ABSTRACT
Background
Yohimbine has been suggested as a possible therapy for type 2 diabetes mellitus. Thus, we evaluated antidiabetic activity of yohimbine in Streptozocin (STZ)-induced rats.
Materials and Methods
Yohimbine’s anti-diabetic effects were investigated by giving male Wistar rats an injection of Streptozotocin (STZ) to cause diabetes and then analyzing their blood samples. Blood Glucose Levels (BGLs) and glucose tolerance was recorded using an FBG and an OGTT, respectively. Triglyceride, Low-Density Lipoprotein (LDL-c), and High-Density Lipoprotein (HDL-c) levels were determined to examine the impact of yohimbine administration on the lipid profile of diabetic rats. Finally, yohimbine’s impact on pancreatic histological damage due to diabetes was investigated.
Results
Overall, our findings imply that yohimbine, an alpha-2 adrenoceptor antagonist, helped diabetic group have better glucose tolerance. Yohimbine-treated mice had lower BGLs and enhanced lipid profiles, with lessened triglyceride and LDL-c levels and improved HDL-c levels. Histopathological analyses showed that the size and density of pancreatic cells had increased and that they had regenerated remarkably.
Conclusion
As a result, we can conclude that yohimbine may have therapeutic value for restoring lipid-carbohydrate equilibrium and in repairing diabetes-related pancreatic damage.
INTRODUCTION
According to the World Health Organization the Diabetes Mellitus (DM) covers a group of metabolic diseases characterized by elevated blood glucose levels. The two main representatives of this group are Type 1 (body does not make enough insulin) and Type 2 (Body unable to utilize the insulin and impairment in the way the body regulates utilization of glucose) diabetes. DM is currently one of the most costly and burdensome chronic diseases (American Diabetes Association, 2011). It is caused by inherited or acquired deficiency in insulin secretion and by decreased responsiveness of the organs to secreted insulin. Type 2 diabetes is a chief cause of disability worldwide. Considerable evidence suggests that it contributes to the onset of cardiovascular disease. Vascular disease is the leading cause of mortality for people with diabetes, accounting for around 70% to 80% of all deaths. Hyperglycemia is the hallmark of diabetes and is hypothesized to predispose to diabetic comorbidities by affecting vascular cellular metabolism, matrix components, and serum lipoproteins. As an example, in the aorta of Streptozotocin (STZ) treated diabetic animals, hyperglycemia raises diacylglycerol levels and stimulates PKC activity. Dyslipidemia and a thickness of the glomerular basement membrane are hallmarks of the diabetes generated by STZ in rats, and similar changes are seen in drug-induced diabetes in rabbits.1
As researchers sought to better understand the progression and consequences of type 1 and type 2 DM in humans, new experimental models that closely resembled these conditions were developed. With the use of Streptozotocin (STZ), created a rat model of diabetes. STZ causes an insulin shortage, like that of type 2 diabetes, by selectively destroying pancreatic beta cells. Moderate hyperglycemia is seen in STZ-induced diabetic rats, along with impaired glucose tolerance and reduced insulin production, giving the animals diabetic condition comparable to that of human beings, and making them a suitable model to explore diabetic complications.2
Yohimbine is an antagonist targeting alpha-2-adrenoceptors, and it has been employed to alleviate erectile dysfunction.3 The most common side effects at high dosages include elevated blood pressure, mild anxiety, and urination that occurs more often. As an aphrodisiac, psychedelic, and supplement, it has several potential uses. Yohimbine has been the subject of substantial research due to its potential beneficial effects on diabetes-related issues such impotence in men (Ernst et al., 1998), infertility in women,4 and leptin levels.5 When given to animals or humans in vivo, or injected to islets in vitro, various alpha-2 adrenoceptor antagonists have been found to increase insulin production. One theory is that blocking alpha-2 adrenoceptors in the islets of Langerhans is responsible for these results by reducing the inhibitory tone caused by intrinsic catecholamines and therefore increasing insulin production.6 In light of these results, the use of alpha-2 adrenoceptor antagonists in the management of T2DM has been proposed.7 Yohimbine has so far been studied as a possible antidiabetic due to this theory. In the current investigation, antidiabetic effects of yohimbine, particularly on insulin, glucose, and lipid profiles of streptozocin-treated diabetic rats have been examined.
MATERIALS AND METHODS
Experimental Procedure
Chemicals
STZ (Hi media), Citric acid (Ranbaxy Fine Chemicals Ltd.,) Tween 80, Hi media, Anesthetic ether, Sodium chloride, nice chemicals, Narsons Pharma.
Yohimbine extract containing 8.25% Yohimbine was procured from Sv Agrofood, Mumbai, India.
Animals
Thirty male Wistar rats were utilized in our experiment, and they were divided into five groups of each containing six animals. Rats were held in sterilized polypropylene cages that were kept at a regulated temperature of 24–26 degrees Celsius. Each cage included three rat inhabitants. The animals received the standard of care as instructed by the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals). A week of acclimatization was given to all the animals prior to commencing the experiment. Before beginning the research, we received official permission from the Institutional Animal Ethics Committee.
