ABSTRACT
Background
Materiovigilance is important for checking the safety and effectiveness of medical devices. There isn’t much research on how pharmacy students understand and perceive about materiovigilance. To understand this study was conducted among M Pharm and D.Pharm students at KLE College of Pharmacy, Hubballi.
Materials and Methods
A cross sectional self-developed questionnaire-based study was conducted by using a set of 15 questions about knowledge, attitudes and perceptions of materiovigilance. The questionnaire was given to 183 students before and after an educational session. SPSS software was used to analyse the results.
Results
Both groups improved their knowledge after the session. D.Pharm students knew more initially, but M Pharm students caught up. In the view of attitude factor both groups felt more positive about materiovigilance after the session whereas D.Pharm students were more positive to start with. M Pharm students had a better view initially and their perception improved more after the session whereas D.Pharm students’ perception slightly went low.
Conclusion
The educational session successfully enhanced both D.Pharm and M Pharm students’ knowledge about materiovigilance. Initially, D.Pharm students had more knowledge and a more positive attitude, while M Pharm students showed a more favourable perception. However, after the session, M Pharm students’ knowledge and perception improved significantly, catching up with their peers. In contrast, although D.Pharm students remained positive, their perception slightly decreased. This indicates that while the educational intervention was beneficial for both groups, there is a need for continued focus on improving perceptions among D.Pharm students.
INTRODUCTION
Materiovigilance aims to monitor and investigate incidents that may arise from the use of medical devices. It facilitates the recall of hazardous or unsafe devices and helps eliminate defects in the manufacturing processes of medical devices, thereby ensuring patient safety (Indushree et al., 2022).
Not only medicines, but medical devices also pose risks to patient safety, security and can potentially cause harm. The devices used in routine practice aid in the detection, diagnosis, prevention and management of various illnesses and disease conditions (Gagliardiet al., 2018). Those of higher risk, or those related with more prominent probability of causing serious or untoward health consequences may sometimes lead to death, involve orthopaedic implants that is of hip or knee joints, also cardiovascular implants used as pacemakers or Implantable Cardioverter Defibrillators (ICDs) (Paneet al., 2017).
Medical devices present significant challenges in ensuring their safe and effective use compared to drugs. In fact, implantable devices often exhibit higher effectiveness than drugs because they are not affected by patient non-adherence. The size, complexity and functionality of medical devices vary greatly, ranging from bandages, disposable gloves and wheelchairs to more complex structures such as active implants (e.g., pacemakers) and automated systems used in cataract surgery (Abhima et al., 2023).
The theoretical framework of materiovigilance is similar to that of pharmacovigilance, which focuses on the safety of medicines. Just as pharmacovigilance involves the collection, assessment, analysis and interpretation of data on Adverse Drug Reactions (ADRs), materiovigilance is concerned with gathering and evaluating data on medical device incidents, problems and Adverse Events (AEs) (Selvamet al., 2024).
As there is smaller number of studies done with respect to responsiveness of materiovigilance, in this way the current study is embraced to survey the knowledge and attitude toward materiovigilance by healthcare specialists in a healthcare sector (Rehmanet al., 2022).
MATERIALS AND METHODS
Study Site
The research was carried out at KLE College of Pharmacy, Vidyanagar, Hubballi.
Study Design
This was a cross-sectional study employing a self-developed questionnaire to assess the knowledge, attitude and perception of materiovigilance among students enrolled in the M.Pharm and D.Pharm programs at KLE College of Pharmacy, Vidyanagar, Hubballi. A set of 15 questions were provided as a part of pre-test to the study participants and were instructed to fill the form (Google form). After the completion of pre-test, participants were exposed to an educational session which covered all the information related to the questions provided with the help of power point presentation. The same set of 15 questions was sent to the participants after the educational session for collecting the responses of post-test.
