ABSTRACT
Background
Momordica dioica (MD) is called the teasle gourd, spiny gourd, and kakrol. Among the important phytochemicals present in MD include alkaloids, flavonoids, phenolics, tannins, saponins, steroids, glycosides, and terpenes. These compounds have a high potential to cure many illnesses. Herbal plant extracts exhibit mucilage, high viscosity, and limited flowability qualities. It is challenging to create the herbal formulation because of these characteristics. Herbal medications may be one of the safest options with the fewest adverse effects.
Objectives
Formulation and Optimization of a novel herbal tablet containing Momordica dioica extract by applying 32 factorial designs for the improvement of hardness and In vitro drug release.
Materials and Methods
The tablets were made using Sodium Starch Glycolate (SSG) as a super disintegrant and Polyvinylpyrrolidone-K30 (PVP-K30) as a binder. The study was designed to optimize the formulation, and the model that resulted from that experiment was validated. Design Expert software 11 used a 3-level 2 factorial (32) design. Several pre- and post-compression characteristics were evaluated to determine the formulation’s quality.
Results
Formulated tablet batches B1 to B9 were tested for various parameters. It was discovered that the values ranged from 3.2 to 3.6 kg/cm2 in hardness, 5.9 to 6.2 mm in thickness, 735-765 mg in weight variation, 18 to 27 sec to wet the tablet, 16 to 19 min to disintegrate, 0.25 to 0.51% in friability, and 98 to 101% in drug content. The best drug release and hardness were achieved with PVP K 30 (6%) and SSG (6%). A promising batch was found as batch B5.
Conclusion
The findings suggest that PVP-K30 and SSG are the two best excipients when making herbal tablets.
INTRODUCTION
Herbal components are employed in many of the conventional treatments today. Almost 25% of American prescription medications contain at least one active component derived from a plant. Some are created using plant extracts, while others are manufactured to resemble organic plant substances. Chemical-based pharmaceuticals are prone to unfavorable side effects. Specific chemical substances that don’t occur naturally in the body tend to be rejected by the body. While phytopharmaceuticals seldom or never cause adverse effects, it is crucial to be aware that there is a chance that other prescription medications will interact chemically with them.1 Early records of herbal medicines in India and China go back 5,000 years, demonstrating the significance of plants in the healthcare system.2 Similarly, India’s herbal remedies are a crucial part of its tradition. People in several nations still rely on herbal remedies to treat their health problems.3 Ayurveda, Yoga, Naturopathy, Unani, Siddha, and Homeopathy are some of the time-tested ancient medical systems of India that are still helpful to people today. WHO research estimates that 80% of the world’s population relies on plant-based medical systems for their essential medical care. 80% of the basic components required to make traditional medicines come from medicinal plants. The usage of these medications correctly and the ongoing availability of pure raw ingredients are key factors influencing their effectiveness.4
Herbal extracts are made from plants that have a substantial impact on human nutrition and have a great deal of promise to treat a variety of ailments.5 There is an increasing demand for phytochemicals from herbal extracts such as phenolics, vitamins, and phytosterols owing to their essential roles in oxidative stress, anti-cancer effect, anti-aging properties, anti-inflammatory effect, etc. Therefore, the use of herbal extracts as a food supplement or a drug constituent instead of synthetic drugs becomes essential day by day due to the side effects of synthetic chemicals.6 There is a rising demand for phytochemicals derived from plant extracts, such as phenolics, flavonoids, vitamins, and phytosterols. Plant extracts are being used more frequently these days as nutritional supplements or components of medications.7
Momordica dioica Roxb. Ex. Wild is a forest plant of the genus Momordica. Indian folk medicine utilizes the fruit, leaf, and tuberous roots of M. dioica to treat diabetes.8 Latin’s word Momordica, which relates to the leaves sharp edges, implies “to bite”.9 Because of the existence of phytoconstituents, it is widely recognized for having a bitter flavour.10 Momordica dioica, also called Teasle Gourd, Kankro, Kakrol, Kantola, Kartoli, Bhatkarela, Ban Karola, Kantroli, Jungli karela, Kaksa, or little bitter-gourd, is a climbing plant. The Indo-Malayan area is where it first appeared.11 It is a tiny, oval to oblong vegetable called locally “Kikoda.” The plant is an annual ascending creeper in the Cucurbitaceae family. When the fruit reaches maturity, it is short-beaked, heavily echinate with soft spikes, and greenish and yellow in colour. This growing creeper is often found in Ceylon, Bangladesh, Pakistan, and the Himalayas.12 Momordica dioica Roxb. is found chiefly in Asia and Africa. The plant’s fruit, which has a variety of medical uses, is typically consumed as green vegetables. The leaves are aphrodisiac and anti-helminthic, while the fruits have laxative, hepatoprotective, alexiteric, diuretic, and stomachic qualities. The root extract has energetic, astringent, and antimicrobial activities. Additionally, it was said to have antioxidant, analgesic, Reno-protective, and anti-inflammatory properties. M. dioica was chosen since it is a common vegetable consumed by locals and has widespread usage in rural regions for various therapies. Additionally, several study findings highlighting the potential of M. dioica from diverse angles, such as plant components, animal models, or conventional drugs.13
Tablets are characterised as solid formulations designed for oral administration and having one or more active components in a single dosage. Compaction is used to create tablets filled with medication and formulation components.14 The leading drug manufacturers are currently looking into medicinal plants as potential sources for novel lead structures and to produce standardised phytotherapeutic molecules with established safety, efficacy, and quality.15 An individual must consume the necessary dose during the specified time to receive the intended benefit from herbal medications.16 The most popular type of product for pharmaceuticals that are taken orally is solid oral dosage forms. Unit dose forms provide the advantages of being handy, secure, and simple to handle and transport.17 An almost tamper-proof dosage form is the tablet. The tablet should be a classy item with a distinct identity, devoid of errors like splits, discolouration, cracks, and contamination, and strong enough to prevent the therapeutic substance from changing. A tablet’s physical properties should be chemically and physically stable throughout time. This dosage form was chosen due to its practicality, simplicity of use, and capacity to hide herbal extracts’ disagreeable tastes and odours.18 The current study aimed to develop and assess a unique herbal tablet using methanolic fruit extracts of Momordica dioica Roxb.19
MATERIALS AND METHODS
Materials
Momordica dioica is sourced from the local market and verified by Dr. R. R. Acharya. Head and Research Scientist (Veg.), Main Vegetable Research Station, Anand Agricultural University, Anand – 388 110, Gujarat (India), in response to a voucher specimen dated 10/05/2021, marked AAU/MVRS/EST/53/2021. Sun-dried fruits were employed for the research. Lactose monohydrate AR, Polyvinylpyrrolidone-K30 (PVP-K30, Sodium Starch Glycolate (SSG), Talc, and Magnesium stearate were purchased from LOBA Chemie Pvt. Ltd., Mumbai, Maharashtra. Methanol and petroleum ether were bought from Merk Life Science Pvt. Ltd., in Vikhori, Mumbai. Aeroperl 300 was received as a gift sample from Evonik Industries in Essen, Germany. All compounds were of an analytical grade. Karela® Tablet (Momordica charantia – 250 mg; Manufactured by The Himalaya Drug Company Private Limited, Bangalore) was purchased from Shri Ayu Pharma Medical Store, Anand, Gujarat, India.
