ABSTRACT
Introduction
A simple, accurate, precise, sensitive, and validated RP-HPLC method was developed for simultaneous estimation of Gallic Acid (GA), Vasicine (VS), Pterostilbene (PTS) and Piperine (PP) from Ayurvedic Proprietary Formulations developed in two different dosage forms viz. Amarantha Gynorite syrup and Amarantha Gynorite capsule.
Materials and Methods
The optimized chromatographic condition was used for separation on Agilent Zorbax SB-C18 (5 um 4.6 x 250 mm), Rheodyne injector with volume of 20 uL and mobile phase consisting of Solvent A (70 percent methanol) and solvent B [10 nm Hexane sulfonic acid, Acetonitrile and Glacial Acetic acid (70:30:1)] in composition of 95: 05 v/v with isocratic elution for 40 min at flow rate of 0.5 mL/min. Quantification was carried out using a photodiode array detector at 280 nm. The method was validated in accordance with International Conference on Harmonization (ICH) guidelines.
Results and Conclusion
No chromatographic interference from syrup and capsule excipients was analyzed. Hence, the present analytical method is applicable for simultaneous determination of GA, VS, PTS and PP in Ayurvedic proprietary formulations.
INTRODUCTION
It has been estimated that not less than 80% of people in the world are relying on herbal medicines. Since last few decades, use of herbal medicines has been increasing for accomplishment of different therapeutic needs.1–3 This influences research and development (R&D) sector of pharmaceutical industries to achieve desired scientific quality standards in herbal medicinal products. Medicinal activity of herbs is primarily depend upon presence of secondary metabolites or phytochemicals. Identification, isolation, quantification, purification, and structural characterization of these phytochemicals with various assessment techniques help to improve the quality of finished products.1–3
Amarantha Gynorite syrup and Amarantha Gynorite capsule are Ayurvedic proprietary formulations manufactured by Ari Healthcare Private Limited for the management of menstrual problems such as dysmenorrhea, dysfunctional uterine bleeding, hypomenorrhea, oligomenorrhea, amenorrhea, PCOS, menorrhagia, metrorrhagia and menometrorrhagia.4–6 Both formulations contain Saraca indica (Ashoka bark) extract, Adhatoda vasica (Vasa leaf) extract, Pterocarpus marsupium (Bijaka stem) extract and Trikatu extract as key ingredients in combination with other ingredients. Gallic Acid (GA), Vasicine (VS), Pterostilbene (PTS) and Piperine (PP) are major marker compounds considerably responsible for pharmacological actions of both the formulations.4–12
Gallic acid or 3,4,5-trihydroxybenzoic acid is one of the most abundant phenolic acids of many plants responsible for anti-inflammatory, free radical scavenging and antioxidant activities.7 Vasicine (peganine) is a quinazoline alkaloid. It is one of the major chemical compounds found in Adhatoda vasica. VS chemically characterized as 1,2,3,9-Tetrahydropyrrolo[2,1-b]quinazolin-3-ol. VS is reported to have antioxidant, anti-inflammatory and immunomodulator activities.8 Pterostilbene (trans-3,5-dimethoxy-40-hydroxystilbene) is a natural polyphenol and a dimethyl ether analog of resveratrol. Various research studies have reported that PTS is useful in the management of gynecological disorders, hematological diseases and vascular dysfunction.9 Research studies showed that pterostilbene treatment significantly increases uterus and ovarian index, FSH, LH and regulates secretion of gonadotropin hormones.10 Piperine (C17H19NO3) is an alkaloid found in Piper longum and Piper nigrum. PP is potent bioavailability enhancer. It exerts antioxidant, antiplatelet, anti-inflammatory, antihypertensive, hepatoprotective, antithyroid and antitumor activities.11,12
Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) is one of the prime options of phytochemical analysis with advancement of isocratic and gradient elution.13–15 It is a sophisticated method available for estimation of GA, VS, PTS and PP individually or in combination with other molecules.13–21 As estimation of GA, VS, PTS and PP individually is time and cost consuming by HPLC; this study was aimed towards the development of cost efficient method with less time requirement for simultaneous separation and determination of GA, VS, PTS and PP. In order to standardize Amarantha Gynorite syrup and Amarantha Gynorite capsule an attempt was made to develop simultaneous determination of four different marker compounds by using RP-HPLC method.
