ABSTRACT
Aim
Oral mucositis is a problem which is found in a cancer patient, taking chemotherapy radiation therapy. This work aims to formulate a mucoadhesive curcumin proniosomal gel by using thiolated chitosan for buccal delivery. Curcumin-loaded proniosomes were optimized using Design-Expert software (version 13) and then mix with thiolated chitosan polymer for improving the bioavailability of curcumin.
Materials and Methods
By using different concentrations of cholesterol, soya-lecithin, and surfactants (Brij 97), a total of fifteen formulations were created by coacervation phase separation method. The impact of the independent variables on the dependent ones, including entrapment effectiveness, vesicle size, and in vitro release, were then characterized.
Results and Discussion
Out of all the experiments, formulation F7 meets the strictest criteria for minimum vesicle size (5.0±0.14), maximum entrapment efficiency (74.12±3.2) and maximum drug release (69.28±2.14). Utilizing the reaction surface plot and expected vs. actual data, the formulation was optimized. The F7 formulation was rendered mucoadhesive by being imbedded in thiolated chitosan before being further defined rheologically. In an in vitro study, Mucoadhesive Proniosomal Gel (TC-CPNG) released the drug at a slower rate than Proniosome (CPNG), releasing only 58 percent of the drug over a 6h period. At varying temperatures, the stability study of TC-CPNG was also monitored.
Conclusion
The result indicates that incorporation of proniosome into thiolated chitosan polymer may considerably increase the drug release rate and provide the sustained release in the buccal cavity.
INTRODUCTION
Edema, erythema, and ulcerations of the oral mucosa are symptoms of the debilitating disorder of oral mucositis.1 It is a condition which occurs during Radiation Therapy (RT) and chemotherapy.2 Within five to fifteen days of tumor treatment, around 20-40% of patients have mucositis problems. The severity of oral mucositis depends upon chemotherapy agents (affects DNA synthesis (S-phase) i.e., 5-fluorouracil, cytarabine and methotrexate), number of dosage and chemotherapy cycles which may vary from patient to patient.3,4 Oral mucositis has been treated using several types of mouthwash but they contain alcohol due to which the patient feels discomfort and pain. Some agents such as allopurinol, chlorhexidine, diphenhydramine, aluminium hydroxide, and palifermin are also used. Due to its fewer adverse effects than chemical medications, there has recently been a rise in interest in evaluating the therapeutic properties of herbs.5,6
The hydrophobic polyphenolic chemical curcumin, with a modest molecular weight, was extracted from the rhizomes of Curcuma longa, family Zingiberacea. It is derived naturally from turmeric. Its IUPAC designation is curcumin (1, 7-bis (4-hydroxy- 3-methoxyphenyl)-1, 6- heptadiene-3, 5-dione).7 The majority of its locations globally are in tropical and subtropical areas. It is often grown in Asian nations, and the Unani, Siddha, and Ayurvedic systems employ it extensively.8 BCS class IV states that curcumin has low permeability and solubility.9 Trials on humans and mice have shown that curcumin taken orally has a lower bioavailability due to intestinal metabolism. By making in the form of liposomes, noisome, and nanoparticles, or by adopting a provesicular method like proniosomes, these impediments may be removed.10,11
Niosomes are non-ionic amphiphilic surfactant-derived closed vesicular bilayer structures. They are similar to liposomes but supersede them in terms of increased chemical stability, greater penetrating property, lower synthesizing cost, easy scaling-up, and affinity towards the target site.12,13 Niosomes are generated in the form of proniosomes due to their physical instability. When the non-ionic surfactant is dissolved in the smallest quantity of accepted solution and the minimum quantity of aqueous phase, a semisolid liquid crystalline product known as a protosome is produced.14 Proniosomes change into niosomes during hydration as a result of the reorganization of surfactant and lipids in an aqueous solution. Niosome limitations, such as fusion, aggregation, and leakage, may be overcome by proniosomes, which also provide ease in terms of transportation, storage, and distribution.15 Drug distribution using the buccal route may avoid the first-pass impact and limit gastric environment degradation, minimizing adverse effects, increasing bioavailability, and eventually lowering the cost of therapy. Proniosomal gel in buccal administration doesn’t need to be hydrated before use. It is possible to use them as a gel base. The formulation adheres better to the buccal mucosa due to the gel base. Both hydrophobic and hydrophilic drugs may be trapped in the proniosomal gel.16 Additionally, mucoadhesion may lengthen the time that a medication is in contact with the buccal epithelium, increasing bioavailability and absorption. Due to its greater mucoadhesion, TCS (Thiolated Chitosan) has become a viable biodegradable polymer for drug delivery. Here, we describe the formulation and characterization of a curcumin proniosomal gel based on TCS for buccal drug delivery. It was made to improve the drug release rate and drug contact time with buccal mucosa.
