ABSTRACT
Introduction
Although adverse drug reaction (ADR) monitoring is widely known, it is not practised in underdeveloped nations due to a lack of awareness and the absence of a central coordinating agency. The recent implementation of the National Pharmacovigilance Program has encouraged ADR monitoring in some centres.
Aim
The purpose of this study was to evaluate the sternness of described ADRs, the additional financial cost associated with ADRs, and the present load of ADRs at the RDT hospital in Battalapalli, AP, India.
Materials and Methods
Over 26 months of hospital admissions of patients, which were managed by hospital staff, a prospective, spontaneous reporting research was carried out.
Results
37 of the 74 adverse drug events (ADEs) that were reported by 56 individuals were indeed ADRs. There were 521 patients admitted, and 9.7% of those ADRs occurred during hospitalisation. Males (56%) had ADRs more often than females (44%). During the hospital stay, no discernible difference between males and females was seen. ADR rates were 19, 20, and 61% for paediatric, geriatric, and adult patients. There were 88 minor responses or 53.7% of the total. The majority of patients (72.6%) recovered from the incident. The majority of the responses show that they were unexpected and possibly avoidable.
Conclusion
According to the study’s findings, 90% of ADRs might be prevented, saving the health system money and decreasing patient expenditures. To prevent unknown and severe ADRs, new medications should be continuously monitored.
INTRODUCTION
The World Health Organization (WHO) describes an adverse drug reaction (ADR) as any harmful, unexpected, or unintentional impact of a medicine that happens at dosages utilised in humans for prevention, verdict, or healing.1 ADRs are a significant contributor to indisposition and significantly tax the system’s meagre healthcare resources.2 Several variables, such as various drug regimens, the sternness of the condition, age, and the kind and quantity of prescribed medications, have an impact on ADR susceptibility. Among developed and developing nations, there are notable disparities in disease prevalence, availability of medications, drug use habits,3,4 and drug management systems, and these variations affect the frequency and makeup of ADRs.5,6
Pharmacovigilance is the science and practice of identifying, evaluating, comprehending, and preventing side effects and other problems associated with drugs or vaccines.7,8 Before they are officially approved for use, all medications and vaccines go through extensive testing for both safety and efficacy through clinical trials.9,10
Recognized as a frequent reason for hospital admissions, adverse drug reactions (ADRs) place a heavy financial burden on hospitals.11,12 The purpose of hospital-oriented ADR watching and recording suites is to detect and measure the hazards related to using medications supplied in a hospital environment. This knowledge may help in recognising and reducing avoidable ADRs and may improve prescribers’ capacity to handle ADRs more skillfully.13
MATERIALS AND METHODS
The investigation was conducted at the RDT Hospital, Bathalapalli, AP, India. This 245-bed secondary care facility serves the less fortunate segments of society. RDT began its first rural hospital and its work with the rural populations to raise awareness in 1978 to fill this gap, but such efforts were insufficient. Vicente and Anne Ferrer established RDT Hospital. To deliver high-quality healthcare at a reasonable cost, they intended to establish medical infrastructure in a remote location. The hospital offers both surgery and general medical sections. The hospital had formulations 159 and 335 of the medicines on hand.
At RDT Hospital in Bathalapalli, Andhra Pradesh, India, a prospective study was carried out for 26 months, from November 2017 to January 2020. Physicians, nurses, and pharmacists worked together to plan the study. A method of spontaneous reporting was used. Both the hospital’s superintendent and the institutional human ethics committee gave their consent. All patients suspected of having ADRs gave their consent before documenting. The study included two male and two female patients from the hospital’s medical wards and intensive care unit. Patients who had either intentionally or unintentionally poisoned themselves were also excluded from the trial, as were drug addicts. The formalised pharmacovigilance programme at the hospital before the trial was not there.