MATERIALS AND METHODS
Instrumentation
The chromatography was performed on water (Waters e2695 Alliance system with a 2996 PDA) HPLC instrument equipped with Photo diode Array Detector with wavelength range of 210 nm to 400 nm and empowers software. Agilent Zorbax SB-C18 (4.6 x 250 mm, 5 μ particle size) was used as stationary phase. Rheodyne manual injector with 100 μL capacity loop was used. Sartorius with weighing capacity of 0.1 mg – 220 g was used as analytical balance, pH meter of Labman and bath sonicator of LABTEK was used in the study.
Reagents and Materials
HPLC-grade solvents such as methanol, acetonitrile, glacial acetic acid, water and hexane-1-sulfonic acid sodium salt were purchased from Merck Life Science Private Limited, Mumbai, India. The reference standards were purchased from Natural Remedies Limited, Bangalore, India. Details of standards are mentioned in Table 1.
| Sl. No. | Name of Standard | Batch Number | Potency (%) |
|---|---|---|---|
| 1 | Gallic Acid (GA) | T12K021 | 99.0 |
| 2 | Vasicine (VS) | T22E155 | 98.2 |
| 3 | Pterostilbene (PTS) | H220659 | 95.0 |
| 4 | Piperine (PP) | T13D040 | 98.0 |
List of standards.
Amarantha Gynorite syrup and Amarantha Gynorite capsule were developed and supplied by Ari Healthcare Private Limited, Pune. Both dosage forms contain Saraca indica (Ashoka bark) extract, Aloe vera (Kumari leaf) extract, Pterocarpus marsupium (Bijaka stem) extract, Symplocos racemosa (Lodhra bark) extract, Caesalpinie bonducella (Latakaranja seed) extract, Adhatoda vasica (Vasa leaf) extract, Carum carvi (Krishna-Jiraka fruit) extract, Apium graveolens (Ajamoda fruits) extract, Trikatu extract as classical Ayurvedic formulation, Pushyanuga Choorna and Tankana and Kasisa Bhasma.
Preparation of Solutions
Preparation of Standard-stock Solutions
Four different standards were prepared namely Gallic Acid (GA), Vasicine (VS), Pterostilbene (PTS) and Piperine (PP). The standard stock solutions were prepared by dissolving 10 mg of each standard in 10 mL of methanol to obtain concentration 1000 μg/mL (1000 ppm).
Preparation of Working-standard Solution
From the standard stock solution 1 mL of each standards solution was pipette out and diluted with methanol up to 10 mL in volumetric flask to obtain concentration 100 μg/mL (100 ppm) as working standard solution.
Preparation of Calibration Curve
A calibration curve was established with seven different concentrations which were prepared by diluting working standard solution at concentrations ranging from 6 to 18 μg/mL. The dilutions are given in Table 2.
| Sl. No. | Concentration (μg/mL) | Dilution | |
|---|---|---|---|
| 1 | 6 | 1.5 | 25 |
| 2 | 8 | 2.0 | 25 |
| 3 | 10 | 2.5 | 25 |
| 4 | 12 | 3.0 | 25 |
| 5 | 14 | 3.5 | 25 |
| 6 | 16 | 4.0 | 25 |
| 7 | 18 | 4.5 | 25 |
Dilutions for linearity.
Preparation of Solvents
Solvent A was prepared using 70% methanol. The ratio of distill water to methanol was 30:70. Solvent B was prepared by solubilizing 4.1248 gm of hexane-1-sulfonic acid sodium salt in mill-Q water (1000 mL), and pH was adjusted with glacial acetic acid to 3.5. A mixture of hexane-1-sulfonic acid sodium salt and acetonitrile (70:30%v/v) were mixed, filtered and degassed.
Mobile Phase Preparation
Mobile phase was prepared using solvent A and B in ratio of 95:5% v/v.
Method Development
Depending on solubility, stability and suitability of standard solution, various compositions of mobile phase were performed to obtain sharp peak with good resolution. Individual standards and sample solutions were allowed to run in different mobile phase compositions to obtain better results. The optimized method was preferred because it gave sharper peak and good resolution at absorbance of 280 nm.
Chromatographic Conditions
The details on optimized parameters of chromatographic conditions for estimation of GA, VS, PTS and PP are mentioned in Table 3.
| Sl. No. | Parameter | Optimized condition |
|---|---|---|
| 1 | Mobile Phase | (95:05 v/v) |
| 2 | Column | Agilent Zorbax SB-C18 (5 µm 4.6 x 250 mm) |
