ABSTRACT
Introduction
Ovarian cancer is the fifth most common cause of cancer which causes more deaths than any other cancer of the female reproductive system because there is no effective screening and most women are diagnosed at advanced stages. The probability of survival at 5 years is less than 30%, and the limitation is that it will not respond to chemotherapy protocol and surgery as well. Nandhi mezhugu is a Siddha herbo-mineral drug widely used to treat cancer. Hence, the present study was intended to evaluate the anti-cancer activity of Nandhi mezhugu on (PA-1) cell line through MTT assay.
Materials and Methods
Stock solutions were prepared from the formulation at various concentrations with serial dilution. Compared with the control and Methotrexate, the extracts were tested using MTT assay at different concentrations.
Results
This study substantiated that the percentage of cell viability of cell line viability decrease with increase in concentration of the test drug NM. Least viability of cell was observed at the concentration of 200ug/mL was 73.02 ± 4.584%, followed by this at 100 ug and 50 ug shows 82.36 ± 3.084%, 87.81 ± 2.657, similarly 10 ug/mL shows 95.89 ± 1.054% cell viability in MTT assay.
Conclusion
Thus, the current study brings forth scientific evidence for the efficacy of Nandhi mezhugu against the ovarian cancer (PA1) cell line.
INTRODUCTION
Ovarian cancer is the fifth common cause of death in women worldwide.1 In 2020, there are approximately 21,750 new ovarian cancer cases, which comprise 1.2% of all cancer cases. The estimated number of deaths related to it are 13, 940.2 Because most of the ovarian cancers are not detected until the disease has metastasized beyond the ovary, the 5-year survival rate for all cases is less than 30%.3 This leads to cause the poor outcome of this disease. Ovarian cancer may occur at any age, but it is more common in post-menopausal women.4
The low diagnostic value of the existing cancer screening tests contributing further to this agony. Detailed gynecological evaluation along with transvaginal ultrasound and laboratory marker like cancer antigen-125 (CA-125) assay are the key early detection strategies have not significantly reduced the morbidity or mortality of this malignancy.5 In Conventional medicine, it includes surgical debulking followed by chemotherapy.6
Chemotherapy is one of the common methods of treating cancer. However, the chemotherapeutic drugs are highly toxic and cause terrible side effects. Various new strategies are being developed to control and treat several human cancers.7 Nowadays there is an increased trend towards the herbal medicines for the treatment of cancer which is less toxic and more affordable.8
Siddha system of medicine is the traditional medicine to cure many chronic diseases. Many Siddha formulations used to treat the cancer. It acts as the therapeutic agents or co-therapy along with chemotherapy to increase the quality of life.9 Nandhi mezhugu (NM) is one of the Siddha herbo-mineral drug indicated for various types of diseases including ovarian cancer, testicular cancer, cancer penis, cancer cervix, oral and cheek cancers, etc., as mentioned in the text Siddha vaidya thirattu.10 Hence, the present study was taken up for evaluating the anti-cancer potential of Nandhi mezhugu in ovarian cancer line (PA-1) by MTT assay.
MATERIALS AND METHODS
Trial drug
The trial drug was prepared as per the Siddha text “Siddha vaidya thirattu” and it was procured from IMPCOPS Pharmacy (Indian Medicine Practitioners Co-operative Society), Chennai.
Preparation of test solutions
Serial dilutions of test formulation (10, 50, 100 and 200μg/mL) and methotrexate 10μg/mL.
In vitro anti-cancer activity
Human ovarian (PA-1) cancer cell culture and media
PA-1 cell lines were procured from National Centre for Cell Science, Pune, India, stock cells were cultured in medium supplemented with DMEM (Dulbecco’s Modified Eagle Medium), penicillin (100 IU/mL), streptomycin (100μg/mL) in a humidified atmosphere of 5% CO2 at 37°C until confluent. The cell was dissociated with Trypsin Phosphate Versene Glucose (TPVG) solution (0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS). The viability of the cells is checked and centrifuged. Further 50,000 cells/well was seeded in a 96 well plate and incubated for 24 hr at 37°C, 5% CO2 incubator.
Source of reagents
DMEM, FBS, Pen strip, Trypsin procured from Himedia.