ADR observation was raised in experimental sessions through hospital well-being and associated health-concerned workers. Clinical pharmacists regularly provide clinical pharmacy services, which included attending ward rounds with the doctors. These pharmacists pushed the doctors to bang potential adverse drug events (ADEs) through the ward rounds. Additionally, when clinical pharmacists had suspicions of ADEs, they immediately informed the treating physicians so that they could plug out the notification forms if they shared those suspicions. Additionally, nurses completed the announcement procedures. Clinical pharmacists did not complete the statement systems themselves. Different forms were created with the study’s objectives in mind. ADR assessment and classification forms, patient and their reply details forms, and announcement forms were among them. The contributing wards maintained notice forms. All inpatients were evaluated for ADRs through the trial epoch. The patient’s prior medical and medication histories were acquired in the suspicious cases. Daily patient monitoring and interviews were conducted.
While their stay in the hospital and health records were examined, the alleged ADRs underwent thorough examination and documentation. All pertinent information was recorded, including all medications taken by the patient before the response started, their dosage, method of administration, frequency, the date the feedback started, and the patient’s drug and food allergies. The patient’s medical history and any comorbidities were also noted. At the RDT hospital, a board of juries comprised of four clinicians and a clinical pharmacist was formed to analyse ADR bangs to determine causality and settle ADRs. The panel convened once a month to discuss and evaluate the ADE issues.14 The ADRs that were thus established were confidential and given a sternness rating. When there was a variance of opinion among the reviewers over whether a certain incident qualified as an ADR, the matter was debated until an agreement was reached. The comments made by the treating physician were given more weight in this regard. When an agreement could not be reached, the report was labelled “unsubstantiated.” The contributory link between the ADR and the alleged pharmacological healing was evaluated. The following are the harshness levels of these reactions.15,16
Slight reactions that were self-preventive, able to resolve on their own with time, and did not contribute to the lengthening of the stay. Sensible ADRs were classified as requiring obligatory prolonged therapeutic intervention and one day in the hospital but were specifically treated to avoid a worse outcome or resolved in 24 hours. Severe ADRs were distinct as they were life-threatening, caused damage, were essential for a lengthy hospital stay, required rigorous medical care, or resulted in the patient’s demise.17
Patient consequences were listed as demise, fully healthier (since the patient improved while in the hospital), improving (meaning the patient partially healed while in the hospital), and inexact (not recognised in the chart after the early crash).18,19
The quantity consumed on patients alienated by the entire patients (n = 521) was used to determine the cost associated with addressing the documented ADRs. The cost of dealing was regarded as zero when the offending substance was withdrawn and the prescribed course of handling was maintained. The hospital costs were calculated for all situations involving outlays for medicines, testing in the lab, syringes, etc. This extra price of the overhaul was included in the overall cost if the patient had to be moved from the ward to which he or she had been known to the critical care unit to manage ADRs.
However, as hospital room rent varies depending on the kind of room the patient remained in, it was not factored into the cost calculation. Also excluded from the cost calculation were nursing and doctor services. Thank you cards were delivered to everyone who completed the notice list, and printed information sheets with updates on the ADRs produced at the hospital were frequently distributed.
Statistical analysis
Rates of ADR-connected charges and ADR incidence through the hospice break were premeditated as a fraction of the inpatient was preserved. The t-test of a pupil was utilised to associate means.20,21 The c2 test was applied to other variables. The arithmetical implication was demarcated as a two-tailed P value of <0.05.
RESULTS
The assessment details of ADR are as per Table 1.
| Age group | Number of ADR reports | Gender | Number of ADR reports |
|---|---|---|---|
| Paediatric | 14 | Male | 42 |
| Adult | 45 | Female | 32 |
| Geriatric | 15 | ||
| Total | 74 | 74 |
The brutality, outcomes and usage for the ADR were as per Table 2 and Figure 1.