| 3 | Flow rate | 0.5 mL/min |
| 4 | Absorbance | 280 nm |
| 5 | Injection Volume | 20 µL |
| 6 | Column Temperature | 25°C |
| 7 | Sample Temperature | 25°C |
Optimized condition for standard solution.
Preparation of sample and test solution
Accurately 100 mg capsule powder and 5000 mg of syrup solution were weighed in 50 mL of volumetric flask. About 40 mL of methanol was added and solutions were sonicated on ultrasonic water bath for 30 min at room temperature. Then solutions were allowed to cool at room temperature. Further, volume was made up to the mark with methanol. The resulting solutions were filtered with Whatman’s filter paper number-41 and through 0.45 μ syringe filter. The resulting solutions were used as test solutions.
Assay % of Standard-solution from Formulations
Both the formulations (Amarantha Gynorite syrup and Amarantha Gynorite capsule) were analyzed to determine the contents of GA, VS, PTS and PP as per method described under chromatographic conditions using RP-HPLC. Every single analysis was repeated three times and results were expressed in percent assay.
Analytical Method Validation
Specificity
As per ICH, specificity was carried out to make sure the identity of an analyte. Specificity of method was determined by comparing retention time and chromatogram of standards such as GA, VS, PTS and PP with diluent, placebo and sample solutions (Amarantha Gynorite syrup and Amarantha Gynorite capsule).
Linearity
As per ICH guidelines, determination of linearity was performed with minimum 7 concentrations. Linearity was performed by plotting peak area against concentration of standards and finding regression coefficient (R2).
Precision
Precision was performed in terms of system precision, method precision and intermediate precision. In system precision, the sample was analysed for six times using above mentioned procedure. Assay for each analyte was expressed in terms of % RSD. In method precision, sample was analysed for six times using above mentioned procedure. Assay for each analyte was expressed in terms of % RSD. Intermediate precision was performed on different systems, i.e., Waters e2695 Alliance system with a 2996 PDA and 2489 Ultraviolet (UV) detector by different analysts by analysing six different samples of extracts and was expressed in terms of % RSD.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
The values of Limit of Detection (LOD) and Limit of Quantitation (LOQ) were determined based on the standard deviation of response and slope of calibration graph. The quantitation was done with the help of equations. LOD = 3.3 σ/s; LOQ = 10 σ/s. Where, σ standard deviation of response estimated based on the calibration curve and s is slope of calibration curve.
Accuracy
Accuracy should be reported as percentage recovery by assay of known added amount of analyte in the sample. Accuracy should be assessed using a minimum of 9 determinations over 3 concentration levels covering specified range. In present work, percentage of recovery was calculated by performing recovery studies in triplicates at three different concentration levels viz. 80%, 100%, 120% by adding known number of standard solutions of GA, VS, PTS and PP. These analysed samples and obtained results were compared with expected results.
Robustness
Robustness of an analytical procedure is a measurement of its capacity to remain unaffected by small, but deliberate variation in method parameters and provides an indication of its reliability using normal usage. For assessment of the robustness of developed analytical method following parameters were deliberately changed viz. flow rate, wavelength and column temperature.
RESULTS
The composition of mobile phase in RP-HPLC method was optimized by testing different solvent compositions of varying polarity, column chemistry, column temperature and pH of mobile phase. Significant efforts were taken to obtain maximum results by using present method, which produced highly symmetrical peaks and showed prominent resolution between each standard and formulations. The scanning wavelength of 280 nm was selected to provide comparable results. At this wavelength all analytes showed optimum response. The details are mentioned in Figures 1, 2 and 3.
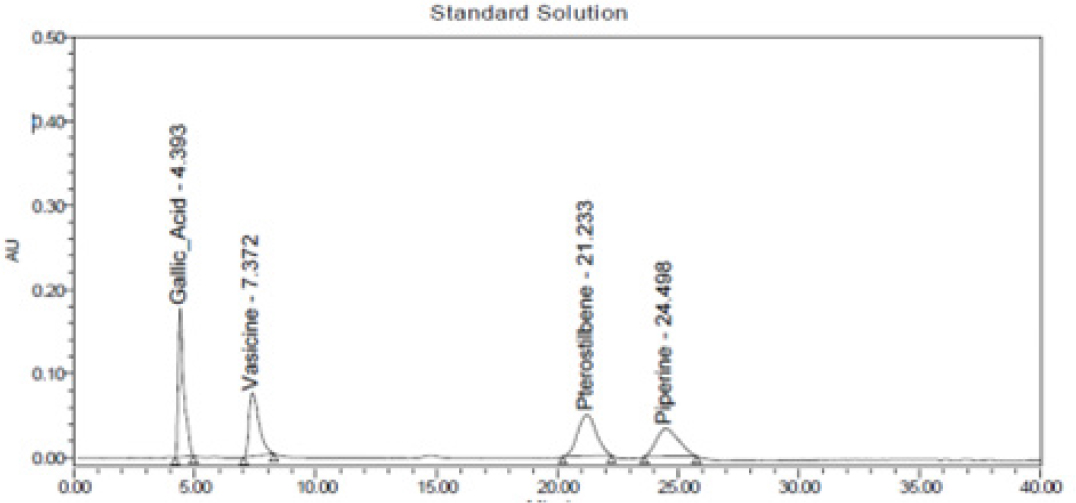
Figure 1:
Chromatogram of standard solution.
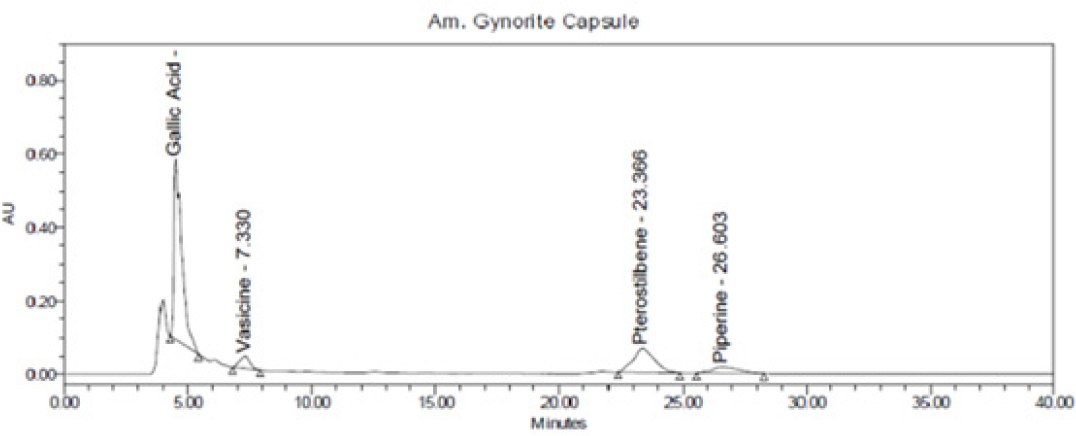
Figure 2:
Chromatogram of Amarantha Gynorite capsule.
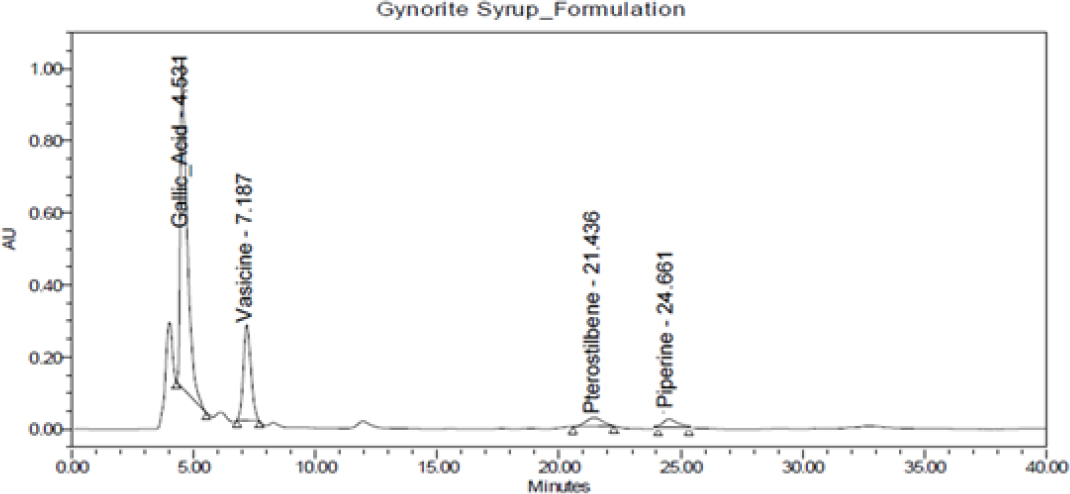
Figure 3:
Chromatogram of Amarantha Gynorite syrup.
Specificity Assessment
Specificity of RP-HPLC method was demonstrated by separation of analyte from other potential components such as placebo, diluent and other active constituent of formulation. A volume of 20 μL of potential components was injected and chromatogram was recorded. Peaks of these components were not found at retention time of 4.2 min, 6.8 min, 21.1 min and 23.8 min of GA, VS, PTS and PP; respectively. Hence, present method was considered as specific on specificity parameters. The details are mentioned in Figures 4 and 5.
Figure 4:
Chromatogram of blank.
Figure 5:
Chromatogram of placebo.
Linearity Assessment
The calibrations graph was linear with concentration range of 6-18 μg/mL. The linear regression data for calibration curves, Limit of Detections (LOD) and Limit of Quantifications (LOQ) were presented in Table 4 and Figure 6.
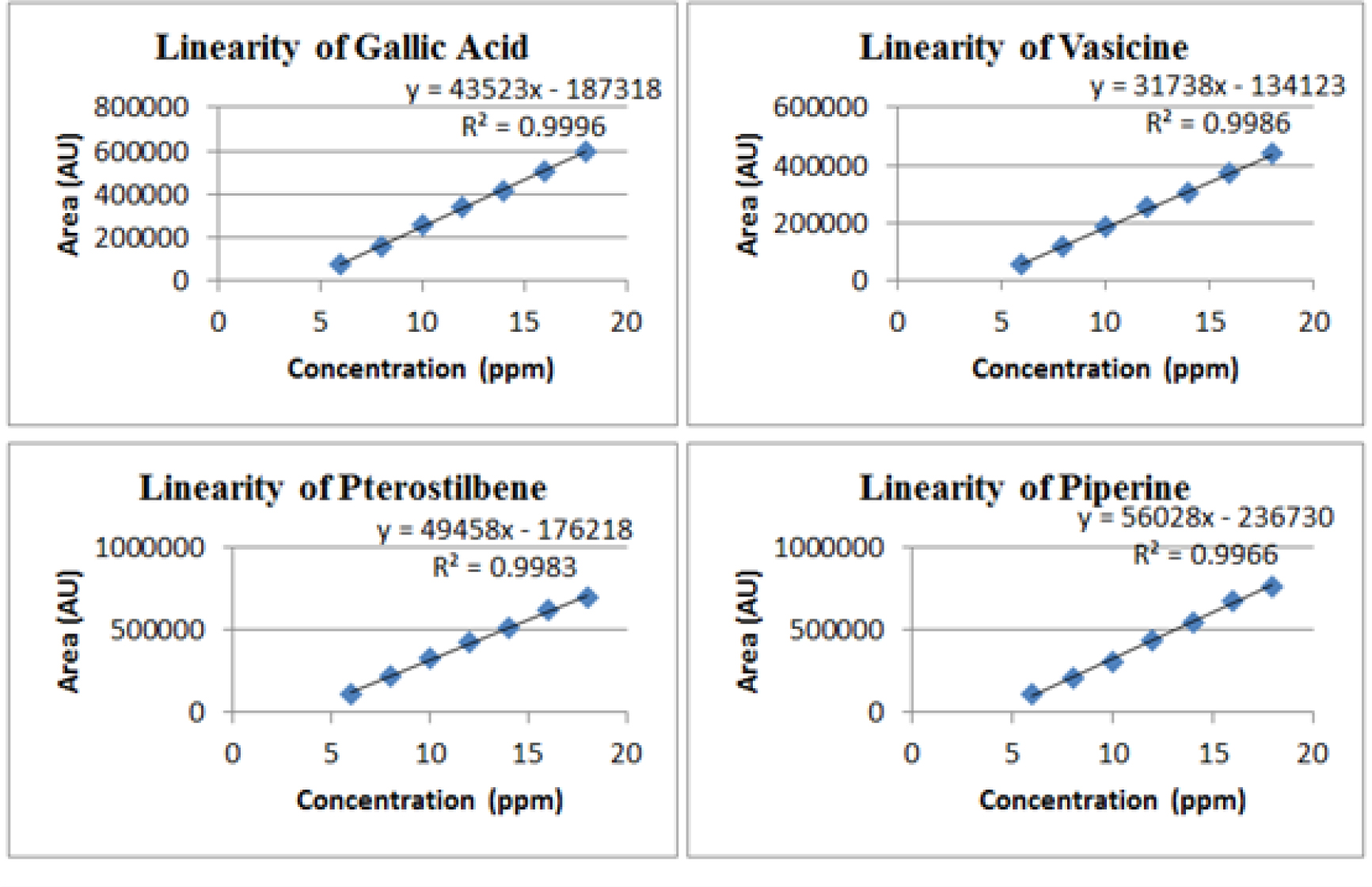
Figure 6:
Linearity graph of GA, VS, PTS and PP.
| Sl. No. | Parameter | Results | |||
|---|---|---|---|---|---|
| Gallic Acid | Vasicine | Pterostilbene | Piperine | ||
| 1 | Linearity Range (μg/mL) | 6 -18 | 6 -18 | 6 -18 | 6 -18 |
| 2 | Correlation coefficient (R2) | 0.9996 | 0.9986 | 0.9983 | 0.9966 |
| 3 | y-intercept | 43523 | 31738 | 49458 | 56028 |
| 4 | Slope | 187318 | 134123 | 176218 | 236730 |
| 5 | LOD | 0.3125 | 0.5759 | 0.6499 | 0.9150 |
| 6 | LOQ | 0.9471 | 1.7450 | 1.9694 | 2.7728 |
Linear regression data for calibrations curve (
n
= 7).
Precision Assessment
A volume of 20 µL of standard solution from 100 µg/mL of stock solution was injected under optimized chromatographic condition to evaluate system suitability, method precision and intermediate precision. An intermediate precision was performed on two different systems. The percentage of Relative Standard Deviation (RSD), USP tailing and plate count were found within acceptance limit. The details are mentioned in Tables 5, 6 and 7.
| Sl. No. | Name of Standard | Retention Time (RT) | Mean Peak Area | % RSD | USP Tailing | Plate Count |
|---|---|---|---|---|---|---|
| 1 | Gallic Acid | 4.393 | 2785223.6 | 0.78 | 1.02 | 25698 |
| 2 | Vasicine | 7.372 | 2304964.6 | 0.46 | 1.16 | 361032 |
| 3 | Pterostilbene | 21.23 | 2950334.4 | 1.06 | 1.06 | 541186 |
| 4 | Piperine | 24.49 | 2542287.6 | 1.27 | 1.12 | 189866 |
System suitability parameter.
| Sl. No. | Name of Standard | % RSD |
|---|---|---|
| 1 | Gallic Acid | 0.524 |
| 2 | Vasicine | 1.121 |
| 3 | Pterostilbene | 0.645 |
| 4 | Piperine | 1.159 |
Method precision parameter.
| Sl. No. | Name of Standard | % RSD for System-1 | % RSD for System-2 | Overall % RSD |
|---|---|---|---|---|
| 1 | Gallic Acid | 0.524 | 1.471 | 1.002 |
| 2 | Vasicine | 1.121 | 0.964 | 1.042 |
| 3 | Pterostilbene | 0.645 | 1.596 | 1.105 |
| 4 | Piperine | 1.159 | 0.982 | 1.071 |
Intermediate precision parameter.
Repeatability Assessment
The repeatability of proposed method was ascertained by injecting five replicates of 100 μg/mL concentration and calculating peak area by proposed RP-HPLC method. From this peak area % RSD was calculated. The details are mentioned in Table 8.
| Sl. No. | Concentration | Peak Area of Standard Solutions | |||
|---|---|---|---|---|---|
| GA | VS | PTS | PP | ||
| 1 | 100 | 2795852 | 2309238 | 2957946 | 2559864 |
| 2 | 2796556 | 2292583 | 2895912 | 2546379 | |
| 3 | 2789514 | 2315689 | 2956128 | 2491567 | |
| 4 | 2797215 | 2312745 | 2966452 | 2535816 | |
| 5 | 2746981 | 2294568 | 2975234 | 2577812 | |
| Mean | 2785223.6 | 2304964.6 | 2950334.4 | 2542287.6 | |
| SD | 21599.10 | 10667.74 | 31357.08 | 32411.85 | |
| % RSD | 0.78 | 0.48 | 1.06 | 1.27 | |
Repeatability of standard solution.
Accuracy Assessment
The accuracy study was carried out by spiking known number of standards into placebo solution at 80%, 100% and 120% of working concentration, respectively. The overall recovery percent were calculated. The details are mentioned in Table 9.
| Standard Solutions | Recovery Level | Peak Area | % Recovery | Average % Recovery | Overall Recovery |
|---|---|---|---|---|---|
| Gallic Acid | 80% – 01 | 153264 | 99.24 | 99.67 | 100.16 |