Ethical Approval
Ethical clearance was granted by the Institutional Ethical Committee of KLE College of Pharmacy, Hubballi. The approval reference number KLECOPH/IEC/2023-24/01.
Study Period
The study was conducted over a period of six months at KLE College of Pharmacy, Vidyanagar, Hubballi, Karnataka. The study involved planning of one month, data collection and interpretation for three months and result analysis two months.
Study Criteria
Inclusion Criteria: Students enrolled in the M.Pharm and D.Pharm programs at KLE College of Pharmacy, Hubballi. Exclusion Criteria: Participants of the pilot study who were already provided with the educational session and students unwilling to participate in the study were excluded.
Study Procedure
15 questionnaires, each containing five questions, were developed by the faculty of the Department of Pharmacy Practice to assess three factors: knowledge, attitude and perception. The knowledge domain employed a binary response format, with “Yes” assigned a value of 1 and “No” assigned a value of 0. The attitude and perception domains utilized a 5-point Likert scale, ranging from ‘Strongly Agree’ (5) to ‘Strongly Disagree’ (1).
Internal consistency was evaluated using Cronbach’s alpha (α) via IBM SPSS 27.0, with values ranging from 0 to 1. A higher alpha value indicates stronger internal consistency, with values between 0.8 and 0.9 signifying good internal consistency. The pilot survey, conducted with 21 participants, yielded a Cronbach’s alpha of 0.827, indicating a respectable level of internal consistency for the MvPI questions.
Participants initially completed a pre-test questionnaire. Subsequently, they attended an educational session on materiovigilance, which included a PowerPoint presentation covering the definition, objectives, regulatory bodies, history, reporting procedures and safety monitoring of medical devices. After the educational session, the same set of 15 questions was administered again via Google Forms to assess post-test responses.
Statistical Analysis
Data were entered into a Microsoft Excel spreadsheet and continuous variables were presented as mean±standard deviation. Group differences were analysed using a t-test and appropriate descriptive and inferential statistical analyses were conducted using Excel and SPSS version 27.
Sample size
Sample size is calculated using the formula;
Where, Z is critical value,
d is allowable error,
p is sample proportion,
α is level of significance.
RESULTS
Demographic details
The study involved 183 participants, with a higher proportion of females (56.83%) compared to males (43.16%). Participants’ ages ranged from 18 to 25 years, with the largest groups being 18-year-olds (19.12%) and 19-year-olds (18.57%). Most participants were D.Pharm students (67.21%), while the rest were M.Pharm students (32.78%). A significant majority of the participants came from non-medical backgrounds (85.24%). In terms of socioeconomic status, a large portion belonged to the lower class, earning less than 1 lakh Rupees per year (42.07%), while others were distributed among lower middle class (16.93%), upper lower class (12.02%), upper middle class (22.40%) and upper class (6.55%). Additionally, the study had more urban residents (61.20%) than rural residents (38.79%), reflecting a greater urban representation (Table 1).
| Demographics | Characteristics | N=183 (%) |
|---|---|---|
| Gender | Female | 104 (56.83) |
| Male | 79 (43.16) | |
| Age in years | 18 | 35 (19.12) |
| 19 | 34 (18.57) | |
| 20 | 22 (12.02) | |
| 21 | 26 (14.20) | |
| 22 | 14 (7.65) | |
| 23 | 29 (15.84) | |
| 24 | 15 (8.19) | |
| 25 | 08 (4.37) | |
| Study group | M.Pharm | 60 (32.78) |
| D.Pharm | 123 (67.21) | |
| Parents profession | Non-medical background. | 156 (85.24) |
| Medical background. | 27 (14.75) | |
| Socioeconomic status | Lower class (<1 lakh Rupees per year). | 77 (42.07) |
| Lower middle class (2-5 lakh Rupees per year). | 31 (16.93) | |
| Upper class (>10 lakh Rupees per year). | 12 (6.55) | |
| Upper lower class (1-2 lakh Rupees per year). | 22 (12.02) | |