Preparation of the extracts
Momordica dioica Roxb. sun-dried fruits were ground into powder using a mechanical grinder. The powdered material (100 g) was gradually extracted with Petroleum ether (900 mL) and methanol (900 mL) using a Soxhlet extractor. The Whatman filter paper was used to filter the extracts. The extracts were vacuum dried after being reduced in a rotary evaporator and then stored at a temperature of 4-5°C until use.20
Preliminary Phytochemical Analysis of Extract
Isolation of Marker compounds
The key bioactive component of the herbal tablet made from Momordica dioica extract, which is also present in Momordica charantia, is charantin. For that reason, the isolation of charantin was done from Momordica charantia. Market-purchased fresh, immature Momordica charantia fruits were divided into small pieces and dried in a hot air oven below 60°C. The dry material was ground into a coarse powder before being percolated with solvent petroleum ether (60-80°) and 80% ethanol respectively. The ethanolic extract was concentrated under a vacuum, suspended in 95% ethanol, and made alkaline with KOH to a pH of around 10, whereas the petroleum ether extract was discarded. The suspension was diluted with water and extracted with ether after 48 hr. The ether extract was cleaned with water, 5% hydrochloric acid, and then water. It was then dried over anhydrous sodium sulfate. After distilling the ether away, the residue was repeatedly recrystallized from 95% ethanol. Momordica charantia ingredient was identified as Charantin (Rf 0.45).23 Also, determine the presence of Charantin in ethanolic extract of MD fruits by HPTLC method.24
Residual solvent analysis GC-HS
The residual solvents will be analyzed using a gas chromatography headspace with a flame ionization detector to determine the amount of methanol left in the methanolic extract of Momordica dioica. Moreover, to confirm the methanol residue acceptable limits in accordance with ICH and pharmacopeial standards.25
Calibration curve of methanolic extract of MD in Gastric pH 1.2 and pH 6.8
Three duplicate experiments assessed a linear relationship between the MD extract concentration and absorbance across the concentration range of 50 to 300 μg/mL. Several aliquots of 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mL were transferred from the stock solution (1000 µg/mL) into a 10 mL volumetric flask and brought to the proper volume with the appropriate buffer. At 270 nm, the calibration curve for absorbance vs. concentration was drawn.26
Development of Formulation
Wet granulation was used to create herbal pills. The components were all put through sieve number 80. Exact amounts of the extract were weighed out and applied to the lactose, and the necessary quantities of PVP K30 were added as a binder and methanol as a solvent. Sieve number 20 was used to create the granules. The produced granules were dried for two hours at room temperature. SSG as a super disintegrant, magnesium stearate, talc, and Aeroperl was then added and blended for two to three minutes. The pre-compression investigations are then performed on these dried granules. Table 1 lists all nine formulations (B1– B9) created using various PVP K30 and SSG concentrations.27
| Ingredients (mg) | B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 | B9 |
|---|---|---|---|---|---|---|---|---|---|
| Herbal extract | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| PVP K30 | 22.5 | 22.5 | 22.5 | 45 | 45 | 45 | 67.5 | 67.5 | 67.5 |
| SSG | 30 | 45 | 60 | 30 | 45 | 60 | 30 | 45 | 60 |
| Talc | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Magnesium stearate | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Aeroperl | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Lactose | 425 | 410 | 395 | 402.5 | 387.5 | 372.5 | 380 | 365 | 350 |
| Total weight | 750 | 750 | 750 | 750 | 750 | 750 | 750 | 750 | 750 |
Determination of Pre-compression and Post compression Parameters
The resultant granules were examined for Micrometrics characteristics, including bulk density, tap density, angle of repose, Hausner’s ratio, and Carr’s index, which were measured to determine the compressibility index and flow property. As quality control metrics for the tablet, weight variation, average weight, hardness, disintegration time, friability, wetting time, thickness, and drug content were evaluated. Lastly, oval capsule-shaped tablets with an average weight of 750 mg were compressed using a 10-station single rotary tablet press with 19 mm punches.28,29
In vitro Dissolution Study
Studies on drug release were carried out utilizing USP equipment II (Paddle). Dissolving research was conducted with a 900 mL phosphate buffer solution with a pH of 6.8 at a temperature of 37 ± 0.50°C and 50 rpm. Samples (5 mL) were extracted and replaced with phosphate buffer at the indicated time intervals. Withdrawn samples were tested using the UV technique after being filtered using a Whatman filter paper. The cumulative percentage of drug release was computed using an equation derived from a calibration curve. Similar in vitro drug release tests were conducted on herbal tablets at phosphate buffer solution pH 6.8, pH 7.4 and gastric pH 1.2.30
Wetting Time
In a small Petri dish with 6 mL of distilled water, a piece of tissue paper is folded twice and put inside. Three tablets from each batch were individually placed in the middle of the paper, and the amount of time it took for the water moves upward from the lower edge is recorded as the wetting time.18
Optimization of Formulation Using 32 Full Factorial Design
Quality by Design procedures was carried out following ICH Q8 R2. 32 full factorial designs were used in the data analysis by design expert software. In this design, two factors were examined at three levels for each, and experimental trials were run in all 9 possible configurations. As independent variables, the amounts of PVP K 30 (%) (X1) and SSG (%) (X2) were chosen. Moreover, as a dependent variable, there is Disintegration time in minutes (Y1) and wetting time in seconds (Y2). The dependent and independent variables, coded values, and converted values are listed in Table 2. The Design-Expert software was used to do Multiple Regression analysis (MLR) and Analysis of Variance (ANOVA) to determine the connection between the two independent variables (X1 and X2) and dependent variables (Y1 and Y2) in the factorial design. The outcomes of multiple linear regression (the correlation coefficient and coefficient values) and analysis of variance (Fisher’s ratio and p values) were considered to choose the optimum mathematical model.
| Independent Variables | Coded Value | Transformed Value (%) | ||||
|---|---|---|---|---|---|---|
| High | Medium | Low | High | Medium | Low | |
| (X1) Amount of PVP K 30 in % | +1 | 0 | -1 | 9 | 6 | 3 |
| (X2) Amount of SSG in % | +1 | 0 | -1 | 9 | 6 | 3 |
| Dependent variables | Response | |||||
| Y1 | Disintegration time in minutes | |||||
| Y2 | Wetting time in seconds | |||||
Linear model
X1 and X2 are the coded levels of the independent variable, β0 is the intercept, which represents the arithmetic average of all quantitative results of factorial runs, and β1 and β2 are the coefficients calculated from the Momordica dioica extract experimental data (s). The interaction is denoted by the words X1X2. ANOVA (analysis of variance) was used to determine the statistical validity of the polynomials, and grid and feasibility searches were carried out to identify the composition of the best formulation. The Design-Expert software produced 3-D contour plots.27,29,30
FTIR study (Fourier Transform Infra-Red)
FT-IR spectrum analysis was used to assess the drug’s compatibility with the excipients; this study was conducted to determine whether the drug’s chemical makeup changed due to the addition of the excipients. Momordica dioica extract and tablet FTIR spectra were obtained in the 4000 to 400 cm-1 range. The materials were put in the sample container after being combined with potassium bromide powder, and the spectra were then taken. The peak values (wave number) and potential functional group are displayed in spectra that contrast with the standard value. These findings demonstrate that the material was a pure methanolic extract compared to the chemical structure.31,32
Differential Scanning Calorimetry (DSC)
To track the thermal changes, a differential scanning calorimeter was employed. Differential scanning calorimetry examined the interactions between the excipient and the drug. Each drug has its thermograph. The thermogram was compared to the standard for Momordica dioica extract. Using a DSC7020-HITACHI, Japan equipment, investigations of pure drugs and tablets containing combinations of drugs and excipients were conducted. Under inert nitrogen, the samples were heated to temperatures ranging from 20°C to 300°C at a rate of 10°C/min.33
Stability studies
A drug’s capacity to maintain its physical, chemical, therapeutic, and toxicological parameters of a specific formulation and container is known as stability. The improved formulation underwent a stability assessment for 90 days at 40±2°C and relative humidity of 75±5%. The formulations were then assessed for changes in the drug content, physicochemical characteristics, and in vitro drug release.34
RESULTS AND DISCUSSION
Preliminary Phytochemicals Analysis
The percentage yield of petroleum ether extract obtained from MD fruit is 5.03% w/w, which is yellowish-brown and solidifies at 28°C. In contrast, the percentage yield of methanol extract is 16.45% w/w, which is semi-solid dark brown, and bitter in taste.