| 80% – 02 | 154156 | 99.57 | |||
| 80% – 03 | 154925 | 100.20 | |||
| 100% – 01 | 254834 | 100.12 | 100.68 | ||
| 100% – 02 | 256891 | 100.57 | |||
| 100% – 03 | 254326 | 101.37 | |||
| 120% – 01 | 339726 | 100.36 | 100.12 | ||
| 120% – 02 | 337451 | 100.14 | |||
| 120% – 03 | 335423 | 99.54 | |||
| Vasicine | 80% – 01 | 116375 | 101.03 | 100.54 | 100.44 |
| 80% – 02 | 115253 | 98.76 | |||
| 80% – 03 | 118851 | 101.84 | |||
| 100% – 01 | 182530 | 100.77 | 99.07 | ||
| 100% – 02 | 182651 | 101.85 | |||
| 100% – 03 | 182856 | 100.95 | |||
| 120% – 01 | 258815 | 101.28 | 101.72 | ||
| 120% – 02 | 258752 | 102.10 | |||
| 120% – 03 | 257926 | 101.78 | |||
| Pterostilbene | 80% – 01 | 221623 | 101.91 | 101.40 | 100.69 |
| 80% – 02 | 219834 | 101.19 | |||
| 80% – 03 | 219681 | 101.12 | |||
| 100% – 01 | 319869 | 101.74 | 101.34 | ||
| 100% – 02 | 321968 | 100.400 | |||
| 100% – 03 | 323981 | 101.89 | |||
| 120% – 01 | 421888 | 98.38 | 99.35 | ||
| 120% – 02 | 424172 | 99.74 | |||
| 120% – 03 | 424932 | 99.92 | |||
| Piperine | 80% – 01 | 230823 | 99.89 | 100.56 | 101.03 |
| 80% – 02 | 235870 | 100.93 | |||
| 80% – 03 | 237447 | 100.87 | |||
| 100% – 01 | 304171 | 101.80 | 101.35 | ||
| 100% – 02 | 304473 | 100.94 | |||
| 100% – 03 | 302701 | 101.32 | |||
| 120% – 01 | 441351 | 101.88 | 101.10 | ||
| 120% – 02 | 431293 | 99.56 | |||
| 120% – 03 | 441279 | 101.87 |
Accuracy Recovery Study.
Robustness Assessment
The robustness of RP-HPLC method was evaluated by analyzing the system suitability parameters by varying flow Rate (±2%), detection wavelength (±2%) and column temperature flow rate (± 2%) and wavelength (±5%). By alteration of these parameters, no change was observed in % RSD of peak area. The change in the retention time was found to be significant. The details are given in Table 10.
| Standard Solutions | Parameter condition | RT | Mean Area | SD | % RSD | Average % RSD |
|---|---|---|---|---|---|---|
| Gallic acid | Flow Rate (± 0.2) | |||||
| 0.3 mL/min | 5.53 | 2315946 | 9365.75 | 0.39 | 0.80 | |
| 0.5 mL/min | 4.42 | 2285871 | 12128.27 | 0.52 | ||
| 0.7 mL/min | 3.85 | 2333997 | 50555.8 | 1.47 | ||
| Detection Wavelength (± 2.0 nm) | ||||||
| 278 nm | 4.61 | 2395871 | 26632.26 | 1.11 | 0.79 | |
| 280 nm | 4.42 | 2285871 | 12128.27 | 0.52 | ||
| 282 nm | 4.59 | 2362428 | 16843 | 0.71 | ||
| Column Temperature (± 5.0°C) | ||||||
| 20°C | 4.60 | 2151774 | 31319.2 | 1.45 | 0.95 | |
| 25°C | 4.42 | 2285871 | 12128.27 | 0.52 | ||
| 30°C | 4.387 | 2142126 | 18762.11 | 0.87 | ||
| Vasicine | Flow Rate (± 0.2) | |||||
| 0.3 mL/min | 8.85 | 1219238 | 13664.92 | 1.12 | 0.78 | |
| 0.5 mL/min | 7.37 | 1238759 | 6785.26 | 0.55 | ||
| 0.7 mL/min | 5.99 | 1215711 | 11729.21 | 0.69 | ||
| Detection Wavelength (± 2.0 nm) | ||||||
| 278 nm | 7.21 | 1248768 | 15346.96 | 1.23 | 0.89 | |
| 280 nm | 7.37 | 1238759 | 6785.26 | 0.55 | ||
| 282 nm | 7.14 | 1233708 | 11234.36 | 0.91 | ||
| Column Temperature (± 5.0°C) | ||||||
| 20°C | 7.215 | 1128597 | 18297.73 | 1.62 | 1.23 | |
| 25°C | 7.37 | 1238759 | 6785.26 | 0.55 | ||
| 30°C | 7.14 | 1177325 | 17804.28 | 1.51 | ||
| Pterostilbene | Flow Rate (± 0.2) | |||||
| 0.3 mL/min | 27.71 | 2052411 | 12611.14 | 0.61 | 1.15 | |
| 0.5 mL/min | 21.23 | 1984869 | 24979.75 | 1.25 | ||
| 0.7 mL/min | 19.68 | 1962876 | 31329.55 | 1.59 | ||
| Detection Wavelength (± 2.0 nm) | ||||||
| 278 nm | 22.63 | 1974435 | 31794.35 | 1.61 | 1.32 | |
| 280 nm | 21.23 | 1984869 | 24979.75 | 1.25 | ||
| 282 nm | 21.97 | 1974917 | 22004.62 | 1.11 | ||
| Column Temperature (± 5.0) | ||||||
| 20°C | 21.38 | 1872245 | 18345.22 | 0.98 | 0.98 | |
| 25°C | 21.23 | 1984869 | 24979.75 | 1.25 | ||
| 30°C | 20.70 | 1906682 | 13612.2 | 0.71 | ||
| Piperine | Flow Rate (± 0.2) | |||||
| 0.3 mL/min | 31.75 | 2553685 | 29619.92 | 1.15 | 1.05 | |
| 0.5 mL/min | 24.49 | 2541818 | 25934.82 | 1.02 | ||
| 0.7 mL/min | 22.34 | 2532589 | 24889.49 | 0.98 | ||
| Detection Wavelength (± 2.0nm) | ||||||
| 278 nm | 25.82 | 2538589 | 26285.9 | 1.03 | 0.97 | |
| 280 nm | 24.49 | 2541818 | 25934.82 | 1.02 | ||
| 282 nm | 25.04 | 2567650 | 22432.97 | 0.87 | ||
| Column Temperature (± 5.0) | ||||||
| 20°C | 25.17 | 2452065 | 38719.79 | 1.58 | 1.39 | |
| 25°C | 24.49 | 2541818 | 25934.82 | 1.02 | ||
| 30°C | 24.32 | 2452065 | 38719.79 | 1.57 | ||