MATERIALS AND METHODS
Materials
Promed provided a free sample of curcumin (Delhi, India). Brij 97, soya lecithin, cholesterol was brought from central drug house Pvt. Ltd., Chitosan, TGA (Thioglycolic Acid) and EDAC were brought from Hi-Media Laboratories. We bought products from Rankem in New Delhi, including potassium hydrogen phosphate, hydrochloric acid, disodium hydrogen phosphate, ethanol, and sodium hydroxide. Every other chemical and solvent was of the highest quality suitable for analytical use.
Methods
Experimental Designs for optimization of C-PNG
Box Benhken’s design of trials (as indicated in Table 1), a grouping of mathematics and statistical methods, was used to generate the optimum formulation. The effect of 3 independent variables including surfactant (A) and cholesterol (B) and Soya lecithin (C)were chosen based on analyses performed before the experimental design was put into place. The impact of the independent factors (Brij 97, cholesterol, and Soya lecithin) on the dependent variables (drug release, entrapment efficiency, and vesicle size) was studied using an experimental design. Design expert software used a box Behnken design to create 15 batches (F1-F15) (Design Expert 13, Stat-Ease and Minneapolis, MN). Data were analyzed using design experts’ numeric optimization tools to determine the most effective formulation. Lower vesicle size, high entrapment effectiveness, and a high percentage of drug release after 8h led to the selection of the optimized batch.
| Independent variables | Units Low (-1) | High (+1) | |
|---|---|---|---|
| Factor | g | g | |
| A: X1 | Brij 97 | 0.9 | 1.8 |
| B: X2 | Cholesterol | 0.1 | 0.2 |
| C: X3 | Soya lecithin | 0.45 | 1.8 |
| Response | Dependent variables | Goal | |
| Y1 | Vesicle size | nm | Minimize |
| Y2 | Entrapment efficient | % | Maximize |
| Y3 | Drug release | % | Maximize |
Preparation of C-PNG
Table 2 displays the results of the trial runs recommended by the Design Expert for creating the curcumin-loaded proniosomal gel by coacervation phase separation. Appropriately measured quantities of lipids (carrier), cholesterol, surfactant, and CU were added in wide-mouthed dried vials with a sufficient amount of solvent. After closing the vials with solvent to avoid solvent loss, the surfactant mixture was warmed to 60°C to 70°C and stirred continuously with a glass rod for approximately 10 min. The clear solution was obtained by adding the aqueous phase (0.1 percent glycerol solution) to the surfactant solution after it had been dissolved in the water bath.17 The resulting dispersion was left to cool until it gelled. Entrapment efficiency, vesicle size, and drug release were all measured over 15 batches.
| Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | Response 3 | |
|---|---|---|---|---|---|---|
| Run | A: Brij 97 | B: cholesterol | C: soya lecithin | R 1 |
R 2 |
R 3 |
| g | g | g | µm | % | % | |
| 1 | 0 | 0 | 0 | 8.2±1.12 | 69.45±0.31 | 52.26±0.84 |
| 2 | 0 | 0 | 0 | 8.2±0.13 | 69.45±0.33 | 52.26±0.83 |
| 3 | 1 | 0 | 1 | 7.8±0.05 | 67.31±1.32 | 63.84±0.41 |
| 4 | 0 | 1 | 1 | 8.16±0.45 | 56.29±0.7 | 49.04±2.15 |
| 5 | -1 | 0 | -1 | 11.2±1.12 | 65.32±2.1 | 54.97±1.94 |
| 6 | -1 | 0 | 1 | 9.6±2.31 | 62.28±1.5 | 55.61±0.34 |
| 7 | 1 | 0 | 1 | 5.0±0.14 | 74.12±3.2 | 69.28±2.14 |
| 8 | -1 | 1 | 0 | 10.4±2.41 | 50.74±1.1 | 42.18±3.29 |
| 9 | 0 | -1 | 1 | 8.3±0.02 | 54.87±0.9 | 48.98±0.45 |
| 10 | 1 | 1 | 0 | 6.1±0.48 | 60.42±1.4 | 51.1±1.48 |
| 11 | 0 | 1 | -1 | 8.9±1.16 | 65.64±2.6 | 49.2±0.12 |
| 12 | 0 | -1 | -1 | 7.3±3.12 | 55.06±1.2 | 47.41±3.18 |
| 13 | -1 | -1 | 0 | 9.9±2.81 | 59.45±0.6 | 51.82±0.06 |
| 14 | 1 | -1 | 0 | 6.4±0.08 | 49.56±3.5 | 75.96±1.19 |
| 15 | 0 | 0 | 0 | 8.2±1.12 | 69.45±0.3 | 52.26±0.84 |
Drug loaded Proniosomal gel evaluation
Vesicle size
Percent Entrapment Efficiency
Using a phosphate buffer of pH 6.8, 10mL of Proniosomal gel (0.2g) was prepared. After 10 min of sonication in a sonicator, the aqueous dispersion was centrifuged at 20,000rpm for 30 min at 20°C. The free drug content in the supernatant was determined using a UV spectrophotometer set at 425nm.20 To determine the drug encapsulation rate (percent EE), we used the following formula:
In vitro drug release of CPNG and TCPNG
Franz diffusion cells (MGI® Borosilicate Glass) were used for in vitro release investigations of proniosomal gel. The receptor compartment could hold 15mL of fluid. There was a donor compartment exposure area of 1.41cm2. Diffusion studies in phosphate buffer at pH 6.8 were performed on cellophane membranes soaked for 24hr to facilitate in vitro drug release.21 Between the donor and the receptor chamber, a cellophane membrane was attached for dialysis. The pH and temperature of the donor and the receptor parts were both 6.8 and 37±2°C.An electric thermostatic hot plate equipped with a magnetic stirrer was used to produce heat. For 6 hr, a Teflon-coated magnetic bead attached to the stirrer was used to continuously mix the receptor fluid.22 To keep the volume of the receptor phase constant, 2mL aliquots were removed from the compartment every hour and replaced with an equivalent amount of diffusion medium. The specimens withdrawn were analyzed spectrophotometrically (Shimazdu-1700) at 425nm.