The petroleum ether extract contains steroids, and terpenoids. At the same time, methanol extract contains Alkaloids, Carbohydrates, glycosides, Cardiac glycosides, steroids, terpenoids, proteins, tannins, saponins, flavonoids, phenol, and proteins.
The isolated marker is Light Yellowish White shiny crystal, % Yield was 0.090 of the dried fruits, melted at 270°C with decomposition, giving a positive Libermann-Burchard Test, Decolorizing dilute potassium permanganate and bromine water. The UV Spectrum of marker shows absorbs exactly at 272 nm. TLC of charantin with solvent system Methanol: Benzene (2:8) showed Rf value 0.45 (Figure 1).
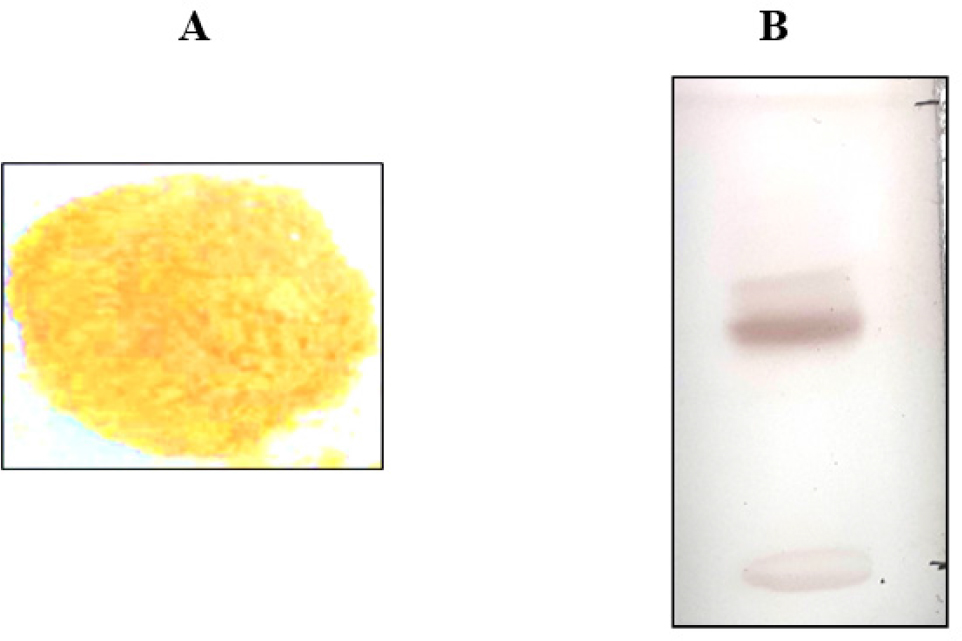
Figure 1:
Powder of Isolated Marker (A) and TLC of Isolated marker (B).
Residual solvent analysis GC-HS
The residual solvent analysis of methanol extract was carried out by GC-HS technique (Figure 2). The residual methanol concentration was found to be 24.88 ppm and this is the allowable permissible intake of methanol according to ICH: Impurities: Q3C (R6).
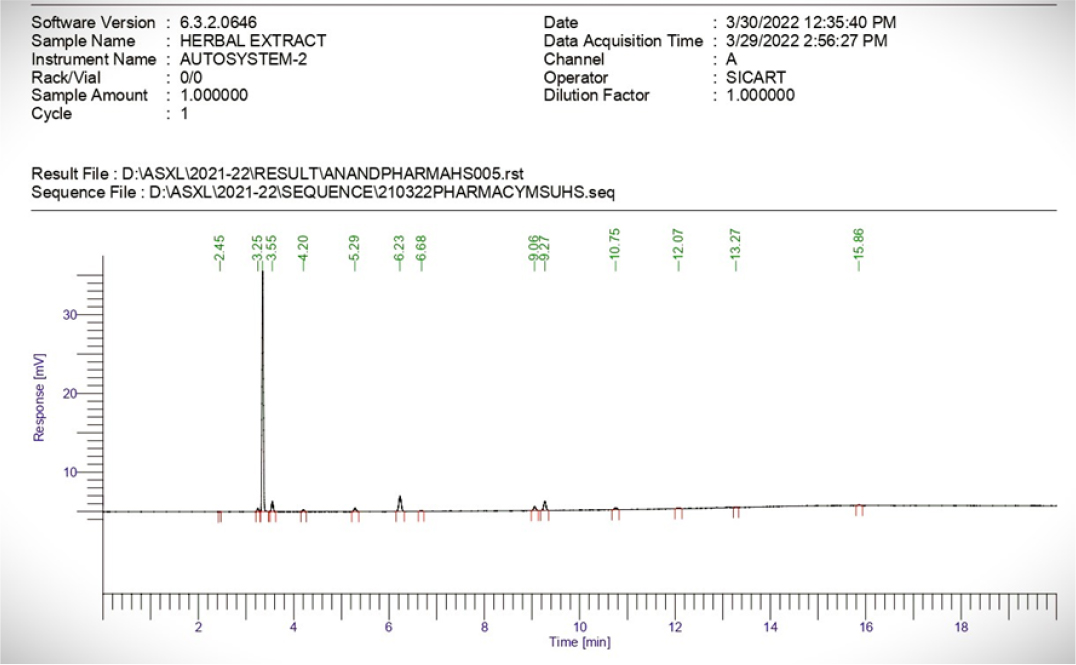
Figure 2:
Residual solvent analysis of methanol extract.
Calibration curve of methanolic extract in Gastric pH 1.2 and pH 6.8
The calibration curve of Methanolic extract in gastric pH 1.2 exhibited maximum absorbance at 270 nm and obeyed Beer’s law in the concentration range of 50 to 300 μg/mL. Linear regression of absorbance on concentration gave equation 0.0028x + 0.0093 with a correlation coefficient of 0.995. Methanolic extract in Phosphate buffer pH 6.8 exhibited maximum absorbance at 270 nm and obeyed Beer’s law in a concentration range of 50 to 300 μg/mL. Linear regression of absorbance on concentration gave equation 0.0032x – 0.0225 with a correlation coefficient of 0.998. The standard graph was created by placing concentrations on the X-axis and absorbance on the Y-axis. The result shown in Figure 3.