Diabetes induction procedure
Preparation of STZ, 60 mg/kg, in a 50 mM sodium citrate buffer, pH 4.5, promptly prior to administration is recommended. Because citrate buffer melts at higher temperatures, ice was constantly added to the glass beaker holding the buffer to keep it at a constant, cold temp. Citrate buffer was injected intraperitoneally into normal rats as a control group.
Study Animals
All of the rats in the study were starved for 6 to 8 hr on day 1 before receiving a dose of Streptozotocin (STZ). As usual, there was access to water. Citrate buffer was made by placing 30 mg of STZ into a microcentrifuge tube and covering it with tin foil; one tube was created for each rat. STZ should be dissolved up to 30 mg/mL in 50 mM sodium citrate buffer pH 4.5 prior to administration.
60 mg/kg (2.0 mL/kg) of STZ solution was administered intraperitoneally.7 With a 23-G needle and a 1 mL syringe in the experimental groups, whereas an equivalent amount of citrate buffer was injected intraperitoneally in the control group.
Animals were relocated to respective enclosures and given their regular diets along with 10% sucrose water. The second day of the experiment, ordinary water was substituted by water with 10% sucrose.8 After inducing diabetes, rats were recruited for the research if their Fasting Blood Glucose value (FBG) was more than 126 mg/dL on day 4.
Experimental design
Group I: Control animal model.
Group II: Streptozotocin-induced diabetic rats (Diabetic control).
Group III and IV: Yohimbine-treated groups (25 mg/kg and 50 mg/kg).
Group V: Glibenclamide (5 mg/kg).9–14
On the first, seventh, fourteenth, and twenty-first days, blood was obtained from a tail prick to measure FBG on glucose strips. In the last week of the research, an OGTT was conducted.
Serum was obtained from all the rat groups at the conclusion of the trial and analyzed for lipid profile (Triglycerides, HDL, and LDL) through micro capillary bleeding from the retro-orbital plexus.
Methodology
Oral Glucose Tolerance Test (OGTT)15-19
Rats fasting overnight under controlled conditions had their tails pricked to measure their oral glucose tolerance. Blood glucose levels were first assessed in the fasted state, and then 2 g/kg of glucose was orally delivered. After then, the subjects’ blood sugar levels were checked again at 30, 60, and 120 min.
Each group’s AUC was determined using the given mathematical formula.
A, B, C and D represents BGLs at 0, 30, 60, and 120 min correspondingly.
Estimation of blood lipid profile
In order to determine the concentrations of triglycerides, HDL-c, and LDL-c in the blood, we followed the instructions included in the ERBA diagnostic kits.
Histopathology
In order to examine the histopathology of the rat pancreas tissues, they were preserved in 10% formalin. The pancreas was extracted from the rat’s abdomen shortly after sacrificing, placed in 10% neutral formalin, subsequently dehydrated in graded alcohol (80%-100%), treated and cleansed using xylene, and lastly embedded in liquid paraffin. After being deparaffinized in xylene solution, filtered through several grades of alcohol, and stained using hematoxylin and eosin, the pancreas was ready for histological examination. Light microscopy was used to analyze the sample. No abnormalities in histopathology were overlooked. repair, islet regeneration, minor necrosis, degeneration, and vacuolation.
Statistical analysis Results are presented as a mean ± SEM. For multiple group comparisons, we utilized a one-way ANOVA and Tukey’s post hoc test in GraphPad Prism 5, with a p value below 0.05 being deemed significant.
RESULTS
Effect of Yohimbine on FBG levels
FBG levels were increased following the induction of diabetes, treatment with Yohimbine LD, Yohimbine HD, and standard drug was responsible for a decrease in BGLs on the 7th day (p < 0.001, p < 0.01, and p < 0.001) as against control group. On the 14th and 21st day all the treatment groups exhibited a remarkable drop in blood glucose levels as against control group (p < 0.001) (Figure 1 and Table 1).