Anti-proliferation assay
The monolayer cell culture was trypsinized and the cell count was adjusted to 1.0 x 105 cells/mL using respective media containing 10% FBS. To each well of the 96 well microtiter plate, 100μL of the diluted cell suspension (50,000 cells/well) was added. After 24 hr, when a partial monolayer was formed, the supernatant was flicked off, washed the monolayer once with medium and 100μL of different test concentrations of test drugs were added on to the partial monolayer in microtiter plates. The plates were then incubated at 37°C for 48 hr in 5% CO2 atmosphere. After incubation the test solutions in the wells were discarded and 100 μL of MTT (5 mg/10 mL of MTT in PBS) was added to each well. The plates were incubated for 4hr at 37°C in 5% CO2 atmosphere. The supernatant was removed and 100μL of DMSO was added and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader at a wavelength of 570 nm. The percentage growth inhibition was calculated using the following formula and concentration of test drug needed to inhibit cell growth by 50% (IC50) values is generated from the dose-response curves for each cell line.11,12
Asample – Absorbance sample; Ab – Absorbance background; Ac – Absorbance control.
IC50 Value
The half maximal inhibitory concentration (IC50) is a measure of the effectiveness of a compound in inhibiting biological or biochemical function. This quantitative measure indicates how much of a particular drug or other substance (inhibitor) is needed to inhibit a given biological process (or component of a process, i.e., an enzyme, cell, cell receptor or micro-organism) by half.
The IC50 of a drug can be determined by constructing a dose-response curve and examining the effect of different concentrations of antagonist on reversing agonist activity. IC50 values can be calculated for a given antagonist by determining the concentration needed to inhibit half of the maximum biological response of the agonist. IC50 values for cytotoxicity tests were derived from a nonlinear regression analysis (curve fit) based on sigmoid dose response curve.
MTT Assay
The in vitro determinations of anti-proliferative effects of the test formulation have been performed by counting viable cells after staining with a vital dye. The MTT system is a means of measuring the activity of living cells via mitochondrial dehydrogenases. The MTT method is simple, accurate and yields reproducible results. The key component is (3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) or MTT, is a water soluble tetrazolium salt upon incubation MTT is converted to an insoluble purple formazan by cleavage of the tetrazolium ring by mitochondrial dehydrogenase enzymes of viable cells. The resulting-colored solution is spectrophotometrically measured. An increase or decrease in cell number results in a concomitant change in the amount of formazan formed, indicating the degree of cytotoxicity caused by the test material.13
RESULTS
In vitro anti-cancer evaluation of test drug NM on the cell viability against Human ovarian (PA-1) cell line was performed at varying concentration ranges from 10 to 200 µg/mL. The result obtained from this study reveals that the percentage of cell viability of PA-1 cell line viability decrease with increase in concentration of the test drug NM. Least viability of cell was observed at the concentration of 200µg/mL was 73.02 ± 4.584%, followed by this at 100 µg and 50 µg shows 82.36 ± 3.084%, 87.81 ± 2.657, similarly 10 µg/mL shows 95.89 ± 1.054% cell viability in MTT assay (Table 1, Figure 1, 2). The corresponding IC50 value was found to be 402.1 ± 71.53 µg/mL. Morphological Comparison of Human ovarian (PA-1) cell line treated with test drug NM at varying concentrations and standard Control mentioned in Figure 3.
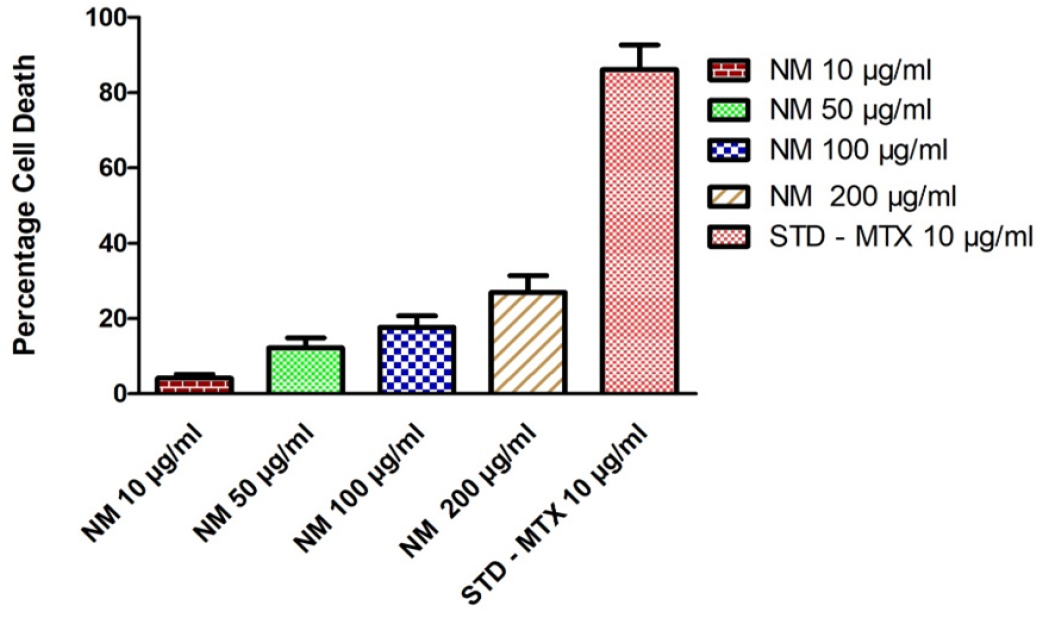
Figure 1:
Anti-Cancer activity of NM on cell viability of Human ovarian (PA-1) cell line.