Robustness Assessment.
DISCUSSION
Market is flooded with Ayurvedic proprietary medicines indicated for menstrual irregularities/PCOS. These Ayurvedic medicines are available in various dosage forms such as tablet, capsule, granules, syrup, and many others. Among these dosage forms, syrup-based formulations are being preferred by consumers. Most of these Ayurvedic syrup formulations are prepared using crude herbs. During manufacturing process, only water-soluble components of crude herbs are available, whereas those components which are not soluble in water will not be available in the final formulation. This may impact efficacy of the product.
In order to make a comprehensive formulation, Amarantha Gynorite Syrup and Amarantha Gynorite Capsule are prepared using standardized herbal extracts. Standardization of polyherbal Ayurvedic medicines has become very essential as it makes sure about the quality of product; broadly it covers qualitative and quantitative part of analysis13–21. Qualitative and quantitative analysis of polyherbal formulation is extremely intricate and it also involves lots of time, and various resources including infrastructure, man power, consumables, etc. Through this research, a single RP-HPLC method which is simple, cost and time effective for analysis of various marker compounds including Gallic Acid (GA), Vasicine (VS), Pterostilbene (PTS) and Piperine (PP) from Amarantha Gynorite Syrup and Amarantha Gynorite Capsule is developed.
The individual standards and samples were run in different mobile phases. From different mobile phases optimized mobile phase was chosen with detection absorbance 280 nm. A satisfactory separation and usual resolutions peak were observed at optimized conditions and at detection wavelength 280 nm. Finally, a cost-efficient RP-HPLC method was developed with less time requirement for simultaneous separation and determination of GA, VS, PTS and PP. This new isocratic RP-HPLC was found to be economical, simple, precise, accurate and reproducible. It was suitable for qualitative and quantitative analysis of GA, VS, PTS and PP.
The injection volume 20 µL was loaded for quantitative analysis and to ensure quality and safety of formulations. The retention time was found to be 4.40 min for GA, 7.37 min for VS, 21.23 min for PTS and 24.98 min for PP. The optimized method was validated as per ICH guidelines. In specificity studies, no interference peak was observed in placebo, blank and both the formulations. Linearity for range of 6-18 μg/mL with correlation coefficient was found to be 0.9996, 0.9986, 0.9983 and 0.9966 for GA, VS, PTS and PP, respectively. Precision of proposed RP-HPLC method was carried out in terms of system suitability studies, method precision, intermediate precision and repeatability. The % RSD was found to be within the acceptance criteria i.e., < 2.0%.
During accuracy recovery studies, the overall recovery percent were found to be 100.16% for GA, 100.44% for VS, 100.69% for PTS and 101.03% for PP; respectively. The LOD, LOQ values were found to be 0.3125 µg/mL and 0.9471 µg/mL for GA; 0.5759 µg/mL and 1.7450 µg/mL for VS, 0.6499 µg/mL and 1.9694 µg/mL for PTS, and 0.9150 µg/mL and 2.772 µg/mL for PP; respectively.
The method was found to be robust with deliberate change of ±2% in flow rate, wavelength, and column temperature.
The quantity of GA, VS, PTS and PP were found to be 0.45% and 0.36%, 1.45% and 0.76%, 2.70% and 2.40% and 0.31% and 0.09% in Amarantha Gynorite syrup and Amarantha Gynorite capsule, respectively. Absence of interference peak at respective retention time indicated that the developed method can be used for routine analysis for both the Ayurvedic proprietary formulations. During the study, low cost, faster analysis, satisfactory precision, accuracy and robustness were the main features of RP-HPLC method.
CONCLUSION
A novel, isocratic RP-HPLC method for qualitative and quantitative analysis of various marker compounds including GA, VS, PTS and PP from Amarantha Gynorite Syrup and Amarantha Gynorite Capsule was developed. The method is economical, simple, precise, accurate and reproducible.
References
- Susan S.. Importance and effectiveness of herbal medicines. J Pharmacogn Phytochem. 2019;8(2):354-7. [Google Scholar]