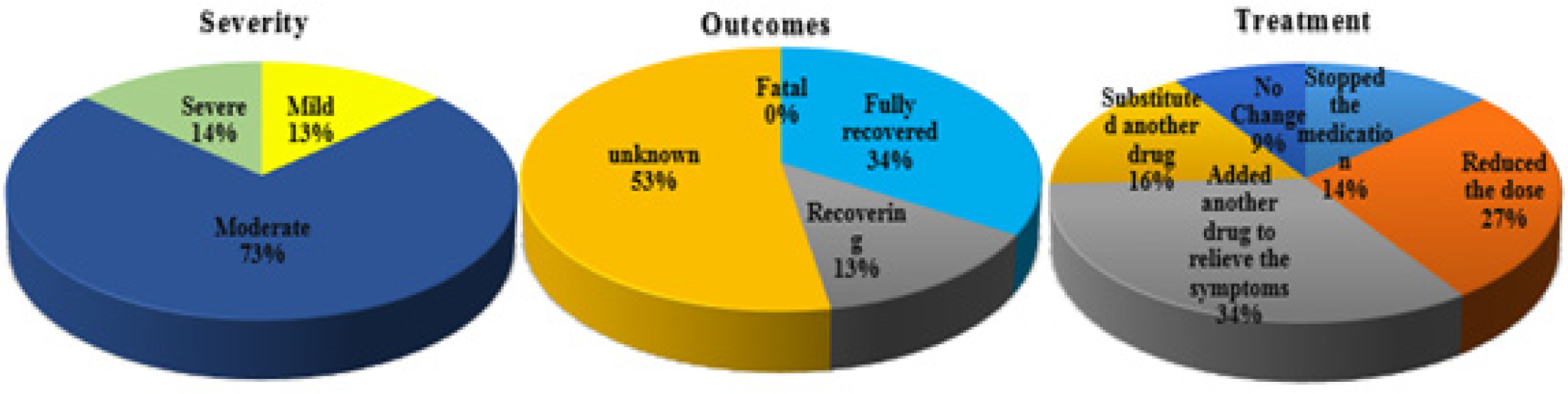
Figure 1:
Graphical glimpses of the severity, outcomes and treatment for ADR.
| Parameter | ADR | |
|---|---|---|
| Number | % | |
| Severity | ||
| Mild | 10 | 13.51 |
| Moderate | 54 | 73.00 |
| Severe | 10 | 13.51 |
| Outcomes | ||
| Fatal | 00 | 00.00 |
| Fully recovered | 25 | 33.78 |
| Recovering | 10 | 13.51 |
| Unknown | 39 | 52.70 |
| Treatment | ||
| Stopped the medication | 10 | 13.51 |