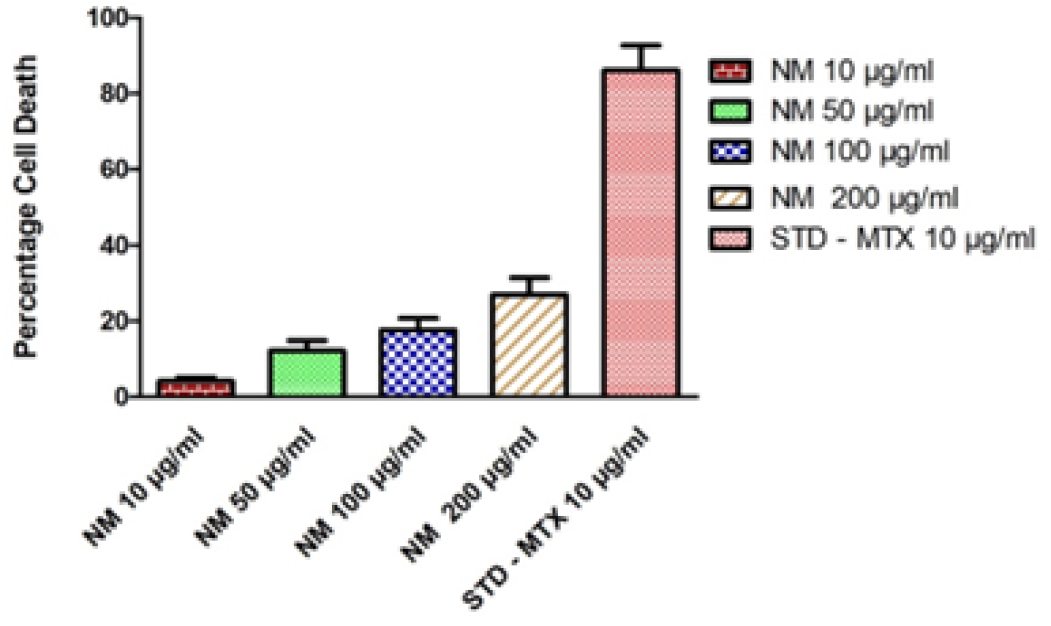
Figure 2:
Anti-Cancer activity of NM on cell death of Human ovarian (PA-1) cell line.