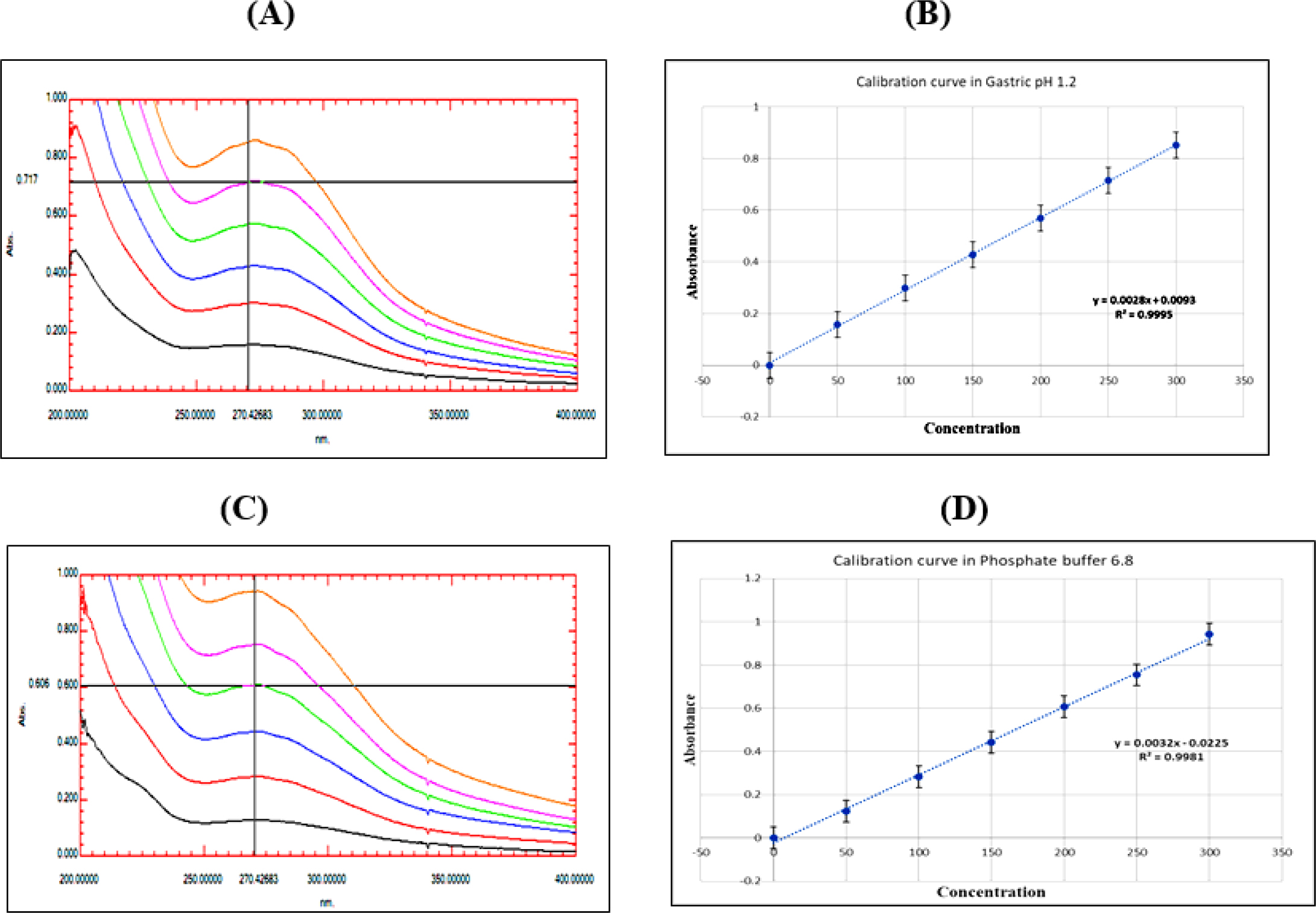
Figure 3:
Methanolic extract (A) UV absorbance in gastric pH 1.2 (B) Calibration curve in gastric pH 1.2 (C) UV absorbance in Phosphate buffer pH 6.8 (D) Calibration curve in Phosphate buffer pH 6.8.
Composition of Herbal tablet
Evaluation of Herbal Tablet
The produced granules for the compression of immediate-release tablets were assessed for different pre- and post-compression parameters.
The Table 3 presents the pre-compression values. According to the USP requirement, all the batches met Angle of repose (25-30) as Excellent, (31-35) Good, Carr’s index (12-16) as Good, (5-15) Excellent, and Hausner’s ratio (1.12-1.18) as Good, (1-1.11) Excellent. So, we draw the conclusion that all of the batches had efficient precompression settings.
| Parameters | Batch 1 | Batch 2 | Batch 3 | Batch 4 | Batch 5 | Batch 6 | Batch 7 | Batch 8 | Batch 9 |
|---|---|---|---|---|---|---|---|---|---|
| Angle of Repose (degrees) | 29.68 ± 0.43 | 29.68 ± 0.42 | 29.68 ± 0.58 | 29.24 ± 0.67 | 29.68 ± 0.48 | 29.68 ± 0.55 | 29.68 ± 0.68 | 29.68 ± 0.55 | 29.68 ± 0.45 |
| Bulk Density (g/mL) | 0.46 ± 0.07 | 0.48 ± 0.06 | 0.48 ± 0.05 | 0.48 ± 0.08 | 0.52 ± 0.03 | 0.54 ± 0.05 | 0.46 ± 0.04 | 0.48 ± 0.07 | 0.54 ± 0.06 |
| Tapped Density (g/mL) | 0.52 ± 0.06 | 0.54 ± 0.08 | 0.54 ± 0.05 | 0.54 ± 0.08 | 0.56 ± 0.06 | 0.59 ± 0.05 | 0.54 ± 0.07 | 0.56 ± 0.06 | 0.59 ± 0.04 |
| Carr’s Index | 11.53 ± 0.15 | 11.53 ± 0.25 | 11.11 ± 0.32 | 11.11 ± 0.16 | 7.14 ± 0.26 | 8.47 ± 0.18 | 14.81 ± 0.29 | 14.28 ± 0.27 | 8.47 ± 0.35 |
| Hausner’s Ratio | 1.13 ± 0.04 | 1.12 ± 0.06 | 1.12 ± 0.07 | 1.12 ± 0.06 | 1.07 ± 0.04 | 1.09 ± 0.06 | 1.17 ± 0.06 | 1.16 ± 0.04 | 1.09 ± 0.07 |
The following post-compression assessment characteristics were also evaluated: weight variation, average weight, hardness, Disintegration Time (DT), friability, Wetting Time (WT), thickness, and drug content, as indicated in the Table 4. The tests for weight variation and hardness were passed by all of the batches.
| Parameters | Batch 1 | Batch 2 | Batch 3 | Batch 4 | Batch 5 | Batch 6 | Batch 7 | Batch 8 | Batch 9 |
|---|---|---|---|---|---|---|---|---|---|
| Weight variation | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| Average weight (mg) | 755.3 | 753.5 | 756.2 | 749.5 | 752.4 | 753.2 | 751.6 | 754.4 | 751.7 |
| Hardness (kg/cm2) | 3.6 ± 0.04 | 3.2 ± 0.02 | 3.6 ± 0.05 | 3.4 ± 0.07 | 3.6 ± 0.02 | 3.4 ± 0.05 | 3.6 ±0.02 | 3.4 ± 0.05 | 3.6 ±0.07 |
| Disintegration Time (Minutes) | 16.58 ± 0.06 | 17.2 ± 0.09 | 18.15 ± 0.06 | 17.29 ± 0.04 | 17.6 ± 0.05 | 19.00 ± 0.07 | 17.29 ± 0.06 | 18.5 ± 0.07 | 19.28 ± 0.06 |
| Friability (%) | 0.25 ± 0.07 | 0.5 ± 0.08 | 0.25 ±0.05 | 0.38 ± 0.03 | 0.25 ± 0.03 | 0.5 ± 0.07 | 0.51± 0.08 | 0.5 ± 0.04 | 0.49 ± 0.03 |
| Wetting time (Seconds) | 24 ± 0.06 | 26± 0.04 | 27 ± 0.02 | 20 ± 0.03 | 23 ± 0.07 | 25 ± 0.06 | 18 ± 0.05 | 20 ± 0.03 | 22 ± 0.05 |
| Thickness (mm) | 6.2 ± 0.06 | 6 ± 0.07 | 5.9 ± 0.05 | 6.1 ±0.06 | 6 ± 0.04 | 6 ± 0.08 | 6.2 ± 0.05 | 6.1 ± 0.07 | 6 ± 0.04 |
| Drug content (%) | 98.3 ± 0.56 | 96.4 ± 0.59 | 101± 0.51 | 97.6 ± 0.54 | 99.4 ± 0.58 | 98.2 ± 0.62 | 99.6 ± 0.47 | 97.5 ± 0.72 | 96.8 ± 0.77 |
The tablet is light brown in color, capsule shape, characteristic odor, bitter taste, and 2.96% w/w moisture.