Synthesis of Thiolated chitosan TCS
Established procedures were used in developing the TCS.23 1% v/v chitosan polymer solution was mixed with 1mL of TGA (1.0% w/v). The carboxylic acid moieties of TGA were then activated by adding 50mM of the EDAC. 5 M NaOH was used to bring the pH of the completed reaction solution to 5.0. The resulting solution was stirred continuously for 3-4 hr at room temperature throughout incubation. Then, the TCS polymer is treated to eliminate the free TGA moieties, dialysis was done twice by the medium containing 5Mm HCl and 1% w/v NaCl. The dialyzed product was then withdrawn, lyophilized, and kept at 4°C for later use.23
Swelling studies
A gravimetric approach was used to compare the swelling characteristics of TCS and CS. TCS and unmodified chitosan controls (30mg each) were crushed into 5.0mm diameter flat-sided tablets and then immersed in phosphate buffer saline (pH 6.0) at 37±0.5°C.24 After withdrawing the swollen pills from the medium at consistent intervals, we measured how much water they had taken in using the following formula:
Where Wt = Tablet weight at a specific time
Wo = Initial tablet weight
Preparation of Thiolated chitosan based CPNG
4% (w/v) distilled water was utilized to hydrate the TCS polymer. After that, gradually add triethonalamine while stirring with a glass rod until a clear gel has formed. After that, TCS CPNG was made by combining TCS gel with CPNG in a 1:2 weight ratio.25
Characterization of TC-CPNG
pH
A digital pH meter was employed to assess the acidity or alkalinity of each gel composition. To make a solution, we mixed 1 gram of gel with 10mL of water. The pH of formulation was tested three times, and the average was used.
Viscosity
When measuring the viscosity of 0.1 g of TCS-CPNG proniosomal gel, we used a Brookfield viscometer with spindle number 7, connected it to a flask holding the gel, and then adjusted the speed of the rotation to 20rpm.26
Spreadability of TCS-CPNG
Proniosomal gel was placed in the cup of the texture analyzer and its top probe was oriented to measure its spreadability. Gently, the top probe was lowered into the cup holding the proniosomal gel. The formulation’s spreadability was evaluated by measuring the amount of force needed to spread it. All proniosomal gel’s dispersibility was measured.27
In vitro Curcumin release study from TCS-CPNG
Franz diffusion cell analysis was used to measure the curcumin release from TCS-CPNG in the F7 formulation (cholesterol soya lecithin, surfactant). The cellophane membrane was used for the study to find out the drug release. Donor and receptor compartments were separated by a cellophane membrane. Before experimenting, the cellophane membrane was pre-soaked in phosphate buffer, pH 6.8, for 24hr. At 0.5, 1, 2, 3, 4, 5, 6 and 8 hr, 2mL specimens were taken and substituted with an equivalent amount of receptor media. The quantity of curcumin release was calculated after collecting and analyzing the samples using a UV-spectrophotometer set at 425 nm. The drug release data were analyzed using many mathematical models (zero order, 1st order, the Higuchi model, etc.,) to determine the process of drug release from the engineered system.28
Stability Studies
The drug retention behaviour of the improved proniosomal gel was evaluated by storing it at refrigeration (4-8°C), oven temperature (45±2°C), and room temperature (25±2°C) for 1–3 months to see how well the gel preserved the drug. All of the formulations were kept in glass vials with aluminium foil seals for the duration of the process. After 1, 2, and 3 months, the drug concentration and average vesicle diameter changes were measured again.29
Box Behnken Batches of CPNG
This design was followed to test the effects of three independent variables with two levels on several distinct responses. Brij 97, cholesterol, soya lecithin, drug release, vesicle size, and entrapment efficiency are all examples of independent variables (% release 6 hr). The Design Expert Software created 15 batches (F1-F15) based on 2 levels (Design Expert 13, Stat-Ease, Minnepolis, MN). A three-dimensional response surface map was formed with the collected data to determine the nature of the connection between the factors and their interaction.