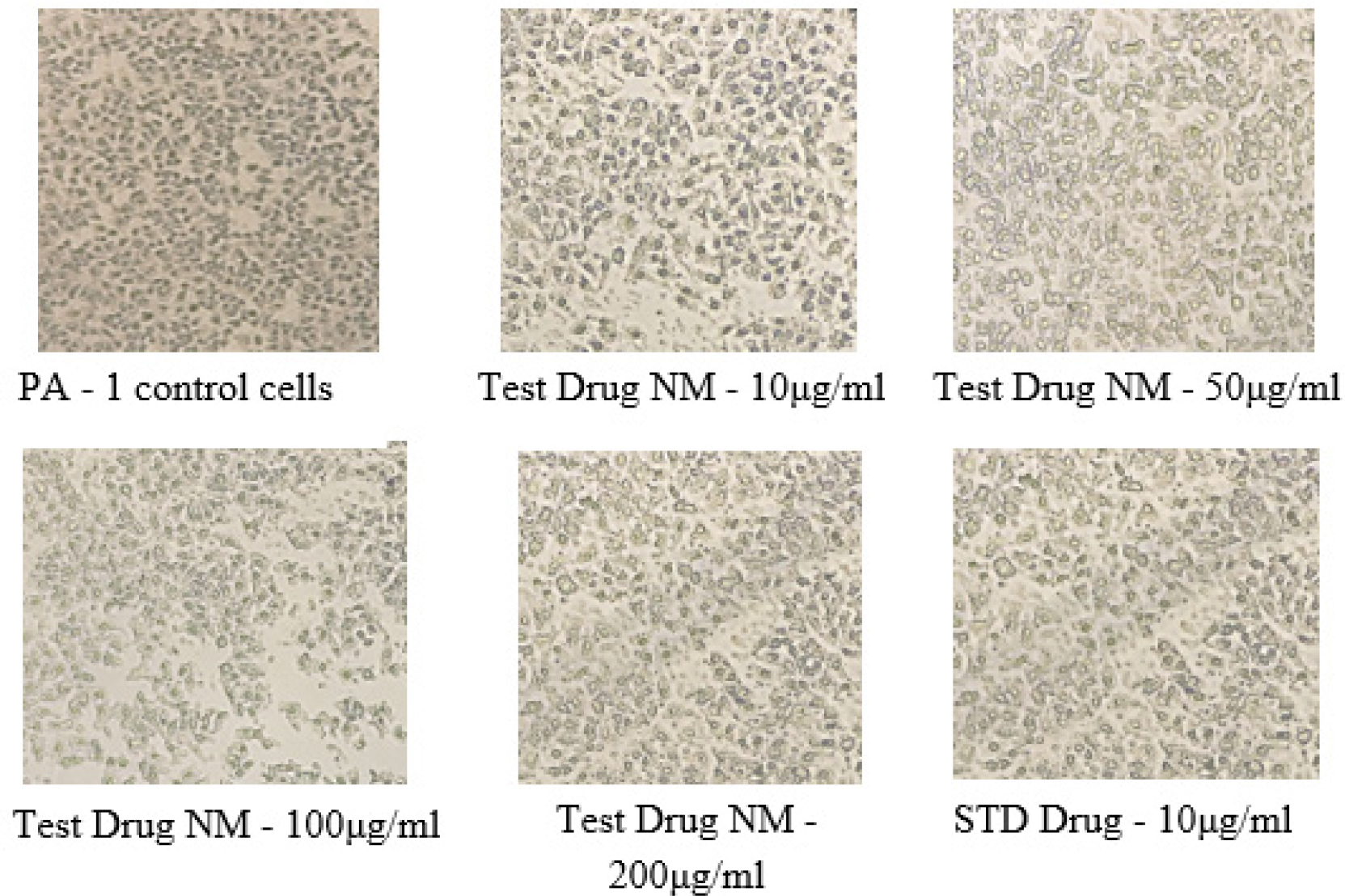
Figure 3:
Morphological Comparison of Human ovarian (PA-1) cell line treated with test drug NM at varying concentrations and standard Control.
| Sl. No | Concentration µLg/mL | % Cell Viability | % Cell Death | IC50 value of NM |
|---|---|---|---|---|
| 1 | 10 µg/ml | 95.89 ± 1.054 | 4.109 ± 1.053 |
402.1 ± 71.53 |
| 2 | 50 µg/ml | 87.81 ± 2.657 | 12.19 ± 2.658 | |
| 3 | 100 µg/ml | 82.36 ± 3.084 | 17.64 ± 3.084 | |
| 4 | 200 µg/ml | 73.02 ± 4.584 | 26.98 ± 4.584 | |
| 5 | STD (Methotrexate 10 µg/ml) | 13.86 ± 6.539 | 86.14 ± 6.54 |
DISCUSSION
Cancers are the group of disease associated with the unregulated and unrestricted proliferation of cells.14 Cancer is the primary cause of morbidity and mortality in the world.15 In developed nations, especially for women over 40, ovarian cancer is the second most frequent malignancy after breast cancer which has the highest mortality rate.16 Chemotherapy is thought to be one of the most potential cancer treatment methods. Cancer patients’ symptoms and quality of life are considerably improved by chemotherapy, although the survival rate can only be slightly increased.17 Chemotherapeutic agents usually associated with some side effects. To curb this issue, scientists have been conducted many anticancer researches in herbs and alternative medicine to evaluate the natural chemotherapeutic agents.18
Nandhi mezhugu is the Siddha medicine comprises the potential herbs and some metallic, mineral compounds which is used against the all types of cancer. In the current study, the MTT assay was used to determine the impact of different concentrations of NM on the viability of PA-1 cell lines. It is one of the most common employed in vitro models to assess the cytotoxic potential of plant extracts against cancer cell lines.19 Many of the herbs present in the test drug have phytochemicals possess with antioxidant and anticancer agents. Antioxidants have the efficacy to prevent and cure cancer and other diseases by protecting cells from damage produced by ‘free radicals’ which are extremely reactive oxygen molecules.19 Previous studies have substantiated the antioxidant activity of some ingredients of the test drug. Cuminum cyminum oil revealed the highest antioxidant activity in all conducted assays especially in β-carotene bleaching test and Ferric-Reducing Antioxidant Power (FRAP) Assay.20 Antioxidant activity study of Syzygium aromaticum oil showed high DPPH scavenging capacity and low hydroxyl radical inhibition.21 Aqueous and ethanol extracts of Piper nigrum exhibited the powerful antioxidant activity in all assays.22 Various extracts of Piper longum, Elettaria cardamomum and Nigella sativa oil showed good antioxidant activity.23–25
The cytotoxic and anti-proliferative effects of most of the ingredients of this formulation have been reported. Most of the scientists throughout the world working in this field to find a lead compound which can block the development of cancer in humans.26 Chemical compounds and phytoconstituents found in the ingredients of the test drug may have contributed to its anticancer activity. Semecarpus anacardium is the principal ingredient of this formulation and numerous reports in recent years revealed