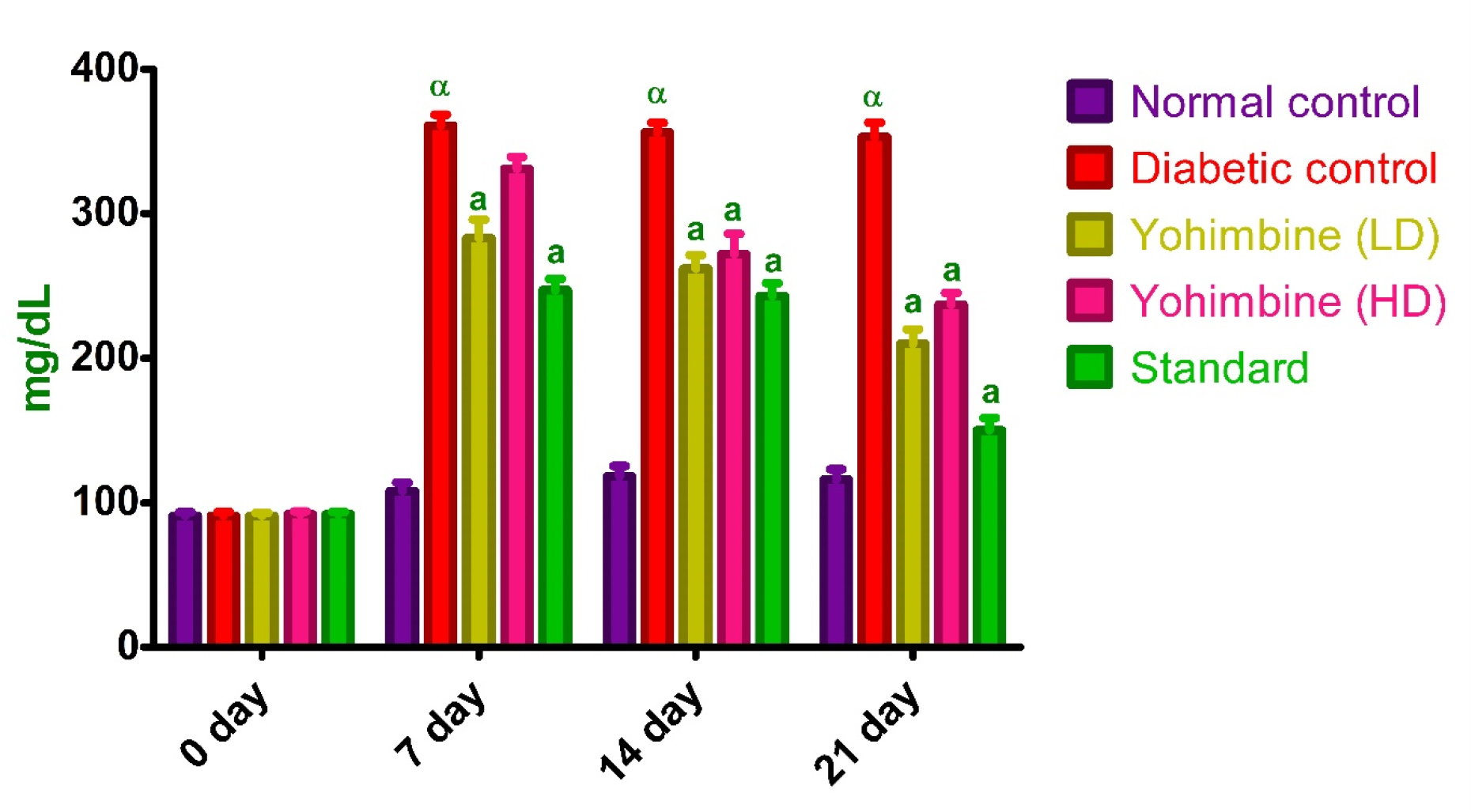
Figure 1:
Effect of Yohimbine on FBG levels.
| Day | Normal group | Diabetic group | Yohimbine (LD) group | Yohimbine (HD) group | Standard group |
|---|---|---|---|---|---|
| 0 | 91.0 ± 2.5 | 91± 2.7 | 91± 2.1 | 92±2.0 | 92± 1.6 |
| 7 | 108± 6.1 | 361 ± 7.5α | 283± 13a | 331± 8.2 | 247± 7.9a |
| 14 | 118± 7.4 | 356± 6.8α | 262± 9.3a | 272± 14a | 243± 9.0a |
| 21 | 116±7.2 | 353± 10α | 210± 10a | 237± 8.2a | 150 ± 8.7a |
Effect of Yohimbine on OGTT
Induction of diabetes resulted in increased OGTT levels as against control group (p < 0.001), administration of yohimbine LD, yohimbine HD, and Standard drug was responsible for the decline in OGTT levels as against diabetic group (p < 0.001) (Figure 2 and Table 2).