Vesicle Size
The mucosal release of vesicles was greatly enhanced by decreasing their size. As can be seen in Figure 1, the mean vesicle size (Y1) across all factorial batches ranged from 5.06µm to 11.28µm. The response surface quadratic model yielded an F value for vesicle size of 11.94, with a p-value of 0.0069 indicating statistical significance. As model terms, A was determined to be considerable with a p-value of 0.0002. The final equation discovered in relation to codes is as follows.
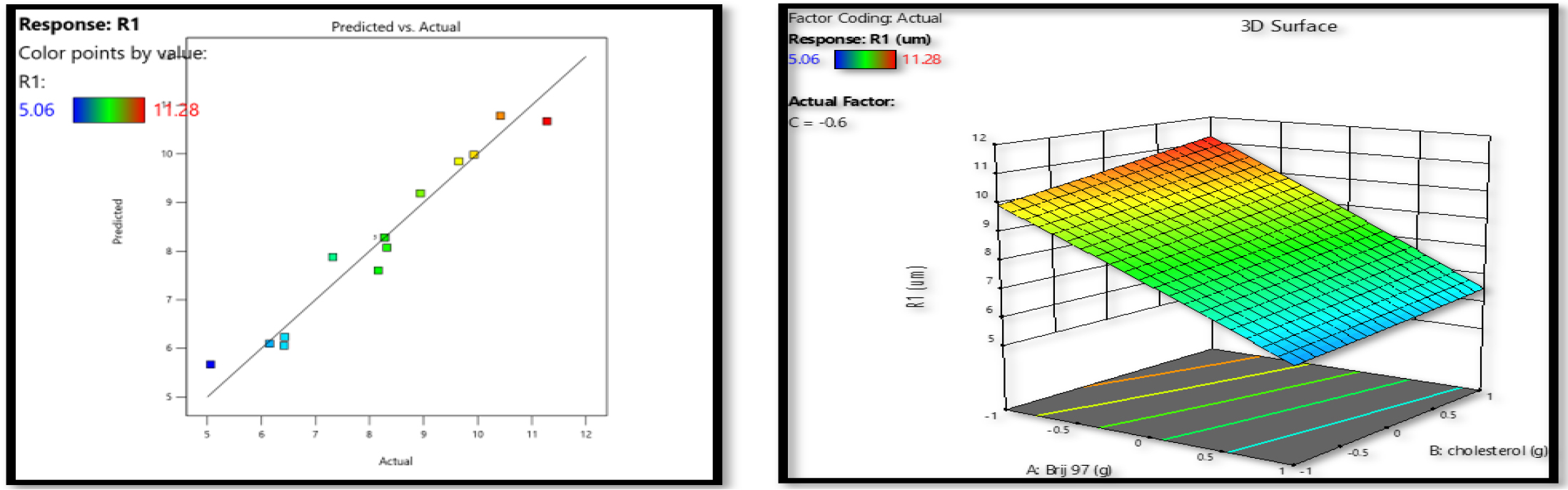
Figure 1:
Surfactant (Brij 97) and cholesterol’s influence on response Y1 was shown using a response surface plot and a linear correlation plot of expected vs actual data.