| Upper middle (5-15 lakh Rupees per year). | 41 (22.40) | |
| Residence | Rural | 71(38.79) |
| Urban | 112(61.20) |
Demographic details of M.Pharm and D.Pharm.
Knowledge factor Assessment of Knowledge regarding MvPI among M.Pharm and D.Pharm students
The Figures 1 and 2 shows the comparison of knowledge between M.Pharm and D.Pharm students about the Materiovigilance Programme of India and medical device reporting showed different results in various areas. For awareness of the Materiovigilance Programme, D.Pharm students did better in the post-test, scoring 95.12% correct answers compared to 90% for M.Pharm students. In understanding medical device classification, D.Pharm students had a higher pre-test score (71.54%) than M.Pharm (48.33%). Although M.Pharm students improved to a slightly higher post-test score (96.67% vs. 94.31%), both groups showed significant progress. When it came to knowing how to report adverse events, M.Pharm students excelled in the post-test with 96.67%, while D.Pharm students scored 91.06%. For exposure to reporting forms for adverse events, D.Pharm students scored higher in the pre-test (60.16%) compared to M.Pharm (43.33%) and after training, D.Pharm maintained a slight lead (90.24% vs. 85%). Lastly, both groups scored the same in the post-test for awareness of alerts or recalls about medical devices (90.24%), but D.Pharm had a higher pre-test score (59.35% vs. 45%). Overall, both groups improved significantly after training, but their performances varied depending on the specific topics, so it’s hard to say that one group was better than the other overall.
The Table 2 presents the mean scores and standard deviations of M.Pharm and D.Pharm students in pre-test and post-test assessments related to their knowledge about the Materiovigilance Programme of India and medical device reporting. For M.Pharm students, the mean pre-test score was 2.48±1.867, which significantly increased to 4.58±1.030 in the post-test (p<0.001). Similarly, D.Pharm students showed a significant improvement from a mean pre-test score of 39±1.867 to 3.40±1.949 in the post-test (p<0.001). These results indicate that both groups benefited from the training intervention, with statistically significant increases in their knowledge scores.
Attitude factor Assessment of Attitude regarding MvPI among M.Pharm and D.Pharm Students
The Figures 3 and 4 compares the attitudes of M.Pharm and D.Pharm students regarding medical device adverse event reporting before and after a training session. In the pre-test, 54.17% of M.Pharm and 56.91% of D.Pharm students agreed that medical devices can cause adverse events, which increased to 68.33% and 70.73%, respectively, after training. Regarding the importance of reporting adverse events, agreement was 68.34% for M.Pharm and 76.42% for D.Pharm in the pre-test, rising to 71.66% and 74.39% post-training. For the necessity of materiovigilance training, 61.67% of M.Pharm and 79.68% of D.Pharm students agreed in the pre-test, with post-training agreement at 75% and 79.67%. When asked if reporting enhances patient safety, 71.67% of M.Pharm and 84.55% of D.Pharm students agreed before training, increasing to 80% and 80.49% afterward. Finally, 71.66% of M.Pharm and 59.35% of D.Pharm students indicated they would report adverse events in the pre-test, which rose to 80% and 73.98% post-training. Overall, both groups exhibited positive attitudes towards adverse event reporting, with D.Pharm students generally showing higher agreement percentages in the pre-test and both groups demonstrating increased agreement across all questions after the training.
The Table 3 presents the mean scores and standard deviations of M.Pharm and D.Pharm students in pre-test and post-test assessments related to their attitude on the Materiovigilance Programme of India and medical device reporting. For M.Pharm students, the mean pre-test score was 17.28±5.428, which increased to 18.58±6.614 in the post-test, with a p-value of 0.261, indicating no statistically significant change. In contrast, D.Pharm students had a pre-test mean score of 17.14±1.892, which significantly improved to 19.19±2.979 post-test, with a p-value of less than 0.001, indicating a statistically significant increase in knowledge. Overall, while both groups showed improvement, only the D.Pharm students demonstrated a significant enhancement in their attitude scores after the training.