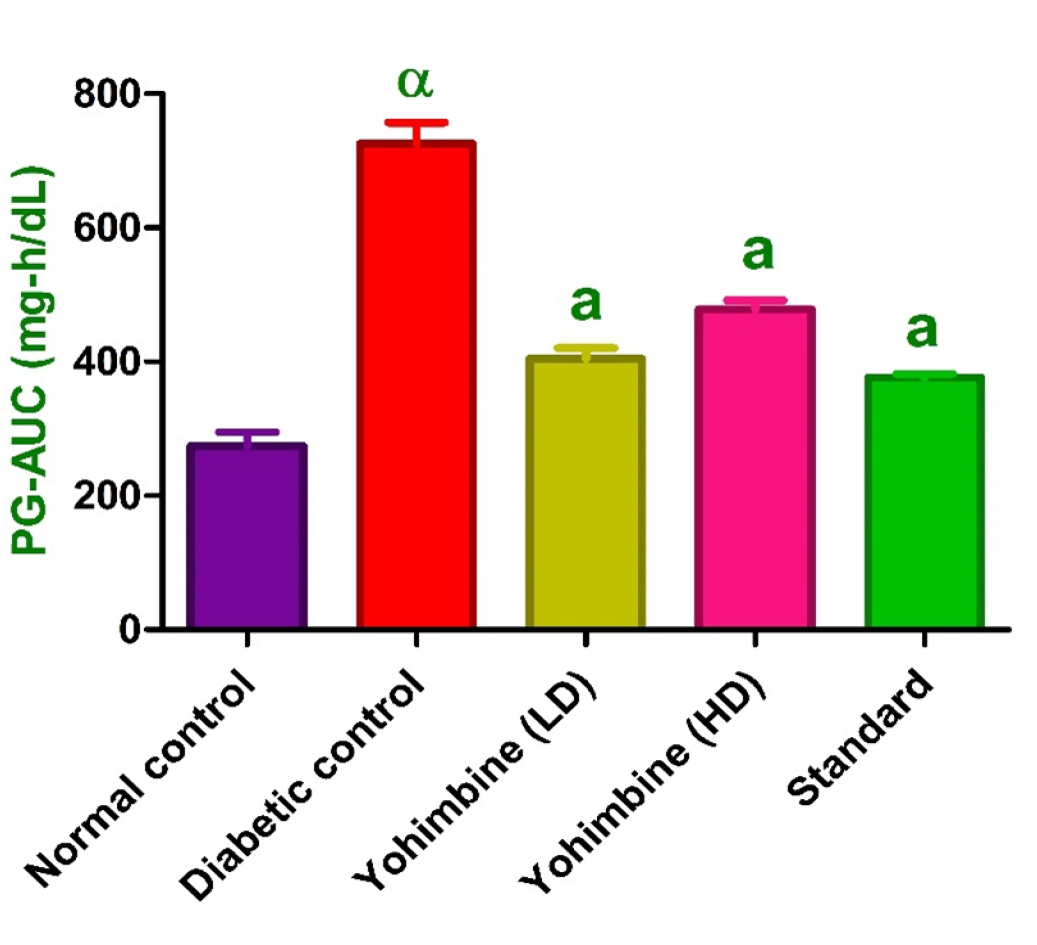
Figure 2:
Effect of yohimbine on OGTT.
| Groups | 0 min | 30 min | 60 min | 120 min | AUC |
|---|---|---|---|---|---|
| Normal control | 112± 10 | 174± 9.3 | 144± 13 | 104± 9.3 | 274± 20 |
| Diabetic control | 333± 7.2α | 387± 13α | 360± 21α | 351± 21α | 726± 31α |
| Yohimbine (LD) | 207± 6.6a | 224± 7.8a | 200± 9.1a | 181 ± 15a | 404± 16a |
| Yohimbine (HD) | 237± 6.7a | 260± 8.4a | 237± 6.1a | 223 ± 7.4a | 479± 13a |
| Standard | 155± 8.7a | 222± 13a | 178± 6.9a | 151 ± 9.6a | 376± 5.6a |
| Parameters | Normal control | Diabetic control | Yohimbine (LD) | Yohimbine (HD) | Standard |
|---|---|---|---|---|---|
| TG (mg/dL) | 100± 3.5 | 189± 4.8α | 151± 4.3a | 159± 6.6b | 172± 6.5 |
| HDLc (mg/dL) | 27± 1.7 | 13± 1.8β | 23± 2.3c | 18± 2.1 | 23± 1.2c |
| LDLc (mg/dL) | 31 ± 1.6 | 102 ± 1.6α | 86± 4.1c | 92± 3.4 | 87± 4.6c |