In vitro dissolution studies
According to the study, the medication release rate rises with the amount of binder and disintegrant at the required concentration. In gastric pH and phosphate buffer 6.8, the drug release profile for batch 5 was found to be 90% of starting level in the first 10 min. In Phosphate buffer 7.4 in 15 min, however, more than 80%. The immediate-release batch 5 tablet contains 6% Polyvinylpyrrolidone (PVP K30) as a binder and 6% Sodium Starch Glycolate (SSG) as a disintegrant contributing to its significant 91% drug release. Compared to the other eight formulations, batch B5 from all the batches exhibits quick drug release (Figure 4).
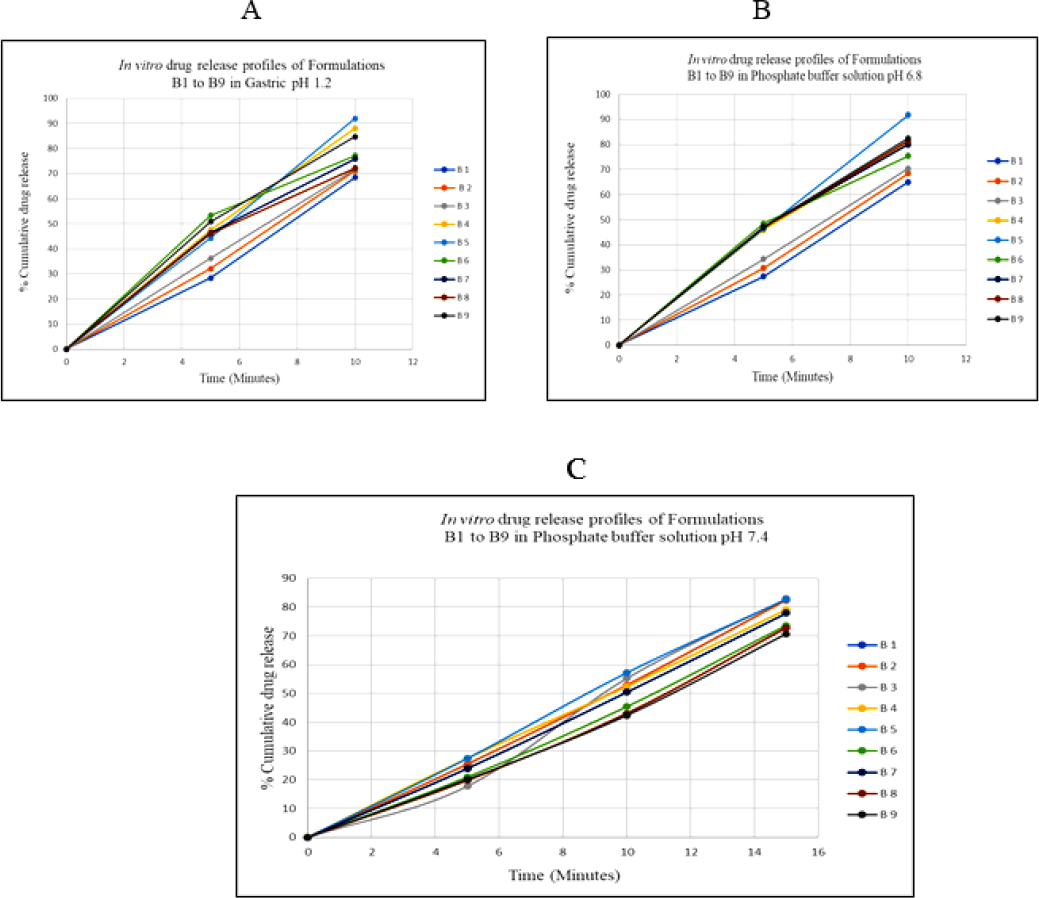
Figure 4:
In vitro drug release profiles of Formulation B1 to B9 (A) Gastric pH 1.2 (B) Phosphate buffer solution pH 6.8 (C) Phosphate buffer solution pH 7.4.
Wetting time
The pills dissolve more quickly, shorter the wetting time is. All formulations had wetting times that were less than 30 sec. The number of excipients in each batch affected the wetting time. Wetting time in batch B5 was 23 sec because of the 6% SSG content.
Optimization of Formulation Using 32 Design
There were 9 trials in total created by the three-level full factorial (32) design. For the nine formulations, Table 5 displays the results of the experimental runs with independent variables and unique herbal tablets containing Momordica dioica extract.
| Batches | X1-Amount of PVP K30 % | X2-Amount of SSG % | Y1- Disintegration Time in minutes n = 3, (± S.D) | Y2- Wetting time in seconds n = 3, (± S.D) |
|---|---|---|---|---|
| B1 | 3 | 4 | 16.58 ± 0.06 | 24 ± 0.06 |
| B2 | 6 | 4 | 17.2 ± 0.09 | 26 ± 0.04 |
| B3 | 9 | 4 | 18.15 ± 0.06 | 27 ± 0.02 |
| B4 | 3 | 6 | 17.29 ± 0.04 | 20 ± 0.03 |
| B5 | 6 | 6 | 17.6 ± 0.05 | 23 ± 0.07 |
| B6 | 9 | 6 | 19 ± 0.07 | 25 ± 0.06 |
| B7 | 3 | 8 | 17.29 ± 0.06 | 18 ± 0.05 |
| B8 | 6 | 8 | 18.5 ± 0.07 | 20 ± 0.03 |
| B9 | 9 | 8 | 19.28 ± 0.06 | 22 ± 0.05 |
The Design Expert program used MLR and ANOVA to determine how the two independent variables (X1 and X2) and the two dependent variables interacted (Y1 and Y2). Table 6 compiles the findings of MLR (correlation coefficient value and coefficient values) and ANOVA (Fisher’s ratio and P values).
| Parameters | Disintegration Time in minutes | Wetting time in seconds |
|---|---|---|
| Intercept | 17.88 | 22.78 |
| A | 0.8783 | 2.00 |
| P value | 0.0001 | 0.0001 |
| B | 0.5233 | -2.893 |
| Model | Linear | Linear |
| R2 | 0.9505 | 0.9811 |
| Adjusted R2 | 0.9340 | 0.9748 |
| Predicted R2 | 0.8984 | 0.9547 |
| Adeq Precision | 20.8141 | 34.8000 |
| PRESS value | 0.6705 | 3.33 |
The contour plot of response Y1 shown in Figure 5(A) indicates no effect on DT when the concentration of PVP K30 and SSG is low. Increase the DT when the concentration of PVP K30 and SSG increases. The contour plot of response Y2 shown in Figure (B) presents the optimum WT when the concentration of PVP K30 and SSG is low. WT increases when the concentration of PVP K30 increases and WT decreases when the concentration of SSG decrease.