Figure 1:
Knowledge assessment of M.Pharm.

Figure 2:
Knowledge assessment of D.Pharm.
Perception factor Assessment of Perception regarding MvPI among M.Pharm and D.Pharm student
The Figures 5 and 6 presents the perceptions of M.Pharm and D.Pharm students regarding various aspects of the Materiovigilance Programme before and after a training session. Initially, 61.67% of M.Pharm students believed that India’s materiovigilance programme can generate evidence-based data on medical device safety, which increased to 79.17% post-test. For D.Pharm students, the pre-test agreement was 52.85%, rising to 90.24% post-test. Regarding the impact of reporting adverse events, 69.99% of M.Pharm students thought one report could make a difference, increasing to 83.33% post-training, while D.Pharm students showed a similar trend, with pre-test agreement at 72.36% and post-test at 83.74%. Awareness of the potential consequences of not reporting adverse events was 60% for M.Pharm students pre-test, increasing to 86.67% post-test and 63.41% for D.Pharm students pre-test, rising to 86.59% post-test. Initially, 60% of M.Pharm students viewed materiovigilance reporting as beneficial for their professional development, which rose to 85.71% post-test and for D.Pharm students, the pre-test agreement was 58.54%, increasing to 84.55% post-test. Finally, 68.33% of M.Pharm students felt there was a need for greater awareness of materiovigilance among pharmacy students, which increased to 83.33% post-test, while D.Pharm students showed similar results, with pre-test agreement at 65.04% and post-test at 89.43%. Overall, both groups demonstrated enhanced positive perceptions regarding the Materiovigilance Programme after the training.
| Sl. No. | Stream | N | Mean±Sd. Dev | p-value | |
|---|---|---|---|---|---|
| Pre | Post | ||||
| 01 | M.Pharm | 60 | 2.48±1.867 | 4.58±1.030 | <0.001* |
| 02 | D.Pharm | 123 | .39±1.867 | 3.40±1.949 | <0.001* |
T-Test Analysis of Knowledge scores in M.Pharm and D.Pharm students regarding MvPI.
| Sl. No. | Stream | N | Mean±Sd. Dev | p-value | |
|---|---|---|---|---|---|
| Pre | Post | ||||
| 01 | M.Pharm | 60 | 17.28±5.428 | 18.58±6.614 | .261 |
| 02 | D.Pharm | 123 | 17.14±1.892 | 19.19±2.979 | <0.001 |
T-Test Analysis of Attitude scores in M.Pharm and D.Pharm students towards MvPI.
The Table 4 presents the results of a t-test analysis comparing the perception factor between M.Pharm and D.Pharm students regarding the Materiovigilance Programme of India and medical device reporting. For M.Pharm students, the mean pre-test perception score was 17.20 (SD=5.784), which significantly increased to 19.75 (SD=5.461) in the post-test, with a p-value of 0.019, indicating a statistically significant improvement. In contrast, D.Pharm students had a mean pre-test perception score of 19.16 (SD=2.732), which decreased to 18.24 (SD=2.722) post-test, with a p-value of 0.004, also statistically significant. This suggests that while M.Pharm students benefited from the training, enhancing their perceptions, D.Pharm students experienced a decline in their perception scores despite the intervention. These findings highlight a noteworthy difference in the impact of the training on the two groups, warranting further investigation to understand the reasons behind the decrease in D.Pharm students’ perceptions.
Comparative analysis of Knowledge, Attitude and Perception (KAP) factors between M.Pharm and D.Pharm students regarding MvPI
The participants responses were categorised into three levels based on their total scores in each domain namely Poor, Moderate and Good. The Table 5 summarizes the structure and scoring for three assessment domains: Knowledge, Attitude and Perception.
The Table 6 compares the Knowledge, Attitude and Perception (KAP) factors between M.Pharm and D.Pharm students, categorized as poor, moderate and good as mentioned in Table 5. In terms of knowledge, D.Pharm students had more participants in the good category, with 76 compared to 53 for M.Pharm. Similarly, for attitude, D.Pharm students had a higher number of participants (88) in the good category than M.Pharm students (42), suggesting that D.Pharm students had a better overall understanding of the Materiovigilance Programme and medical device reporting. However, when it comes to perception, M.Pharm students had more participants (47) in the good category compared to D.Pharm students (65), indicating that M.Pharm students had a better understanding of the consequences and benefits of the Materiovigilance Programme. While the table provides a clear comparison of the KAP factors between the two groups, further analysis would be needed to determine if these differences are statistically significant (Figures 7 and 8).
DISCUSSION
In this study we discuss the Knowledge, Attitude and Perception among the M.Pharm and D.Pharm students in which they were assessed with Pre-test and Post-test respectively. Both the streams had positive impact in all aspects which signifies the importance of educational intervention. The results also showcase the differences in both the streams as seen in KAP of Materiovigilance between the groups where D.Pharm students had an overall higher level of understanding as compared to M.Pharm towards MvPI from the on set.
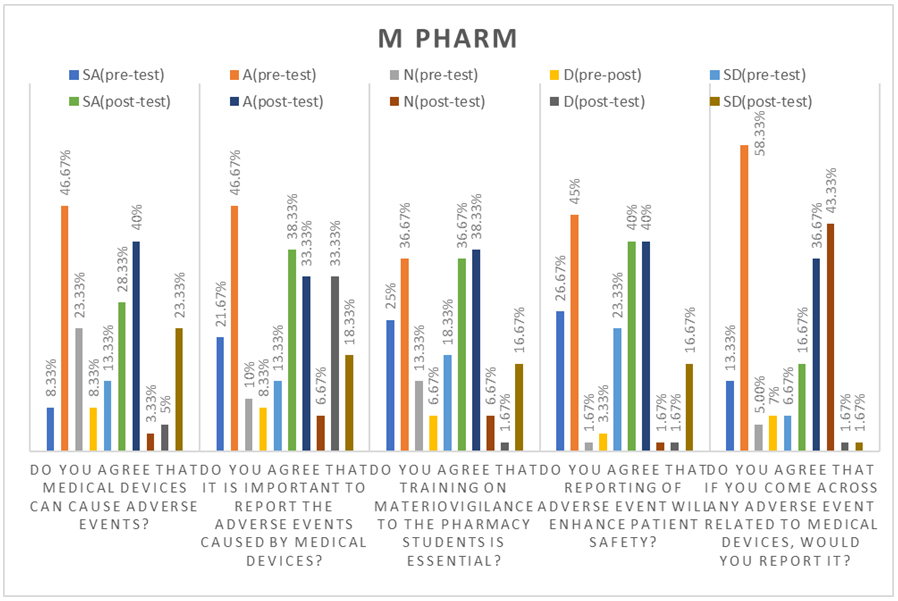
Figure 3:
Attitude assessment of M.Pharm.

