrus
and
D. bipinnata
.
Total phenolic and flavonoid contents
The quantitative estimation of total Phenolic and Flavonoid contents in the ethanolic extract of E. ganitrus and D. bipinnata are shown in Figure 1 and Figure 2 respectively. It was revealed from the graph that total phenolic content was 214.5 mg/g in E. ganitrus and 134.6 mg/g in D. bipinnata (Figure 1). However, flavonoid content was 83.56 mg/g and 67.90 mg/g in E. ganitrus and D. bipinnata ethanolic extracts, respectively (Figure 2). The results showed that the family of Elaeocarpaceae plant i.e., E. ganitrus is the richest source of phenolic compounds. There is an encouraging connection between phenolic content and free-radical scavenging activity.15 (Flavonoids are essential in human diet and are present in plant extracts that have been used for medicinal purpose.16 Further studies are needed with fruit extract of E. ganitrus to identify the unknown flavonoids and phenolic acids in the analysed ethanolic extracts by isolating, characterizing and elucidating the structure of the bioactive compounds of plant in order to prepare natural pharmaceutical products with a of high medicinal value.
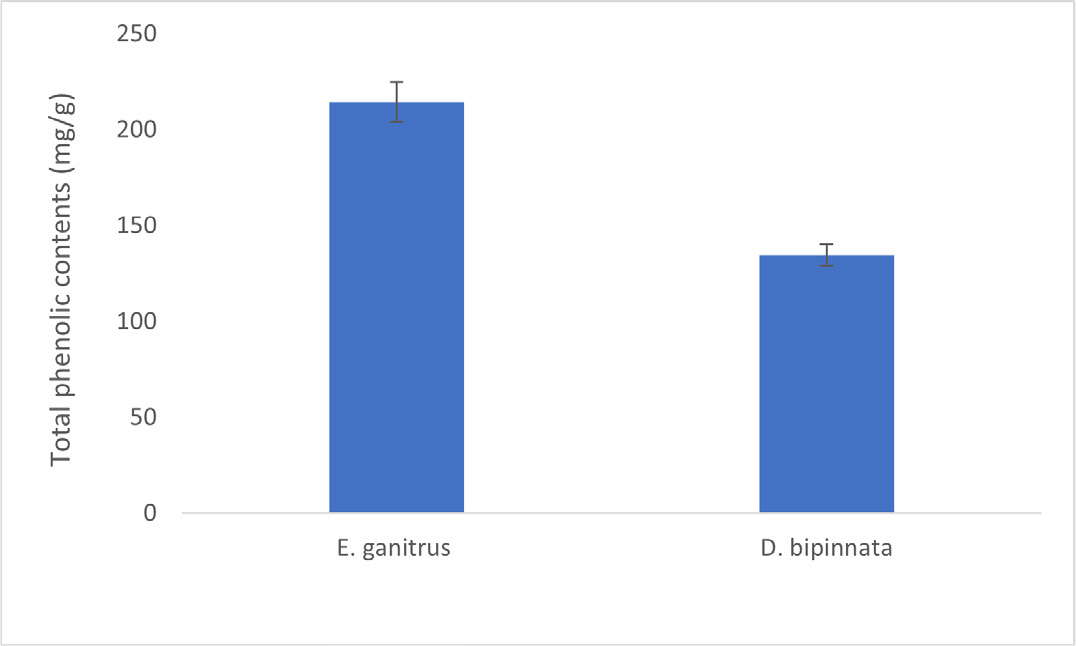
Figure 1:
Total Phenolic content of ethanolic extract of E. ganitrus and D. bipinnata.
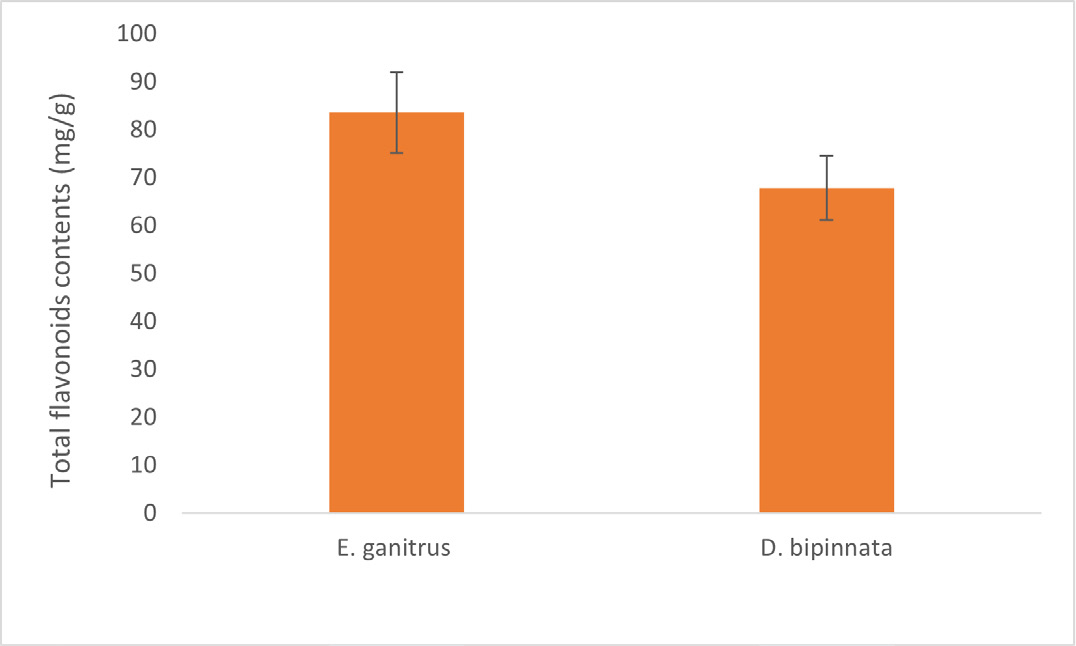
Figure 2:
Total Phenolic content of ethanolic extract of E. ganitrus and D. bipinnata.
CONCLUSION
The present study revealed that E. ganitrus and D. bipinnata extracts were rich in phenolic and flavonoid compounds. The plants extracts also possess several phytoconstituents that may be responsible for their therapeutic activity. The results of these investigations are expected to help in correct identification, characterization and other relevant findings. The outcomes of preliminary phytochemical investigations may guide isolation and purification of lead compounds responsible for traditional therapeutic claims. Further studies are going on concerning this plant in order to identify, characterize and elucidate the structure of bioactive compounds by various techniques such as high performance liquid chromatography and spectroscopy techniques.
References
- Rai N, Chaubey S, Pradhan S.. Critical review article on rudraksha ( Roxb.). Word J Pharm Res.. 2021;10(3):1090-9. [Google Scholar]