Effect of surfactant and cholesterol on vesicle size
This indicated that a rise in Brij 97 concentration (A) decreased vesicle size (Y1). This may be because Brij 97 increases charge, and a higher charge decreases vesicle aggregation and improves system stability.30 Since it is cholesterol that gives the bilayer membrane its stiffness, it is clear that this addition has the most direct influence on the vesicles’ physical stability. CPN grown at lower cholesterol amounts had significantly smaller vesicle size compared to CPN developed at greater cholesterol levels. Increasing vesicle size with elevated cholesterol may be due to the creation of a stiff bilayer structure (Figure 1).31
Entrapment Efficiency
From a pharmacological research perspective, entrapment efficiency was a crucial factor. The ability of the formulation to retain medication inside the carrier system bilayer membrane. As can be seen in Figure 2, the entrapment efficiencies ranged from 49.56 to 74.12 throughout all 15 factorial batches. Variations in the concentration of the independent variables were shown to affect the entrapment CPN efficiency. Entrapment efficiency in the quadratic response model has an F-value of 6.88 and a p-value of 0.0235. In this scenario, the model terms AB and B2 are very important. The final equation found in relation to codes is as follows:
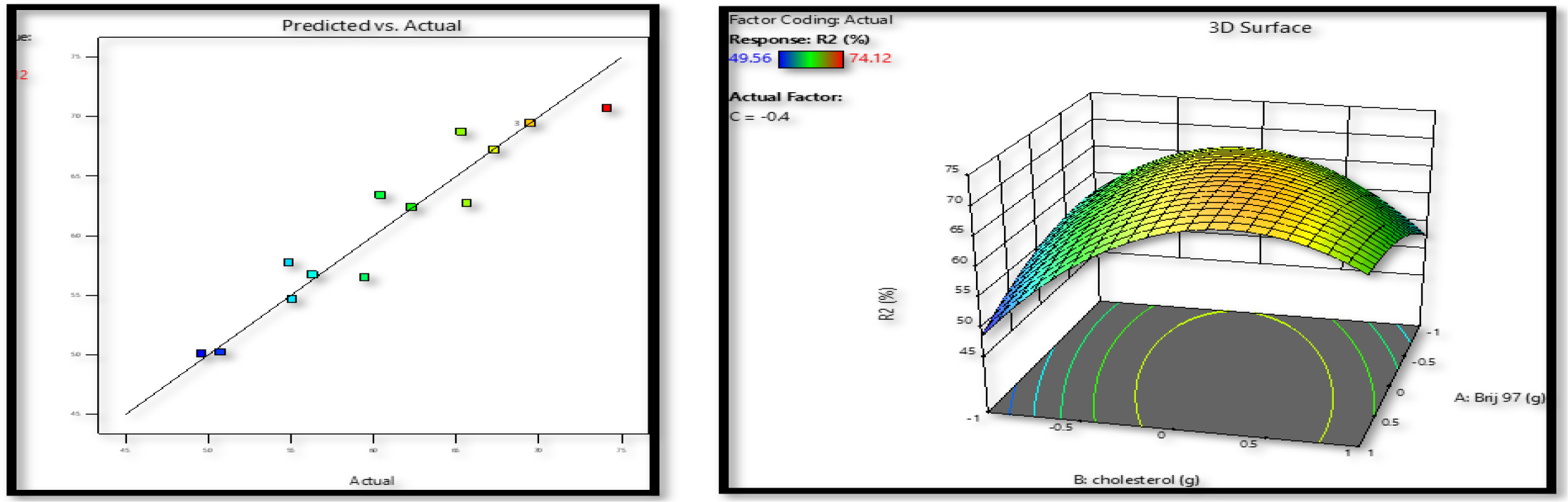
Figure 2:
Effects of surfactant (Brij 97) and cholesterol on response Y2 were mapped out using a response surface and a linear correlation plot of expected vs actual data.
Table 2demonstrates that an increase in the surface area contributes to a rise in entrapment effectiveness for proniosomes with reduced mean vesicle size. As the 3D surface plots illustrate, the entrapment efficiency of Curcumin may be drastically altered by the presence or absence of cholesterol (Figure 2).
Effect of surfactant, cholesterol and soya lecithin on Entrapment Efficiency%
Up to a certain point, the higher the cholesterol content, the greater the entrapment efficiency but on further increasing the concentration from 0.15 to 2.0g, the entrapment efficiency decreases this possibly happens because remove the medicine from the bilayer because cholesterol molecules occupy the same area as the drug. Curcumin’s entrapment effectiveness also dropped significantly with increasing lecithin concentration; however, this impact was much less than that of cholesterol. Niosomes are more likely to aggregate in the presence of a lot of lipids because lecithin enhances the lipophilicity of the vesicles.32 The HLB value of the surfactant is also critical to the proniosome entrapment efficiency. Brij 97 had a high transition temperature of 30°C and an HLB value of 12.4. The percent entrapment effectiveness of Curcumin also increased noticeably with the increase of Brij 97 amount. However, if the concentration of the surfactant is raised even higher, it will break the vesicular mixed micelles, and membrane configuration would develop with the niosomal vesicles; this might reduce the entrapment efficiency.33
Percentage Drug Release
One of the most crucial factors determining proniosome effectiveness is the drug release rate. The CPN release was found between 42-69% in 6hr as illustrated in Figure 3. The outcomes demonstrated that all three of the influencing factors, and the interactions between them, substantially influenced the amount of drug released. Significantly (p<0.05), a positive coefficient was discovered. Important model terms, in this case, are A, AB, B2, and C2. The final equation relating to code representations is as follows.