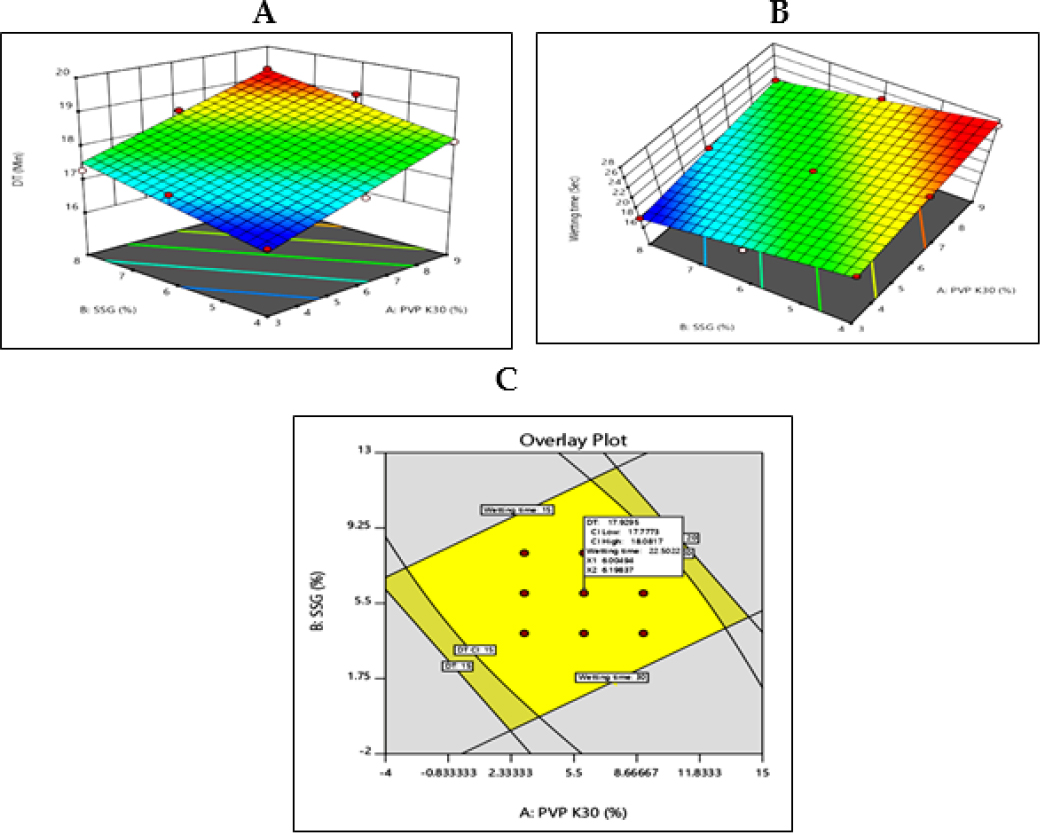
Figure 5:
Graph from design of experiment (A) 3D counter plot of Y1 response (B) 3D counter plot of Y2 response (C) Overlay plot with design space.
The combined effect shows that the DT and WT increase when PVP K30 and SSG increase. A higher % PVP K30 improves the wetting time, and DT becomes optimum when the concentration of PVP K30 and SSG increases.
TLC of Novel Herbal Tablet, Methanol extract of MD Fruit and Isolated Marker (Charantin)
TLC confirm the Phytoconstituents in Novel dosage formulation, Methanolic extract of MD form at Rf value 0.45 (Figure 6) when compared with an isolated marker.

Figure 6:
TLC of Novel Herbal Tablet, Methanol extract of MD Fruit and Isolated Marker.
FT-IR Study
To check for any interactions between the MD extract and excipients in the Innovative herbal formulation, FTIR studies were performed. Figure 7 displays the FTIR spectra of MD extract and herbal pill. The spectrum of the MD extract displays a peak for OH stretching at 3282 cm-1, a peak for CH stretching at 2931 cm-1, a peak for the C=O group at 1874 cm-1, and a peak for C=C bending at 968 cm-1. The IR spectra of the Novel Herbal pill that contains the MD extract showed OH stretching peaks at 3282 cm-1, CH stretching peaks at 2900 cm-1, peak at 1662 cm-1, which corresponds to C=C stretching, OH bending peaks at 1423 cm-1, and C-O stretching peaks at 1041 cm-1.There is no indication of chemical interaction between the extract and excipients, as shown by the retention of different functional groups in the FT-IR spectra of the methanol extract (A) and herbal tablet (B). Since there is no peak deviation, the excipients must be compatible with the medicine and possess a hydrogen bond.
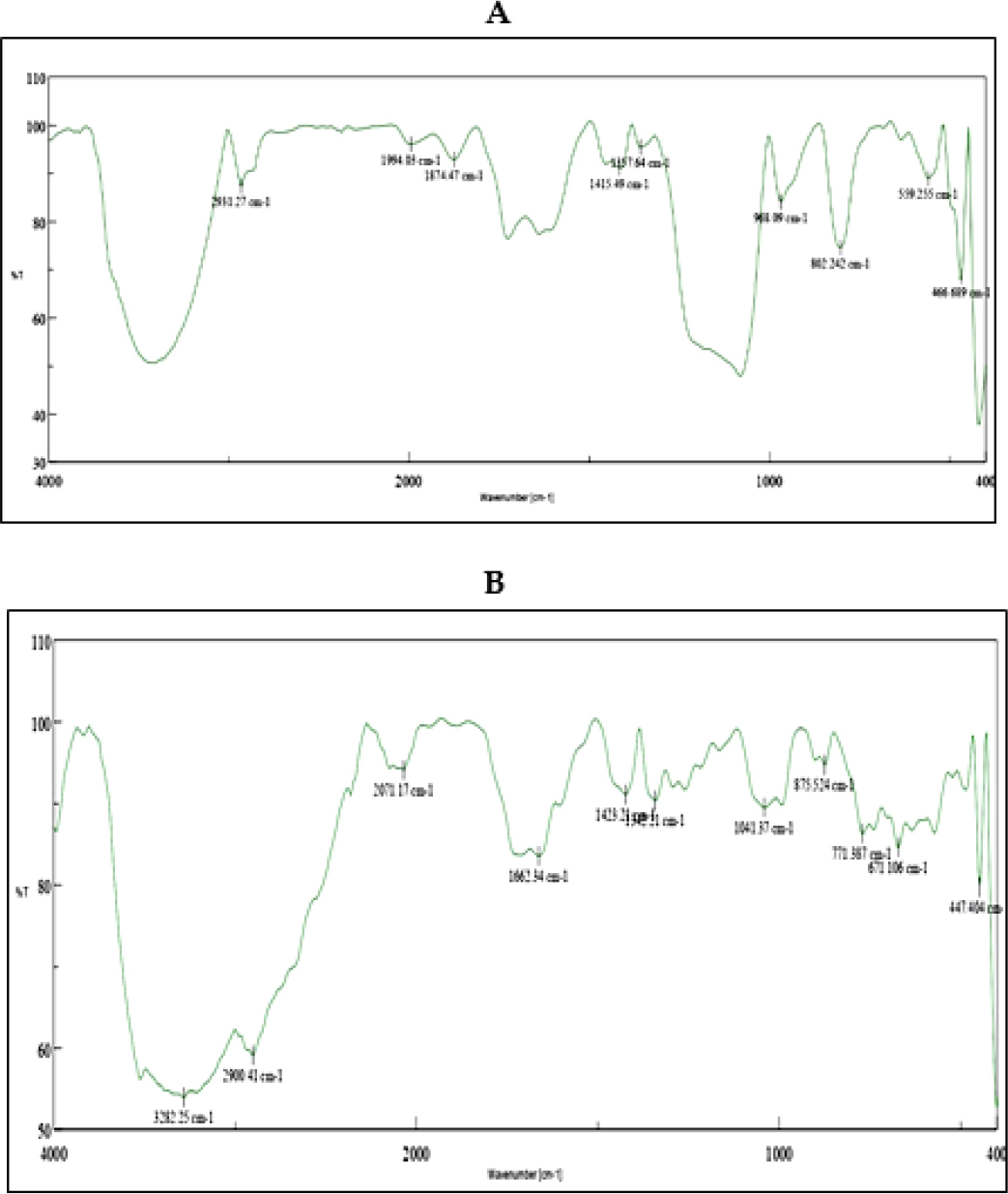
Figure 7:
FT IR spectra of (A) MD Extract (B) Novel Herbal tablet contain MD extract.