Impacts of yohimbine on lipid profile
Triglyceride levels
Triglyceride levels were elevated in diabetic control group as against control group (p < 0.001), yohimbine LD and yohimbine HD administration resulted in drop in triglyceride levels (p < 0.001 and p < 0.01), whereas standard drug did not show any significant decrease when compared to diabetic group (Figure 3).20
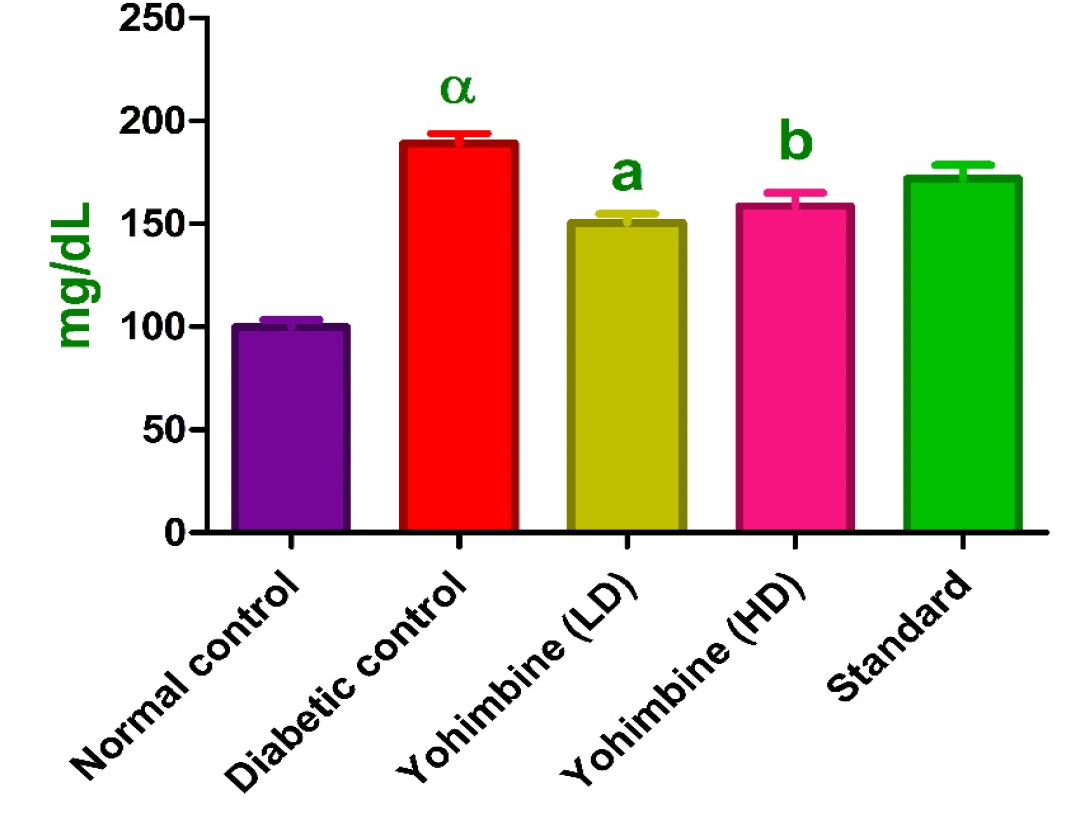
Figure 3:
Effect of yohimbine on TG levels.
HDL-c levels
HDL-c levels were declined in diabetic group (p < 0.01) relative to control group. Yohimbine LD and standard drug administration was responsible for an increase in levels (p < 0.05), whereas yohimbine HD did not show any significant elevation in HDL-c levels relative to diabetic group (Figure 4).
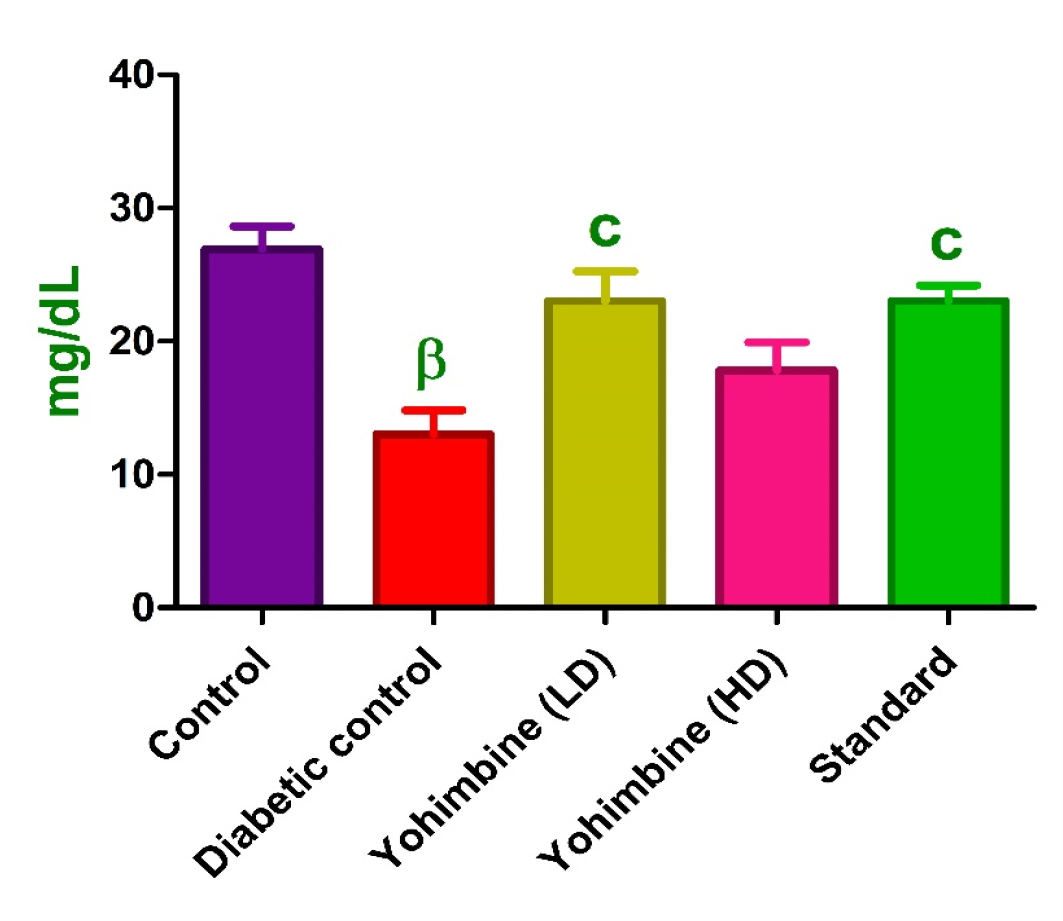
Figure 4:
Effect of yohimbine HDL-C levels.