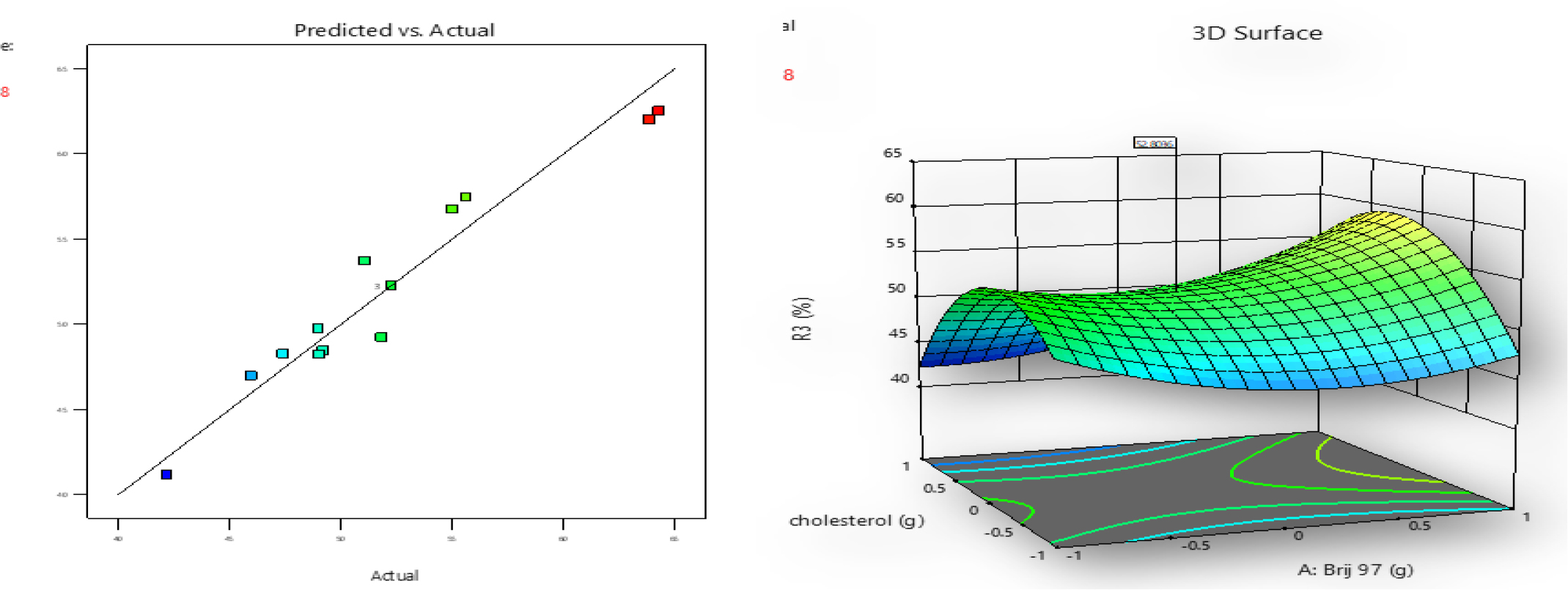
Figure 3:
The impact of surfactant (Brij 97), cholesterol, and soya-lecithin on response Y3 were shown using a response surface and a linear correlation plot between expected and actual data.
Effect of Surfactant, cholesterol and Soya- lecithin on % drug release
An improvement in drug-releasing efficiency has been linked to surfactants with a low transition temperature. Because Brij 97 forms a layer that is both extremely permeable and less stiff, this is the case. In most cases, adding more lecithin led to a significant acceleration of the drug release rate. Possible explanations for this include the permeation-enhancing effects of lecithin.34
As cholesterol levels rise, it was found that the curcumin release rate dropped dramatically. Cholesterol in niosomal formulations is thought to have this effect because it decreases fluidity, resulting in less drug elution from the vesicles.
Optimized formulation selection
Studies were conducted to choose optimum formulations with desirable characteristics, such as high entrapment efficiency, small vesicle size, and high cumulative %drug release. For this formulation, numerical optimization was used to choose the best option. The software was used to create the various batches of proniosomes. The results of the regression analysis showed that every independent variable had a statistically significant impact on each of the dependent variables. The optimized formulation containing Brij 97 (1.8), cholesterol (1.5), soya-lecithin (1.8) having minimum vesicle size (5.0±0.14), entrapment efficiency (81.12±3.2), cumulative percent drug release (71.02±2.14).
SEM Analysis
Surface morphology and precise vesicle size were studied using SEM. This optimized formulation vesicle size was studied by scanning electron microscope.
Characterization of thiolated chitosan
TCS was synthesized by carbodiimide chemistry with EDAC. TCS lyophilization resulted in a white, fibrous substance that was more water-soluble after being processed. Iodometric titration was used to establish that TCS has 285.9±21.151µmol/g of thiol groups linked to the chitosan backbone. The thiol group on the chitosan backbone was efficient for mucoadhesive properties upon buccal administration. The figure shows that the swelling of TCS was more than chitosan.
Characterization of TC-CPNG
pH
The pH of the TC-CPNG was found to be 6.4 which suits the saliva pH.
Viscosity
Using a Brookfield viscometer, we calculated the viscosity of the optimal formulation (CPNG) and the TC-CPNG (20rpm, spindle no. 7). The viscosity of CPNG and TC-CPNG was found to be 34532±453 cps and 28243 ± 211 cps. A very viscous gel resists flow more strongly (similar to increased friction) and hence flows more slowly.