- Heredia D, Green I, Klaasen J, Rahiman F.. Importance and relevance of phytochemicals present in . Array. 2022;2022:5793436 [PubMed] | [CrossRef] | [Google Scholar]

- Agrawal P, Vegda R, Laddha K.. Simultaneous estimation of withaferin A and Z-guggulsterone in marketed formulation by RP-HPLC. J Chromatogr Sci. 2015;53(6):940-4. [PubMed] | [CrossRef] | [Google Scholar]

- Amruta BL, Varghese E, Kulkarni P.. Menstrual health and . Int Ayurvedic J.. 2016;4(4):602-5. [PubMed] | [CrossRef] | [Google Scholar]

- Katole UM, Ovar DD, Shinde MM, Deshmukh GS. Ayurveda view on common menstrual disorders, causes, symptoms and management. World J Pharm Res.. 2021;7(10):246-8. [PubMed] | [CrossRef] | [Google Scholar]

- Vidyasagar GM, Prashantkumar P.. Traditional herbal remedies for gynecological disorders in women of Bidar district, Karnataka, India. Fitoterapia. 2007;78(1):48-51. [PubMed] | [CrossRef] | [Google Scholar]

- Kahkeshani N, Farzaei F, Fotouhi M, Alavi SS, Bahramsoltani R, Naseri R, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22(3):225-37. [PubMed] | [CrossRef] | [Google Scholar]