LDL-c levels
LDL-c levels were lessened in diabetic group (p < 0.001) as against control group. Yohimbine LD and standard drug treated groups exhibited an increase in levels (p < 0.05), whereas yohimbine HD did not show any significant increase in LDL-c levels when compared with diabetic group.21 (Figure 4, Table 3).
Data were expressed as mean±SEM. αp < 0.001, when contrasted with the normal group; cp < 0.05 when contrasted to the diabetic group.
Histopathology
In a normally structured pancreas, both beta cells and non-beta cells are grouped in a dense pattern. The RBCs are in close proximity. In diabetic group, atrophic and vacuolated beta cells were observed in the pancreatic tissue, as well as widespread necrosis of the islets, distort typical endocrine organization, giving the islets a starfish look characterized by necrotic cells, pyknotic nuclei, and acidophilic cytoplasm. In yohimbine-treated groups, the pancreatic tissue has typical endocrine and exocrine pancreas in addition to extensive cellular and architectural repair, minor necrotic degeneration, and vacuolation (Figure 5). Glibenclamide-treated group displayed typical endocrine and exocrine pancreatic tissue, including extensive cellular and architectural.
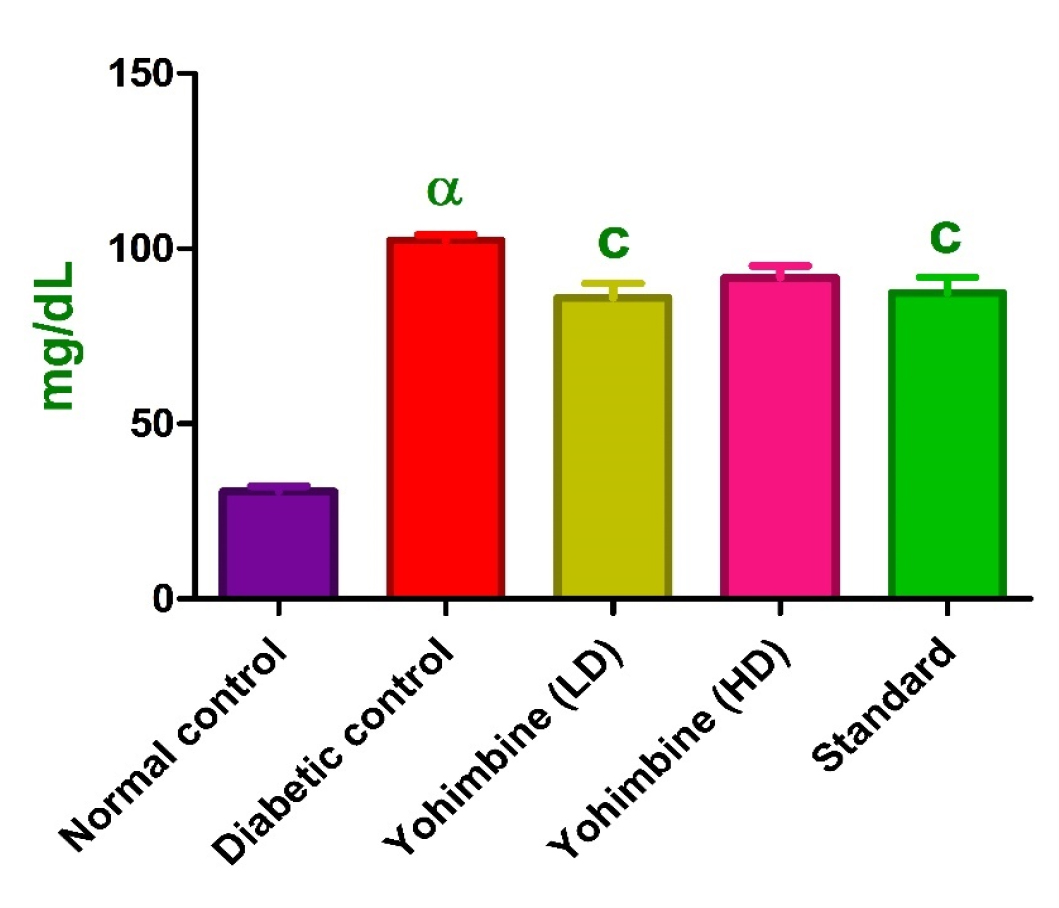
Figure 5:
Effect of yohimbine on LDL-C levels.