DSC Analysis
An endothermic peak at 103°C is visible in the DSC thermogram of the methanol extract (A). Two endothermic peaks at 146.6°C and 194°C are visible on the DSC thermogram of the herbal tablet (B), which suggests the presence of hydrogen bonds with the excipients, which improves the drug release (Figure 8). DSC demonstrates how the medication becomes amorphous or loses its crystalline shape.
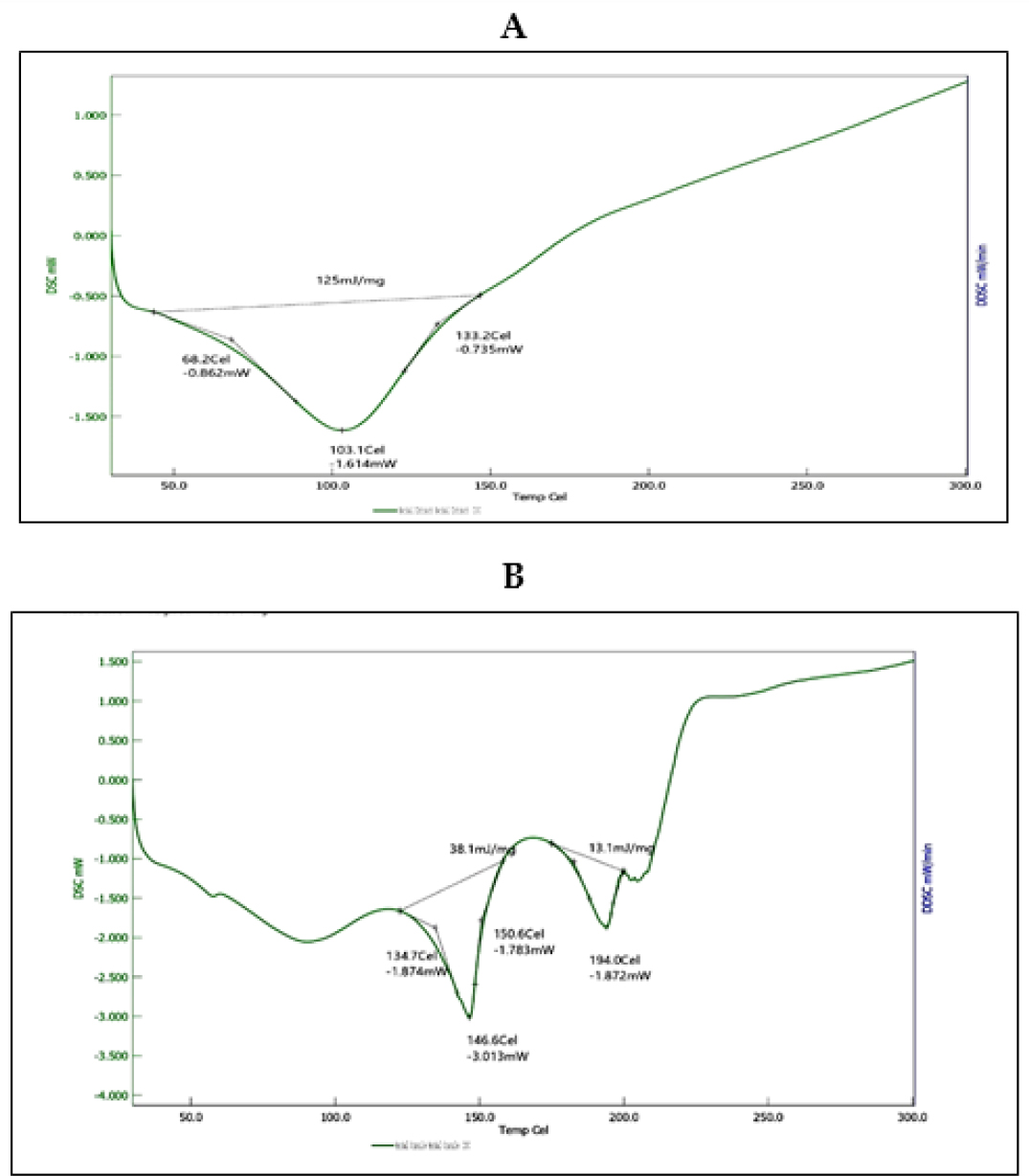
Figure 8:
DSC of (A) MD Extract (B) Novel Herbal tablet contain MD extract.
Stability studies
Stability tests on the improved tablet formulation (B5) were carried out, and after the storage period, no discernible changes in the tablets’ appearance were found. After storage, the drug’s content was discovered to be 98.38% ± 0.73 percent, compared to 99.4% ± 0.58 before storage. In dissolving tests, the amount of extract released from the B5 pill before and after storage was 100% within 15 min. After being kept for three months at 40°C/75% RH, there was no discernible variation in the average amount of MD extract released or the drug content from B5 tablets.
CONCLUSION
In this investigation, PVP K30 was used as a binder and SSG as a disintegrant to create fast-release tablets of MD extract. The pre-compression analysis of the granules from the nine batches reveals that they have satisfactory flow characteristics. All batches’ post-compression levels were confirmed to comply with the rules. Compared to other formulations, batch 5 with an equal amount of PVP K30 and SSG had good hardness, friability, weight variation, wetting time, disintegration time, and in vitro drug release. DSC thermograms and FTIR spectroscopy indicate that the extract is compatible with excipients. The stability investigation of the optimized formulation showed consistent results after three months of storage.