Spreadability
The spreadability of TC-CPNG was found to be 21.40g.cm/sec. The spreadability value suggests that the gel may be applied with minimum force.
In vitro CU release studies
The release of CU at pH 6.8 in vitro from CPNG and TC-CPNG. Under the same conditions, the results show that TC-CPNG only releases 58% of the drug after 6h, whereas CPNG releases 69%. The hydrophobic drug provided delayed drug release from TCS gel surrounding the niosomes, which may explain the long-lasting effects of TC-CPNG.
Stability study
The stability of the TC-CPNG batch of proniosomal gel was studied by evaluating the formulation at 0, 1, and 3-month intervals while stored at 40°C±75% RH. The organoleptic properties and drug content were found to be the same.
CONCLUSION
Cancer patients who have oral mucositis while receiving chemotherapy or radiation treatment were treated with TC-CPNG, which was administered to the buccal mucosa. The formulated optimized proniosomal gel has small vesicles with good encapsulation efficiency to enable buccal distribution, but the drug release rate was low. To, increase the drug release rate a new polymer thiolated chitosan was formed and then encapsulated curcumin proniosome into it. Polymers provide sustained release and prolonged delivery of drugs through buccal delivery. Thiolated chitosan forms strong covalent disulfide linkage with the cystine of mucosa which increases the mucoadhesion time and hence longer attachment with mucosa.
References
- Beech N, Robinson S, Porceddu S, Batstone M.. Dental management of patients irradiated for head and neck cancer. Aust Dent J. 2014;59(1):20-8. [PubMed] | [CrossRef] | [Google Scholar]
- Elad S, Cheng KKF, Lalla RV, Yarom N. Mucositis guidelines leadership group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) Clinical practice guidelines for the management of mucositis secondary to cancer therapy. [PubMed] | [CrossRef] | [Google Scholar]
- Naidu MU, Ramana GV, Rani PU, Mohan IK, Suman A, Roy P., et al. Chemotherapy-induced and radiation therapy–induced oral mucositis-complicating the treatment of cancer. Neoplasia. 2004;6(5):423-31. [PubMed] | [CrossRef] | [Google Scholar]
- Brown TJ, Gupta A.. Management of cancer therapy-Associated oral mucositis. JCO Oncol Pract. 2020;16(3):103-9. [PubMed] | [CrossRef] | [Google Scholar]
- Baharvand M, Sarrafi M, Alavi K, Jalali Moghaddam EJ. Efficacy of topical phenytoin on chemotherapy-induced oral mucositis; a pilot study. Daru. 2010;18(1):46-50. [PubMed] | [Google Scholar]
- Abdollahzadeh Sh, Mashouf R, Mortazavi H, Moghaddam M, Roozbahani N, Vahedi M., et al. Antibacterial and antifungal activities of peel extracts against oral pathogens. J Dent (Tehran).. 2011;8(1):1-6. [PubMed] | [Google Scholar]
- Araújo CC, Leon LL. Biological activities of L. Mem Inst Oswaldo Cruz. 2001;96(5):723-8. [PubMed] | [CrossRef] | [Google Scholar]
- Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee R.. Turmeric and curcumin: biological actions and medical applications. Curr Sci. 2004;87:44-53. [PubMed] | [CrossRef] | [Google Scholar]
- Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol.. 2007;595:453-70. [PubMed] | [CrossRef] | [Google Scholar]
- Nawaz A, Khan G, Hussain A, Ahmad A, Khan A, Safdar A., et al. Curcumin: A natural product of biological importance. Gomal Uni Res. ;201(27):07-14. [PubMed] | [CrossRef] | [Google Scholar]
- Alam MI, Baboota S, Kohli K, Ali J, Ahuja A.. Pharmacodynamic evaluation of proniosomal transdermal therapeutic gel containing celecoxib. Science Asia. 2010;36(4):305-11. [PubMed] | [CrossRef] | [Google Scholar]
- Khatoon M, Sohail MF, Shahnaz G, Ur Rehman F, Fakhar-Ud-Din , Ur Rehman A, et al. Development and evaluation of optimized thiolated chitosan proniosomal gel containing duloxetine for intranasal delivery. AAPS PharmSciTech. 2019;20(7):288 [PubMed] | [CrossRef] | [Google Scholar]
- Ashwani S, Murugesan S, Bharat K.. Proniosome gel: A novel topical delivery system. Int J Recent Adv Pharm Res. 2011;3:1-10. [PubMed] | [CrossRef] | [Google Scholar]