| Reduced the dose | 20 | 27.02 |
| Added another drug to relieve the symptoms | 25 | 33.78 |
| Substituted another drug | 12 | 16.21 |
| No change | 07 | 09.45 |
Various drug classes of ADR were as per Figure 2. The organs involved in the ADR were as per Figure 3.
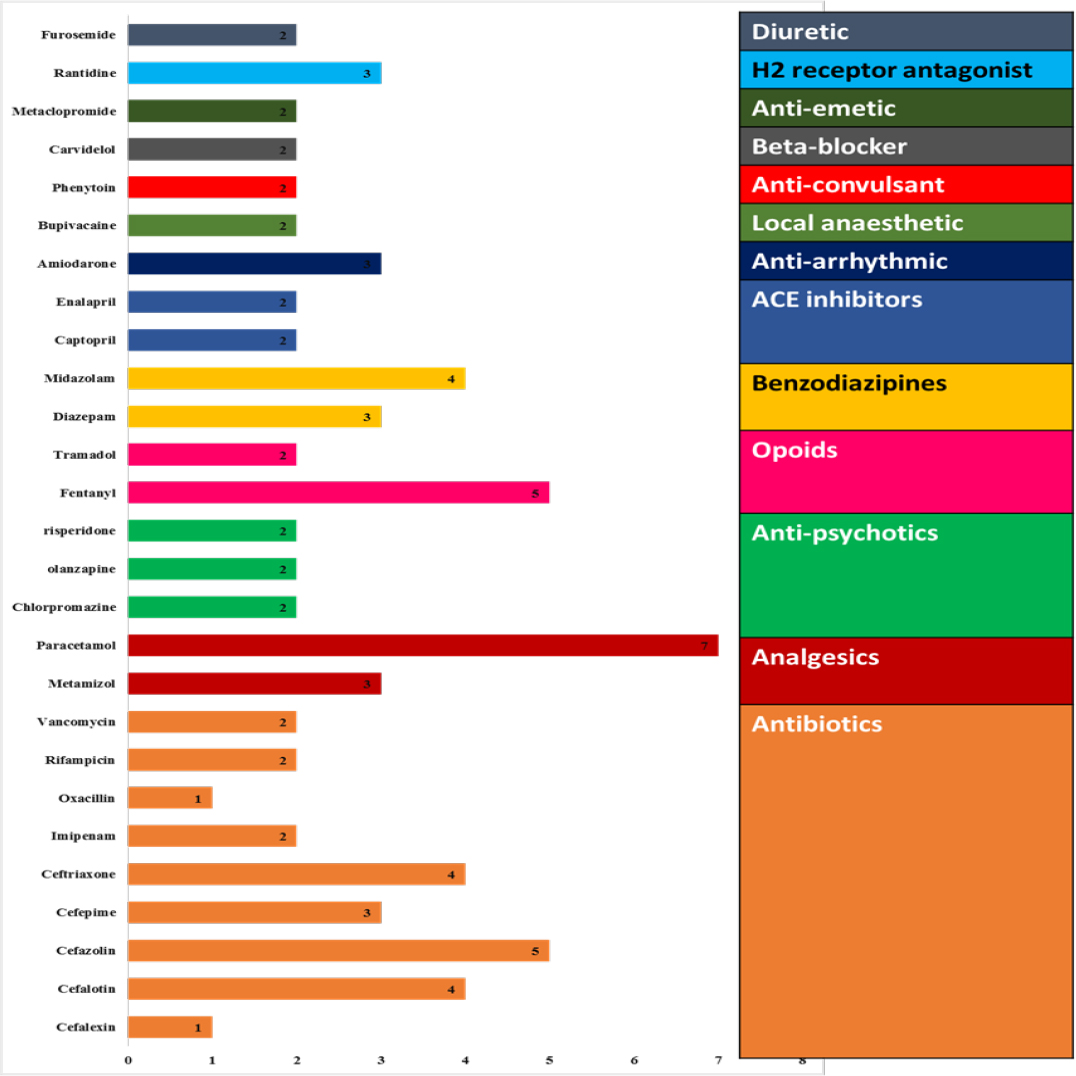
Figure 2:
various drug classes of ADR.
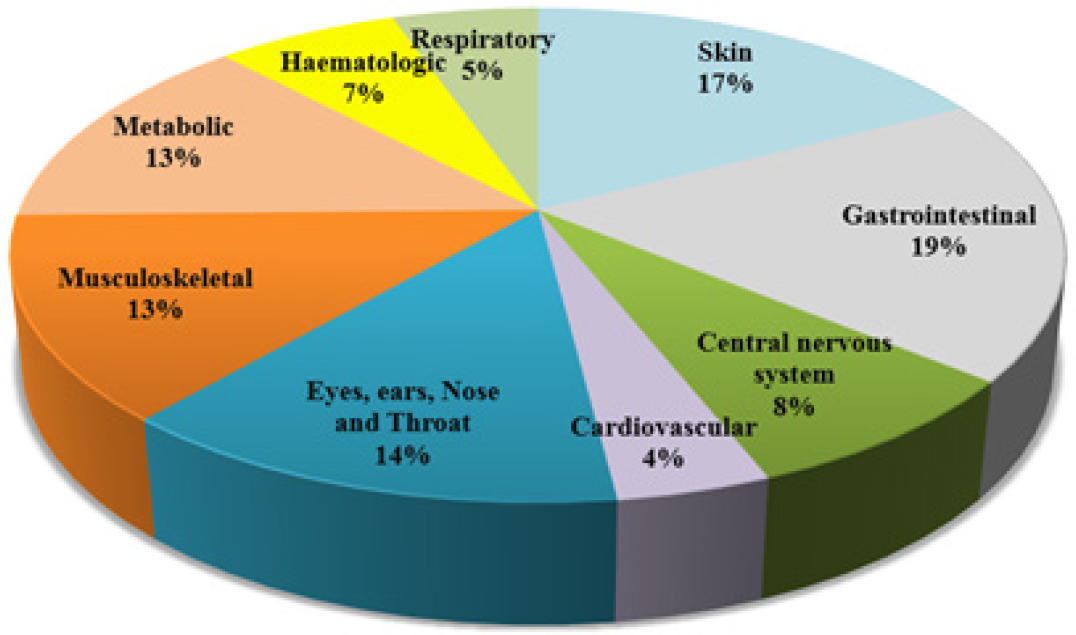
Figure 3:
Organs involved in the ADR.