Figure 4:
Attitude assessment of D.Pharm.
| Sl. No. | Stream | N | Mean±Sd. Dev | p-value | |
|---|---|---|---|---|---|
| Pre | Post | ||||
| 01 | M.Pharm | 60 | 17.20 ±5.784 | 19.75 ±5.461 | .019* |
| 02 | D.Pharm | 123 | 19.16 ±2.732 | 18.24 ±2.722 | 0.004* |
Test Analysis of Perception scores in M.Pharm and D.Pharm students regarding MvPI.
| Domain | Question format | Scoring | Poor category | Moderate category | Good category |
|---|---|---|---|---|---|
| Knowledge | Yes/No (2 options). | Yes=1, No=0 | 0-2 points | 3 points | 4-5 points |
| Attitude | SA, A, N, D, SD (5 options). | 5,4,3,2,1 | 0-16 points | 17-18 points | 19-25 points |
| Perception | SA, A, N, D, SD (5 options). | 5,4,3,2,1 | 0-16 points | 17-18 points | 19-25 points |
Scoring and categorization of KAP.
| Stream | M.Pharm | D.Pharm | ||||
|---|---|---|---|---|---|---|
| Criteria | Poor | Moderate | Good | Poor | Moderate | Good |
| Knowledge | 03 | 04 | 53 | 32 | 15 | 76 |
| Attitude | 12 | 06 | 42 | 23 | 12 | 88 |
| Perception | 10 | 03 | 47 | 36 | 22 | 65 |
Comparison of KAP factors between M.Pharm and D.Pharm.
The analysis of Knowledge tells us that both the groups had significant improvement in the knowledge after the training, in which D.Pharm had outclassed M.Pharm in the post-test by scoring 95.12% correct answers as compared to M.Pharm 90% respectively. In case of reporting of adverse events M.Pharm had more positive knowledge 96.67% as compared to D.Pharm students 91.06%. A comparable study was done by Sivagourounadin et al. in which nurses were found to have adequate knowledge regarding MvPI and NCC but had slightly poor knowledge regarding the functioning of MDAEs 61% which also resulted in lack of reporting of adverse events (Sivagourounadinet al., 2022).
The assessment of attitude showed positive trend of response in both the groups from pre-test to post-test respectively. In this D.Pharm had shown profound attitude in the pre-test as compared to M.Pharm which justifies that D.Pharm had a higher agreement in understanding the reporting of adverse events and the necessity of Materiovigilance program. Thus, both the groups had exhibited positive attitude and approach of adverse event reporting after the training. The study led by Bikash Ranjan et al. in Bhubaneswar had a significant majority in reporting of ADRs (84.4% of medical faculty and 90% of resident doctors) and they believed that medical devices can cause adverse events. Also, 93.3% of resident doctors felt the necessity and were obliged to report the adverse events in a responsible manner (Meheret al., 2022).
These outcomes were found to be consistent in various studies such as study conducted by Panchal et al. which revealed high level of interest in reporting MDAEs 77.6% and the significance of establishing of Materiovigilance by educational intervention had an overall response of 94.8% (Panchalet al., 2022). Hence, these studies describe us that positive Attitude regarding Materiovigilance is necessary for reporting of adverse events associated with medical devices and to enhance the patient’s safety and HRQOL.
The perception-based analysis tells us that in both the groups M.Pharm and D.Pharm there was significant improvement in post-test scores resulting in positive perception of Materiovigilance program. In this study D.Pharm students had shown higher agreement of percentages in several areas, particularly in recognizing the importance of reporting adverse events and the potential for professional growth through materiovigilance. This indicates both had an overall positive perception of Materiovigilance in which their score had climbed to 83.74% as compared to M.Pharm post-test score of 83.33% respectively. In the study conducted by Mangala et al. 71% of health-care practitioners observe patients for MDAEs out of which 38% had actually encountered MDAEs. This study shows doctors had advanced practice as compared to other medical professionals and majority of medical professionals 82% did not receive training on reporting of MDAEs (Srinivaset al., 2023). These studies show marked importance in improving the positive perception about Materiovigilance programme and a need for better training to report adverse events.