- Yasam VR, Jakki SL, Natarajan J, Kuppusamy G.. A review on novel vesicular drug delivery: proniosomes. Drug Deliv. 2014;21(4):243-9. [PubMed] | [CrossRef] | [Google Scholar]
- Rentel CO, Bouwstra JA, Naisbett B, Junginger HE. Niosomes as a novel peroral vaccine delivery system. Int J Pharm. 1999;186(2):161-7. [PubMed] | [CrossRef] | [Google Scholar]
- Monica R, Priyanka K.. Formulation and evaluation of antifungal proniosomal gel for oral candidiasis. 2018;8(4):291-301. [PubMed] | [CrossRef] | [Google Scholar]
- Gupta A, Singh M. Design and development of a proniosomal transdermal drug delivery system for captopril. Trop J Pharm Res.. 2007;1(1):687-93. [PubMed] | [CrossRef] | [Google Scholar]
- Soliman SM, Abdelmalak NS, Abdelmalak E-GON, Abdelaziz N.. Novel nonionic surfactant proniosomes for transdermal delivery of lacidipine: optimization using 23 factorial design and evaluation in rabbits. 2016 [PubMed] | [CrossRef] | [Google Scholar]
- Abdelbary GA, Aburahma MH. Oro-dental mucoadhesive proniosomal gel formulation loaded with lornoxicam for management of dental pain. J Liposome Res.. 2014:1-15. [PubMed] | [CrossRef] | [Google Scholar]
- Rahman SA, Abdelmalak NS, Badawi A, Elbayoumy T, Sabry N, El Ramly AE, et al. Formulation of tretinon –loaded topical proniosomes for treatment of acne: characterization, skin irritation test and comparative clinical study. Drug Deliv.. 2015;22(6):731-9. [PubMed] | [CrossRef] | [Google Scholar]
- Reddy MC, Firoz S.. Formulation and Evaluation of their more versible gel containing fluconazole. Int J Pharm Res Anal.. 2011:15-20. [PubMed] | [CrossRef] | [Google Scholar]
- Basu S, Maity S. Preparation and characterization of mucoadhesive nasal gel of venlafaxine hydrochloride for treatment of anxiety disorders. Indian J Pharm Sci.. 2012;74(5):428-33. [PubMed] | [CrossRef] | [Google Scholar]
- Sohail MF, Shah PA, Tariq I, Saeed-ul-Hassan S, Amin U, Raza SA, et al. Development and evaluation of flurbiprofen microcapsules prepared by modified solvent evaporation technique. Trop J Pharm Res.. 2014;13(7):1031-8. [CrossRef] | [Google Scholar]
- Monica R, Kamble P. Formulation and evaluation of antifungal proniosomal gel for oral candidiasis. J Drug Deliv Ther.. 2018:291-301. [CrossRef] | [Google Scholar]
- Alam MI, Baaboota S, Kohli K, Ali J, Ahuja A.. Pharmacodynamic evaluation of proniosomal transdermal therapeutic gel containing celecoxib. Sciences, (Asia). 2010;36(4):305-11. [CrossRef] | [Google Scholar]
- Ibrahim MMA, Sammour OA, Hammad MA, Megrab NA. Array. AAPS PharmSciTech.. 2008;9(3):782-90. [PubMed] | [CrossRef] | [Google Scholar]
- Thomas L, Viswanand V.. Formulation and optimization of clotrimazole-loaded proniosomal gel using 32 factorial designs. Sci Pharm. 2012;80(3):73-80. [PubMed] | [CrossRef] | [Google Scholar]
- Khatoon M, Sohail MF, Shahnaz G.. Development and evaluation of optimized thiolated chitosan proniosomal gel containing duloxetine for intranasal delivery. AAPS PharmSciTech. 2008;20:2009 [PubMed] | [CrossRef] | [Google Scholar]
- Hao Y, Zhao F, Li N, Yang Y. KaL. Studies on a high encapsulation of colchine by a noisome system. Int J Pharm. 2001;244(1-2):73-80. [PubMed] | [CrossRef] | [Google Scholar]
- Yadav K, Yadav D, Saroha K. Proniosomal gel: A provesicular approach for transdermal drug delivery. Pharm Lett. 2010;2:189-98. [PubMed] | [CrossRef] | [Google Scholar]
- Marwa A, Omaima S, Hanaa E, Mohammed A. Preparation and evaluation of diclofenac sodium niosomal formulations. Int J Pharm Sci Res.. 2013;4:1757-65. [PubMed] | [CrossRef] | [Google Scholar]
- Kumar A, Ahuja A, Ali J. Conundrum and therapeutic potential of curcumin in drug delivery. Cri Rev Ther Drug Carr Syst. 2010;27(4):312 [PubMed] | [CrossRef] | [Google Scholar]
- Taymouri S, Varshosaz J.. Effect of different types of surfactants on the physical properties and stability of carvedilol nano-niosomes. Adv Biomed Res. 2016;5:48 [PubMed] | [CrossRef] | [Google Scholar]
- EL-Hadidy GN, Ibrahim H, Mohamed MD, El-Milligi MF. Microemulsion as vehicles for topical administration of voriconazole: formulation and evaluation. Drug Dev Ind Pharm.. 2011;38(1):64-72. [PubMed] | [CrossRef] | [Google Scholar]