DISCUSSION
56 individuals reported 74 persistent ADRs throughout the 22-month research period. There were 521 patients acknowledged, and 9.7% of ADRs happened when patients were hospitalised. Males (56%) had ADRs more often than females (44%). There was no discernible change between males and females during the hospital stay. ADR rates were 19, 20, and 61% for paediatric, geriatric, and adult patients, respectively. ADRs were caused by a multitude of variables, including the variety of medications used concurrently. We determined the median number of medications per patient thought to be responsible for ADRs, which came to 6.8% of all prescriptions, to evaluate the relevant causes.
The researchers also found that 7.4% of patients who took more than 6 medications had a greater risk of adverse drug reactions. More than half of the observed reactions were reasonable (73.00%), modest (13.51%), and severe (10%), as evidenced by the ruthlessness of the ADRs. In 33.78% of patients with ADRs, full recovery was achieved, 10% were still making progress, and 39% had undetermined outcomes. The alternatives for treatment included stopping the medicine (13.51%), cutting the dose (27.02%), adding another medication to treat the symptoms (33.78%), switching to another medication (16.21%), and not changing anything (9.45%). The table on the following slide lists the medicines that cause ADRs most frequently, along with specifics about each response.
Antibiotics account for about 10.81%; analgesics for about 13.51 %; anti-psychotics for about 8.1%; opioids for 9.45%; benzodiazepines for 9.45%; ACE inhibitors for 5.45%; anti-arrhythmic for 4.05%; local anaesthetics for 2.7%; anti-convulsants for 2.7%; beta-blockers for 2.7%; and anti-emetics for 2.7%.
CONCLUSION
Most ADRs might be avoided, saving the healthcare system money and ultimately benefiting patients. To work together toward ADR prevention, doctors, nurses, and pharmacists should be aware of potential clinical concerns by assessing medications that the patient has recently used. The ongoing monitoring of new drugs is necessary to prevent severe and unidentified ADRs. Most of the incidents were of moderate ruthlessness, and most of the results were unknown. The best result for preventing ADRs was obtained when another medication was added to treat symptoms. The majority of ADR cases were linked to antibiotic use, and the skin is the organ most commonly affected. Males and adults are the demographics most impacted by ADRs.
References
- Mascolo A, Scavone C, Sessa M, di Mauro G, Cimmaruta D, Orlando V, et al. Can causality assessment fulfill the new European definition of adverse drug reaction? A review of methods used in spontaneous reporting. Pharmacol Res. 2017;123:122-9. [PubMed] | [CrossRef] | [Google Scholar]
- Bangwal R, Bisht S, Saklani S, Garg S, Dhayani M. Psychotic disorders, definition, sign and symptoms, antipsychotic drugs, mechanism of action, pharmacokinetics and pharmacodynamics with side effects and adverse drug reactions: updated systematic review article. J Drug Delivery Ther.. 2020;10(1):163-72. [CrossRef] | [Google Scholar]
- Kumar N, Narendra B, Upendra K, Rajesh K.. A review on Adverse drug reactions monitoring and reporting. Int J Pharm Res Technol. 2019;9(2):12-5. [CrossRef] | [Google Scholar]
- Ahad HA, Chinthaginjala H, Dharani BHS. Country Wise Measures in Contrast to The Spread of SARS-COV2/COVID-19. 2022 [CrossRef] | [Google Scholar]
- Alhuzali H, Ananiadou S. Paper presented at: Proceedings of the 18th BioNLP Workshop and Shared Task 2019. [CrossRef] | [Google Scholar]