Figure 5:
Perception assessment of M.Pharm.

Figure 6:
Perception assessment of D.Pharm.

Figure 7:
Post-test KAP of M.Pharm.

Figure 8:
Post-test KAP of D.Pharm.
Lastly, this study marks that both the groups had significantly improved in all the aspects knowledge, attitude and perception showing positive results in post-test which marks the importance of educational intervention regarding Materiovigilance. The study also reveals that D.Pharm students had consistently performed better than M.Pharm students in overall KAP assessments. A study conducted by Nirmalya et al. states that training regarding MDAEs reporting and its promotion can be done by posting posters with instructions. Also, by implementing training, workshops and CME (continuing medical education) Materiovigilance awareness can be improved among the health-care professionals. Strategies to include medical device surveillance and Materiovigilance in the curriculum can definitely workout towards early awareness among all HCPs (Mannaet al., 2023).
CONCLUSION
The comparison of Materiovigilance (Mv) performance between D.Pharm and M.Pharm graduates using Knowledge, Attitude and Perception (KAP) factors reveals that D.Pharm students exhibit superior knowledge, attitudes and perceptions regarding medical device safety and reporting compared to M.Pharm students. D.Pharm students demonstrated higher initial and post-intervention awareness of the Materiovigilance Programme of India, medical device classifications and reporting procedures. They also showed a stronger belief in the importance of reporting adverse events and the need for materiovigilance training. In contrast, M.Pharm students, while improving post-intervention, initially had lower levels of awareness and less belief about the impact of reporting on healthcare systems. Both groups acknowledged the importance of materiovigilance for professional growth and emphasized the need for greater awareness among pharmacy students, marking the necessity for enhanced training across all pharmacy education levels to ensure improved patient safety and healthcare outcomes.
Cite this article:
Sail SS, Gore A, Sayed AA, Khan MR, Swamy AHMV, Dayana BM, et al. KAP Analysis of the Materiovigilance Programme: A Comparative Study between M Pharm and D Pharm Students. Int. J. Pharm. Investigation. 2025;15(2):10-8.
ABBREVIATIONS
| KAP | Knowledge Attitude Perception |
|---|---|
| SPSS | Statistical Package of Social Sciences |
| M.Pharm | Master of Pharmacy |
| D.Pharm | Diploma in Pharmacy |
| ICD | Implantable cardioverter defibrillators |
| ADRs | Adverse drug reactions |
| AEs | Adverse events |
| SD | Strongly disagree |
| D | Disagree |
| N | Neutral |
| A | Agree |
| SA | Strongly agree |
| MvPI | Materiovigilance Programme of India |
| Sd. Dev | Standard Deviation |
| NCC | National Coordinating Centres |
| MDAEs | Medical device related adverse events |
| HRQOL | Health related quality of life |
| CME | Continuing medical education |
| HCPs | Health care professionals |
| Mv | Materiovigilance |
References
- Gagliardi A. R., Ducey A., Lehoux P., Turgeon T., Ross S., Trbovich P., Easty A., Bell C., Urbach D., et al. (2018) Factors influencing the reporting of adverse medical device events: Qualitative interviews with physicians about higher risk implantable devices. BMJ Quality and Safety 27: 190-198 Google Scholar