- Hardainiyan S, Nandy BC, Kumar K.. Array. Int J Pharm Sci Rev Res.. 2015;34(1):55-64. [Google Scholar]

- Pandey K, Singh M, Pandey B, Upadhyaya A, Pande KK. Preliminary phytochemical screening and antimicrobial activities of plant extract of Roxb. Int J Bioassays. 2016;5(9):4885 [CrossRef] | [Google Scholar]

- Singh B, Chopra A, Ishar MP, Sharma A, Raj T.. Pharmacognostic and antifungal investigations of (Rudrakasha). Indian J Pharm Sci.. 2010;72(2):261-5. [PubMed] | [CrossRef] | [Google Scholar]

- Swami G, Nagpal N, Rahar S, Singh P, Singla S, Porwal A, et al. Array. Pharm Lett.. 2010;2(1):297-306. [PubMed] | [CrossRef] | [Google Scholar]

- Maheshwari R, Kumar A, Punar S, Ram L, Sharma R, Kakodia A, et al. A comprehensive review on phytochemical, pharmacological, dielectric and therapeutic attributes of multifarious rudraksha ( Roxb.). Adv image video process.. 2021;9:10 [PubMed] | [CrossRef] | [Google Scholar]

- Arivu I, Muthulingam M.. Detailed study on (Rudraksha) for its medicinal importance-A review. Int J Curr Sci.. 2017;2017(1):16-30. [PubMed] | [CrossRef] | [Google Scholar]

- Padhy S.. Over-religious activity, A threat to biodiversity (3). Conserve ‘Kusha’ () before its Extinction. J Biodivers. 2017;8(1):44-50. [PubMed] | [CrossRef] | [Google Scholar]

- Madhukar Gaikwad P, Pawar SS, Bhimasha Khyade V. Halfa and cogon: the two novel grasses. Int J Curr Microbiol Appl Sci.. 2019;8(1):3014-27. [CrossRef] | [Google Scholar]

- Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from indigenous to Balochistan. Scientific World Journal. 2017;2017:5873648 [PubMed] | [CrossRef] | [Google Scholar]

- Madaan R, Bansal G, Kumar S, Sharma A.. Estimation of total phenols and flavonoids in extracts of Roots and antioxidant activity studies. Indian J Pharm Sci.. 2011;73(6):666-9. [PubMed] | [CrossRef] | [Google Scholar]

- Sakat SS, Wankhede SS, Juvekar AR, Mali VR, Bodhankar SL. Antihypertensive effect of aqueous extract of Roxb. fruits in renal artery occluded hypertensive rats. Int J Pharm Tech Res.. 2009;1:779-82. [PubMed] | [CrossRef] | [Google Scholar]

- Bharti A.. Determination of quercetin in extract of Roxb. seed by using HPTLC method. Int Res J Pharm.. 2013;4(3):186-8. [CrossRef] | [Google Scholar]

- Singh B, Ishar MPS, Sharma A. Estimation of quercetin, an anxiolytic constituent, in . J Pharmacogn Phytochem.. 2013;1:117-21. [CrossRef] | [Google Scholar]

- Oki T, Masuda M, Furuta S, Nishiba Y, Terahara N, Suda I., et al. Involvement of anthocyanins and other phenolic compounds in radical-scavenging activity of purple-fleshed sweet potato cultivars. Food Chem Toxicol. 2002;67(5):1752-6. [CrossRef] | [Google Scholar]

- Gross M.. Flavonoids and cardiovascular disease. Pharm Biol. 2004;42(S1):21-35. [CrossRef] | [Google Scholar]