References
- Byeon JC, Ahn JB, Jang WS, Lee SE, Choi JS, Park JS, et al. Recent formulation approaches to oral delivery of herbal medicines. J Pharm Investig. 2019;49(1):17-26. [CrossRef] | [Google Scholar]
- Parasuraman S.. Herbal drug discovery: challenges and perspectives. Curr Pharmacogenomics Pers Med. 2018;16(1):63-8. [CrossRef] | [Google Scholar]
- Ceballos R LA.. Use of Herbal Medicines and Implications for Conventional Drug Therapy Medical Sciences. Altern Integr Med. 2013;02(06):130 [CrossRef] | [Google Scholar]
- Mishra P, Kumar A, Nagireddy A, Mani DN, Shukla AK, Tiwari R, et al. DNA barcoding: an efficient tool to overcome authentication challenges in the herbal market. Plant Biotechnol J. 2016;14(1):8-21. [PubMed] | [CrossRef] | [Google Scholar]
- Karim N, Jia Z, Zheng X, Cui S, Chen W. A recent review of citrus flavanone naringenin on metabolic diseases and its potential sources for high yield-production. Trends Food Sci Technol. 2018;79:35-54. [CrossRef] | [Google Scholar]
- Islam Shishir MRI, Karim N, Gowd V, Zheng X, Chen W.. Liposomal delivery of natural product: A promising approach in health research. Trends Food Sci Technol. 2019;85:177-200. [CrossRef] | [Google Scholar]
- Sogut O, Aydemir Sezer U, Sezer S.. Liposomal delivery systems for herbal extracts. J Drug Deliv Sci Technol. 2021;61:1-13. [CrossRef] | [Google Scholar]
- Rao PS, Mohan GK. Array. Saudi J Biol Sci.. 2017;24(6):1262-7. [PubMed] | [CrossRef] | [Google Scholar]
- Chekka SV, Mantipelly NK. Array. GSC Biol PharmSci.. 2020;12(2):129-35. [CrossRef] | [Google Scholar]
- Kumar Jha D, Koneri R, Samaddar S.. Medicinal use of an ancient herb : a review. Int J Pharm Sci Res.. 2018;9(2):432-41. [CrossRef] | [Google Scholar]
- Talukdar SN, Hossain MN. Phytochemical, Phytotherapeutical and Pharmacological Study of . Evid Based Complement Alternat Med.. 2014;2014:806082 [PubMed] | [CrossRef] | [Google Scholar]
- Salvi J, Katewa SS. Nutritional Composition of fruits: as a wild vegetable. Nutr Compos Momordica dioica fruits as a wild Veg. 2015;3(2):18-22. [PubMed] | [CrossRef] | [Google Scholar]
- Hassan MM, Uddin S, Bhowmik A, Ashraf A, Islam MM, Rokeya B., et al. Phytochemical screening and antidiabetic effects of fruit rind of roxb. on streptozocin induced type 2 diabetic rats. Heliyon. 2022;8(1):e08771 [PubMed] | [CrossRef] | [Google Scholar]
- Kumar TS, Muthusamy P, Radha R, Ilango K.. Formulation and Evaluation of antidiabetic Polyherbal tablets form some traditional used Herbs. J Phytopharmacol.. 2021;10(3):173-9. [CrossRef] | [Google Scholar]
- Rezghi M, Mortazavi SA, Fahimi S, Choopani R, Sheikholeslami MA, Hamzeloo-Moghadam M., et al. Polyherbal tablet based on Iranian traditional medicine. J Med Plants. 2021;20(77):15-25. [CrossRef] | [Google Scholar]
- Thakur S, Bhardwaj B, Nandy SK. Formulation and characterization of herbal tablets for the management of dengue. EPRA Int J Res Dev.. 2021;6(4):104-13. [CrossRef] | [Google Scholar]
- Kushwaha SK, Kori ML. Development and evaluation of polyherbal syrup from some hepatoprotective medicinal plants. Sch Acad. J Pharmacol. 2014;3(3):321-6. [CrossRef] | [Google Scholar]
- Pandey H, Srivastava S, Mishra B, Saxena R, Tripathi YB. Development and evaluation of herbal tablet loaded with water extract with use of different excipients. Asian J Pharm. 2018;12(9):S786-93. [CrossRef] | [Google Scholar]
- Maurya H, Kumar T. Formulation, standardization, and evaluation of polyherbal dispersible tablet. Int J Appl Pharm.. 2019;11(1):158-67. [CrossRef] | [Google Scholar]
- Nemkul CM, Bajracharya GB, Maeda H, Shrestha I.. Ethnomedicinal knowledge verification for the antidiarrheal and antioxidant effects of mill. Fruits with Identification of Thirty Constituents. Pharmacogn J.. 2021;13(1):37-43. [CrossRef] | [Google Scholar]
- Shaikh JR, Patil M.. Qualitative tests for preliminary phytochemical screening: an overview. Int J Chem Stud. 2020;8(2):603-8. [CrossRef] | [Google Scholar]
- Pillwan SN, Thool ND, Chopkar SH, Mathankar SP, Pise SA, Pise AG, et al. To study extraction, phytochemical screening and formulation from bertoni. Res J Pharm Technol.. 2020;13(12):5757-62. [CrossRef] | [Google Scholar]
- Patel S, Patel T, Parmer K, Bhatt Y. Isolation, characterization and antimicrobial activity of charantin from linn. fruit. Int J Drug Dev Res. 2010;2(3):629-34. [CrossRef] | [Google Scholar]
- Shanmugapriya R, Poornima S.. Detection of charantin in the leaves and fruits of (Cogn.) Roxb. and (Roxb Ex Wild) by Analytical HPTLC. Int J Sci Res Publ. 2014;4(6):1-8. [CrossRef] | [Google Scholar]
- Opuni KFM, Togoh G, Frimpong-Manso S, Adu-Amoah D, Alkanji O, Boateng KP, et al. Monitoring of residual solvent contamination in herbal medicinal products in Ghana: A pilot study. Sci Afr.. 2021;13:e00825 [CrossRef] | [Google Scholar]
- Bhavani A, Hemalatha B, Padmalatha K.. Formulation development and Evaluation of sustained release matrix tablets of cefpodoxime proxetil. Asian J Pharm Technol. 2021;11(4):273-8. [CrossRef] | [Google Scholar]
- Malviya V, Thakur Y, Shrikhande S, Gudadhe K, Tawar M. Formulation and evaluation of natural gum based fast dissolving tablet of meclizine hydrochloride by using 32 factorial design. Asian J Pharm Pharmacol.. 2020;6(2):94-100. [CrossRef] | [Google Scholar]
- Pandey H, Srivastava S, Mishra B, Saxena R, Tripathi YB. Development and evaluation of herbal tablet loaded with water extract with use of different excipients. Asian J Pharm.. 2018;12(2):S786-93. [CrossRef] | [Google Scholar]
- Gaonkar VMP, Mannur VS, Mastiholimath VS, Hullatti KK. Development and evaluation of herbal supplement: A quality by design approach. Indian J Pharm Sci. 2020;82(4):640-9. [CrossRef] | [Google Scholar]
- Dalwadi S, Thakkar VT, Rana HB. Hybrid Liquisolid technique: A novel approach to enhance the performance of antidiabetic drugs. Curr Drug Ther.. 2021;16(5):409-22. [CrossRef] | [Google Scholar]
- Siregar C, Martono S, Rohman A.. Application of Fourier Transform Infrared (FTIR) spectroscopy coupled with multivariate calibration for quantitative analysis of curcuminoid in tablet dosage form. J Appl Pharm Sci. 2018;8(8):151-6. [CrossRef] | [Google Scholar]
- Kansabanik S, Mukhi S, Das C, Das D, Bose A.. Establishment of quality control parameters of . Res J Pharm Technol.. 2019;12(12):5967-71. [CrossRef] | [Google Scholar]
- Aswathy KN, Asdaq SMB, Saritha CK, Thomas L, Haridas N, Viswanad V, et al. Formulation and characterization of fast-disintegrating herbal extract sublingual immunotherapy tablet for peanut-induced allergic asthma. Saudi J Biol Sci.. 2022;29(3):1283-97. [PubMed] | [CrossRef] | [Google Scholar]
- Puri D, Bhandari A, Gaur PK, Yasir M, Kumar SS, Choudhary D, et al. Formulation of Herbal Fast Disintegrating Tablets and its Study for Anti-histaminic Activity in Guinea Pig ileum. Curr Clin Pharmacol.. 2018;13(2):128-35. [PubMed] | [CrossRef] | [Google Scholar]