the anticancer characteristics of this herb in cancer cell lines through decrease in Bcl-2 and increase in Bax, cytochrome c, caspases, Poly (ADP-ribose) Polymerase (PARP) cleavage, and ultimately by internucleosomal DNA fragmentation.27 The presence of alkaloids strychnine and brucine in Strychnos nux-vomica is responsible for the anti-proliferative and cytotoxic activity.28 Plumbagin, a naturally occurring naphthoquinone component found in the roots of the medicinal plant Plumbago zeylanica, showed anticancer activity against almost all the cancer cell lines.29 According to earlier research, the presence of metallic compounds such as Mercury, Sulphur, Red Sulphide of mercury, Zinc sulfate, Mercurous chloride, Mercuric sulfide may exhibit the anticarcinogenic activity.30 The arsenic compounds such as Arsenic monosulphide, Arsenic trisulphide induces apoptosis, inhibits cell growth, inhibits angiogenesis, and may modulate the immune system, as shown by in vitro and in vivo studies. These activities together produce a broad spectrum of anti-cancer effects.31,32 Hence, Nandhi mezhugu presents a strong case of synergism as well as additions of the heterogeneous chemicals from the 53 constituents, the majority of which interfere with cancer in one or more aspects that have been scientifically demonstrated.
CONCLUSION
This study substantiated that the test drug Nandhi mezhugu possess the cytotoxic and anti-proliferative activity against ovarian cancer cell line (PA1). Hence, it can be used as the therapeutic anticancer agent for ovarian cancer. In future, we have made an effort to investigate the anticancer activity of this formulation through both in vivo and in vitro method in various assays. Further work is required to discover the powerful anticancer Siddha formulation and protect people all over the world from cancer.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin.. 2020;70(1):7-30. [CrossRef] | [Google Scholar]
- Shepherd JE. Current strategies for prevention, detection, and treatment of ovarian cancer. J Am Pharm Assoc (Wash). 2000;40(3):392-401. [PubMed] | [CrossRef] | [Google Scholar]
- Roett MA, Evans P.. Ovarian cancer: an overview. Am Fam Phys. 2009;80(6):609-16. [PubMed] | [Google Scholar]
- Modugno F, Edwards RP. Ovarian cancer: prevention, detection, and treatment of the disease and its recurrence. Molecular mechanisms and personalized medicine meeting report. Int J Gynecol Cancer.. 2012;22(S2):S45-57. [CrossRef] | [Google Scholar]
- Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460-73. [CrossRef] | [Google Scholar]
- Liu FS. Mechanisms of chemotherapeutic drug resistance in cancer therapy-a quick review. Taiwan J Obstet Gynecol.. 2009;48(3):239-44. [PubMed] | [CrossRef] | [Google Scholar]
- Gupta S, Zhang D, Yi J, Shao J.. Anticancer activities of . J Herb Pharmacother.. 2004;4(1):21-33. [CrossRef] | [Google Scholar]
- [CrossRef] | [Google Scholar]
- Kuppusamy KN mudaliyar. Siddha Vaidhya Thirattu, Department of Indian medicine and homeopathy, Chennai, Edition. 2009:183-9. [CrossRef] | [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106(3):761-71. [CrossRef] | [Google Scholar]