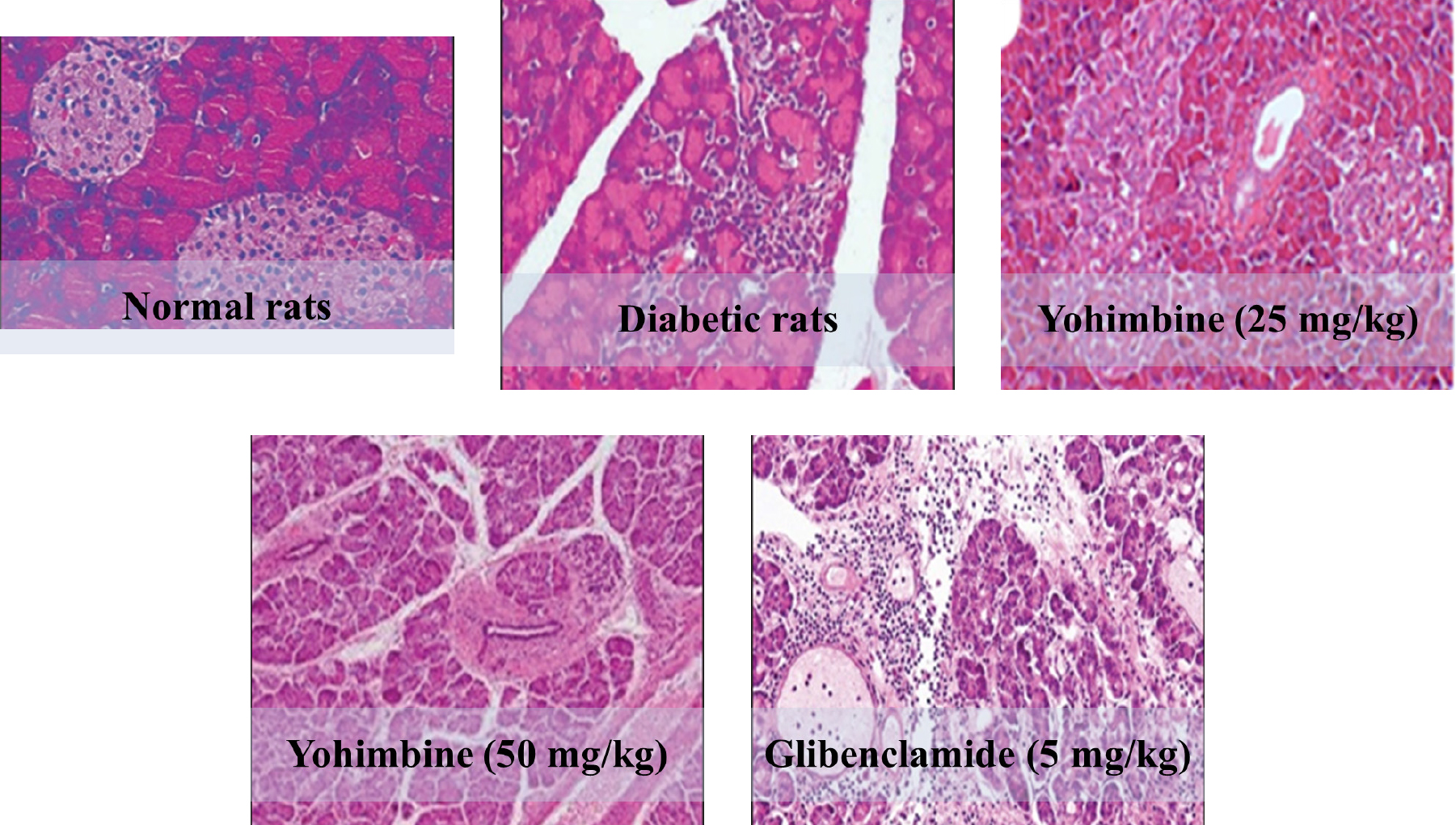
Figure 5:
Histopathology of pancreatic tissue of various untreated and treated groups.
DISCUSSION
In adult rats, a modest rise in FBG level, impaired tolerance to oral glucose, hypoinsulinaemia, and impaired glucose-stimulated insulin secretion were caused by STZ administration, as was previously shown (Angel et al., 1996). Rats given STZ, which destroys pancreatic beta cells, increases FBG levels and alters glucose tolerance, is widely acknowledged as diabetic model (Burcelin et al., 1993). A similar pattern of behavior was seen in the rats given streptozotocin in this investigation. Rapid elevations in fasting glucose and decreases in insulin levels were seen in streptozotocin-treated adult rats.