- Rachana R, Basu S, Pant M, Kumar MP, Saluja S. Review and future perspectives of using vasicine, and related compounds. Indo Glob J Pharm Sci.. 2011;1(1):85-98. [PubMed] | [CrossRef] | [Google Scholar]

- Nagarajan S, Mohandas S, Ganesan K, Xu B, Ramkumar KM. New insights into dietary Pterostilbene: sources, metabolism, and health promotion effects. Molecules.. 2022;27(19):6316 [PubMed] | [CrossRef] | [Google Scholar]

- Vasavirama K, Upender M.. Piperine: a valuable alkaloid from piper species. Int J Pharm Pharm Sci. 2014;6(4):34-8. [PubMed] | [CrossRef] | [Google Scholar]

- Zhang H, Wang C, Li X, Zhang Y. Effects of pterostilbene on treating hyperprolactinemia and related mechanisms. Am J Transl Res.. 2016;8(7):3049-55. [PubMed] | [Google Scholar]

- Chopra B, Dhingra AK, Kapoor RP, Prasad DN. Piperine and its various physicochemical and biological aspects: a review. Open Chem;J. 2016(3):75-96. [PubMed] | [Google Scholar]

- Ögenler O, n İ, Uzel İ. Medical plants used for treatment of gynecological disorders in Ottomans in the 15th century. J Complement Med Res.. 2018;7(2):171-7. [CrossRef] | [Google Scholar]

- Vaidya VN, Tatiya AU, Elango A, Kukkupuni SK, Vishnuprasad CN. Need for comprehensive standardization strategies for marketed formulations. J Ayurveda Integr Med.. 2018;9(4):312-5. [PubMed] | [CrossRef] | [Google Scholar]

- Balekundri A, Mannur V.. Quality control of the traditional herbs and herbal products: a review. Future J Pharm Sci. 2020;6(1):67 [CrossRef] | [Google Scholar]

- . Validation Anal Proc Text Methodol Curr Step 4 versions. 2005;Q2:(R1) [CrossRef] | [Google Scholar]

- Kshirsagar RR, Vaidya SA, Jain V. Development and validation of a novel RP-HPLC method for the simultaneous quantification of ascorbic acid, gallic acid, ferulic acid, piperine, and thymol in a polyherbal formulation. Indian J Nat Prod Resour.. 2020;11(4):307-11. [CrossRef] | [Google Scholar]

- Annapurna MM, Venkatesh B, Teja GR. Development of a validated stability indicating liquid chromatographic method for the determination of pterostilbene. Indian J Pharm Educ Res.. 2018;52(4s):s63-70. [CrossRef] | [Google Scholar]

- Kurangi B, Jalalpure S.. A validated stability-indicating RP-HPLC method for piperine estimation in black pepper, marketed formulation and nanoparticles. Indian J Pharm Educ Res.. 2020;54(3s):s677-86. [CrossRef] | [Google Scholar]

- Fernandes FHA, Batista RSA, de Medeiros FD, Santos FS, Medeiros ACD. Development of a rapid and simple HPLC-UV method for determination of gallic acid in . Rev Bras Farmacognosia. 2015;25(3):208-11. [CrossRef] | [Google Scholar]

- Singh MP, Gupta A, Singh S.. Qualitative analysis of gallic acid by HPLC method in different extracts of roxb fruit qualitative analysis of gallic acid by HPLC method in different extracts of roxb fruit. Fabad J Pharm Sci.. 2019;44(2):101-6. [CrossRef] | [Google Scholar]