- Beaufort CM, Helmijr JCA, Piskorz AM, Hoogstraat M, Ruigrok-Ritstier K, Besselink N, et al. Ovarian Cancer Cell Line Panel (OCCP): clinical importance of morphological subtypes. PLOS ONE.. 2014;9(9):9.e103988 [CrossRef] | [Google Scholar]
- Gonzalez RJ, Tarloff JB. Evaluation of hepatic sub cellular Fractions for alamar blue and MTT reductase activity. Toxicol Vitro. 2001;15:259-9. [CrossRef] | [Google Scholar]
- [CrossRef] | [Google Scholar]
- Singh . Challenges in cancer research in India. Indian J Med Res. 2018;148:362-5. [CrossRef] | [Google Scholar]
- . [Jun 6, 2022];Centers for disease control and prevention. [CrossRef] | [Google Scholar]
- rajesh Gacche N, Shaikh RU, Pund MM. Array. Asian J Trad Med.. 2011;6(3) [CrossRef] | [Google Scholar]
- Skehn P, Storeng R, Scudiero A, Monks J, McMohan D, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer drug screening. J Natl Cancer Inst. 1990;82:1107-12. [CrossRef] | [Google Scholar]
- Caragay AB. Cancer-preventative foods and ingredients. Food Technol. 1992;46:65-8. [CrossRef] | [Google Scholar]
- . Chemical composition and antioxidant activity L. essential oils. Int J Food Prop.. 2016;19(2):438-42. [CrossRef] | [Google Scholar]
- Radünz M, da Trindade MLM, Camargo TM, Radünz AL, Borges CD, Gandra EA, et al. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove ( L.) essential oil. Food Chem. 2019;276:180-6. [CrossRef] | [Google Scholar]
- Gülçin İ. The antioxidant and radical scavenging activities of black pepper () seeds. Int J Food Sci Nutr.. 2005;56(7):491-9. [CrossRef] | [Google Scholar]
- Kumar S, Malhotra S, Prasad AK, Van der Eycken EV, Bracke ME, Stetler-Stevenson WG, et al. Anti-inflammatory and antioxidant properties of Piper species: a perspective from screening to molecular mechanisms. Curr Top Med Chem. 2015;15(9):886-93. [CrossRef] | [Google Scholar]
- Singh G, Kiran S, Marimuthu P, Isidorov V, Vinogorova V.. Antioxidant and antimicrobial activities of essential oil and various oleoresins of (seeds and pods). J Sci Food Agric.. 2008;88(2):280-9. [CrossRef] | [Google Scholar]
- Burits M, Bucar F.. Antioxidant activity of essential oil. Phytother Res.. 2000;14(5):323-8. [CrossRef] | [Google Scholar]
- [CrossRef] | [Google Scholar]
- Mathivadhani P, Shanthi P, Sachdanandam P.. Apoptotic effect of nut extract on T47D breast cancer cell line. Cell Biol Int.. 2007;31(10):1198-206. [CrossRef] | [Google Scholar]
- Rao PS, Ramanadham M, Prasad MNV. Anti-proliferative and cytotoxic effects of root extract on human multiple myeloma cell line – RPMI 8226. Food Chem Toxicol.. 2009;47(2):283-8. [CrossRef] | [Google Scholar]
- Yin Z, Zhang J, Chen L, Guo Q, Yang B, Zhang W, et al. Anticancer effects and mechanisms of action of plumbagin: review of research advances. BioMed Res Int. 2020;2020:1-10. [CrossRef] | [Google Scholar]
- Vinardell MP, Mitjans M.. Antitumor activities of metal oxide nanoparticles. Nanomaterials. 2015;5(2):1004-21. [CrossRef] | [Google Scholar]
- Dong Y, Li X. Prospect of research and clinical application of arsenic compounds in chemotherapy for gynecological malignant tumors, Gynecology and Obstetrics Clinical Medicine. 2022;2(1):23-8. [CrossRef] | [Google Scholar]
- Hu J, Fang J, Dong Y, Chen SJ, Chen Z.. Arsenic in cancer therapy. Anti Cancer Drugs. 2005;16(2):119-27. [CrossRef] | [Google Scholar]