- Singh L, Abdul Ahad H, Pavan Kumar B, Madhusudhan V.. A Professional Opinion on the Delta AY.4.2 Variant: a Global Threat to Humanity. RJPPD. 2022:151-4. [CrossRef] | [Google Scholar]
- de Vries ST, Denig P, Ekhart C, Burgers JS, Kleefstra N, Mol PGM, et al. Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in the Netherlands: an explorative observational study. Br J Clin Pharmacol. 2019;85(7):1507-15. [PubMed] | [CrossRef] | [Google Scholar]
- Ahammed SGJ, Bhupalam P, Abdul Ahad HA, Chinthaginjala H, Rahamathulla S, Yadav S., et al. Black fungus: A lethal communal issue after winning the life battle against COVID-19. Biomed Pharmacol J. 2021;14(4):2095-100. [CrossRef] | [Google Scholar]
- Sato K, Mano T, Iwata A, Toda T.. Neurological and related adverse events in immune checkpoint inhibitors: a pharmacovigilance study from the Japanese Adverse Drug Event Report database. J Neurooncol. 2019;145(1):1-9. [PubMed] | [CrossRef] | [Google Scholar]
- Kumar YB, Ahad HA, Haranath C, Sumanth G, Pasupuleti DS, Reddy SS, et al. Platelet Rich plasma Therapy: A quick note for every health care professional. Int J Life Sci Pharm Res. 2020;10(5):84-9. [PubMed] | [CrossRef] | [Google Scholar]
- Paudyal V, Al-Hamid A, Bowen M, Hadi MA, Hasan SS, Jalal Z, et al. Interventions to improve spontaneous adverse drug reaction reporting by healthcare professionals and patients: systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(9):1173-91. [PubMed] | [CrossRef] | [Google Scholar]
- Yadiki MN, Suggala VS, Puchalapalli DSR, Ahad HA. Temperature and Exposure Time Impact on the Extraction of and Cladodes on% Yield as a Response: screening using Design Expert Software. GJMPBU. 2022:17 [CrossRef] | [Google Scholar]
- Imam F, Sharma M, Khayyam KU, Al-Harbi NO, Rashid MK, Ali MD, et al. Adverse drug reaction prevalence and mechanisms of action of first-line anti-tubercular drugs. Saudi Pharm J. 2020;28(3):316-24. [PubMed] | [CrossRef] | [Google Scholar]
- De Leon J, Ruan CJ, Schoretsanitis G, De las Cuevas C. A rational use of clozapine based on adverse drug reactions, pharmacokinetics, and clinical pharmacopsychology. Psychother Psychosom.. 2020;89(4):200-14. [PubMed] | [CrossRef] | [Google Scholar]
- Malki MA, Pearson ER. Drug–drug–gene interactions and adverse drug reactions. Pharmacogenomics J. 2020;20(3):355-66. [PubMed] | [CrossRef] | [Google Scholar]
- Geer MI, Koul PA, Tanki SA, Shah MY. Frequency, types, severity, preventability and costs of Adverse Drug Reactions at a tertiary care hospital. J Pharmacol Toxicol Methods.. 2016;81:323-34. [PubMed] | [CrossRef] | [Google Scholar]
- Pinheiro SMB, Castro JGD, Momenté VG, Pranchevicius M-CS. Adverse drug reaction monitoring: support for pharmacovigilance at a tertiary care hospital in Northern Brazil. Lobo MGAdA. BMC Pharmacol Toxicol. 2013;14(1):1-7. [PubMed] | [CrossRef] | [Google Scholar]
- Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9(11):1275-87. [PubMed] | [CrossRef] | [Google Scholar]
- Chinthaginjala H, Ahad HA, Sumanth G, Shaik M, Dasari RR. Hunting hantavirus: A quick guide for healthcare professionals before it invades the globe unlike COVID-19. Int J Pharm Phytopharmacol Res. 2020:1-6. [PubMed] | [CrossRef] | [Google Scholar]
- Annepogu H, Ahad HA, Nayakanti D. Determining the best poloxamer carrier for thiocolchicoside solid dispersions. Turk J Pharm Sci.. 2020;17(4):372-80. [PubMed] | [CrossRef] | [Google Scholar]
- Breitung J.. Rank tests for nonlinear cointegration. J Bus Econ Stat. 2001;19(3):331-40. [CrossRef] | [Google Scholar]