- Manna N., Mazumdar S., Panchanan P., Das S.. (2023) A study of assessing knowledge, attitude and practice of materiovigilance among staff nurses in Medical College and Hospital, Kolkata. National Journal of Physiology, Pharmacy and Pharmacology : 1 Google Scholar

- . (2023) attitude and practices of materiovigilance among the medical professionals in a tertiary care centre: a cross-sectional study 5: 1100-1103 Google Scholar

- Meher B. R., Padhy B. M., Srinivasan A., Mohanty R. R.. (2022) Awareness, attitude and practice of materiovigilance among medical professionals at a tertiary care institute of national importance: A cross-sectional study. Perspectives in Clinical Research 13: 94-98 Google Scholar

- . (2022) Knowledge and attitude of materiovigilance among doctors in a tertiary care teaching hospital: A cross-sectional survey. National Journal of Physiology, Pharmacy and Pharmacology : 1 Google Scholar

- Panchal Y., Vyas B., Suthar K., Shah K.. (2022) A study of assessing knowledge, attitude and practice of materiovigilance among medical surgeons of Gujarat. National Journal of Physiology, Pharmacy and Pharmacology : 1 Google Scholar

- Pane J., Coloma P. M., Verhamme K. M. C., Sturkenboom M. C. J. M., Rebollo I.. (2017) Evaluating the safety profile of nonactive implantable medical devices compared with medicines. Drug Safety 40: 37-47 Google Scholar

- Rehman S., Ray A., Pandit S.. (2022) Materiovigilance: Impact of awareness cum sensitization programme on healthcare professionals of a tertiary care teaching hospital in South Delhi. IP International Journal of Comprehensive and Advanced Pharmacology 7: 146-150 Google Scholar

- Selvam S., Prassath R., Babu I. J., Raja S., Rajarathinam N.. (2024) Knowledge attitude and practice of materiovigilance among healthcare professionals in tertiary care hospitals. International Journal of Basic and Clinical Pharmacology 13: 358-363 Google Scholar

- Sivagourounadin K., Rajendran P., Ravichandran M.. (2022) Knowledge, attitude and practice of materiovigilance among nurses at a tertiary care hospital in South India: A cross-sectional study. Journal of Pharmacy and Bioallied Sciences 14: 162-167 Google Scholar

- Srinivas M., Krishnegowda S., Udaykumar P.. (2022) A cross-sectional study to assess the knowledge, attitude and practice of health-care professionals regarding reporting of medical device-related adverse events in a tertiary care center. National Journal of Physiology, Pharmacy and Pharmacology 13: 1429-1433 Google Scholar