The results of the current research show that oral glucose tolerance may be enhanced by pretreatment with selective alpha-2 adrenoceptor antagonists, such as yohimbine, in diabetic rats. Yohimbine-treated mice had lower BGLs and enhanced lipid profiles, with declined triglyceride and LDL-c levels and augmented HDL-c levels. Previous research demonstrated that yohimbine moderated lipid and glucose profiles favorably in diseased rats triggered by a high-fat diet (Dudek et al., 2015). Since yohimbine also interacts with α1 -adrenergic receptors (Flechtner-Mors et al., 2004), it is probable that the impact on lipid profile is attributable to this kind of adrenergic receptor blockage.
The correct amount of STZ utilized in this investigation resulted in practically total necrosis of cells in diabetic, untreated rats. Diabetic rat pancreatic tissue may have fewer islets because larger islets shrink to smaller size. Islet volume density, % of cells, and islet size all increased after yohimbine administration, which may be indicative of regeneration and extensive cellular and architectural repair. If the quantity or size of other kinds of pancreatic cells, especially α-cells, rises without a corresponding rise in the volume density of β-cells in the islets, this may be the cause of the observed phenomenon. In the glibenclamide-treated group, the endocrine and exocrine pancreas seemed to be normal.
CONCLUSION
Overall, our findings imply that yohimbine, an alpha-2 adrenoceptor antagonist, helped diabetic group have better glucose tolerance. Yohimbine-treated rats had declined BGLs and improved lipid profiles, with drop in triglyceride and LDL-c levels and augmented HDL-c levels. Histopathological analyses showed that the size and density of pancreatic cells had increased and that they had regenerated remarkably. As a result, we can conclude that yohimbine may have therapeutic value for restoring lipid and glucose profiles and in repairing diabetes-related pancreatic damage.
References
- Chattopadhyay RR, Bandyopadhyay M.. Effect of leaf extract on serum lipid profile changes in normal and streptozotocin induced diabetic rats. Afr J Biomed Res.. 2005;8(2):101-4. [CrossRef] | [Google Scholar]
- Forero R, Nahidi S, De Costa J, Mohsin M, Fitzgerald G, Gibson N, et al. Application of four-dimension criteria to assess rigour of qualitative research in emergency medicine. BMC Health Serv Res. 2018;18(1):120 [PubMed] | [CrossRef] | [Google Scholar]
- Traish A, Kim NN, Moreland RB, Goldstein I. Role of alpha-adrenergic receptors in erectile function. Int J Impot Res.. 2000;12(S1):S48-63. [CrossRef] | [Google Scholar]
- Ernst E, Pittler MH. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. J Urol. 1998;159(2):433-6. [PubMed] | [CrossRef] | [Google Scholar]
- Kalra B, Kalra S.. Effect of yohimbine on sexual disorders in women with diabetes. Diabetes. 2007:56 [PubMed] | [CrossRef] | [Google Scholar]
- Naghadeh MM, Moghadam FH, Ibrahimi H.. Effects of yohimbine on plasma levels of leptin in normal and streptozotocin induced diabetic rats. Acta Med Iran. 2006:77-82. [PubMed] | [CrossRef] | [Google Scholar]
- Gromada J, Chabosseau P, Rutter GA. The α-cell in diabetes mellitus. Nat Rev Endocrinol. 2018;14(12):694-704. [PubMed] | [CrossRef] | [Google Scholar]
- Abdel-Zaher AO, Ahmed IT, El-Koussi AD. The potential antidiabetic activity of some alpha-2 adrenoceptor antagonists. Pharmacol Res. 2001;44(5):397-409. [PubMed] | [CrossRef] | [Google Scholar]
- Donovan J, Brown P. Parenteral injections. Curr Protoc Immunol.. 2007;73(1):1-6. [CrossRef] | [Google Scholar]
- Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc.. 2021;1(4):e78 [PubMed] | [CrossRef] | [Google Scholar]
- Ebrahimi B, Estaji M, Rajabzadeh A.. The effect of different doses of glibenclamide on blood glucose and islet volume in diabetic rats. 2019;21(68):10-8. [PubMed] | [CrossRef] | [Google Scholar]
- Rahul PK, Manikata VK, Madoosudan AP, Bhanuprakash GR. Geranimum oil ameliorates endothelial function in high fat high sucrose diet induced metabolic complications in rats. J Funct Foods. 2005;15:284-93. [PubMed] | [CrossRef] | [Google Scholar]
- Deba Z, Jambale TA, Swamy PG, Murthy DJ. Study of levels of malondialdehyde, super oxide dismutase and hs-CRP in serum of nonobese patients with polycystic ovarian syndrome. Int J Clin Biochem.. 2017;4:191-4. [PubMed] | [CrossRef] | [Google Scholar]
- Yadav MK, Dwivedi J, Upadhyay PK, Vishwakarma VK. The ceiling effect of curcumin and quercetin in combination on cyclophosphamide induced hepatotoxicity. Ann Phytomed. 2021;10(1):108-13. [CrossRef] | [Google Scholar]
- Angel I, Burcelin R, Girard J, Salomon Z.. Normalization of insulin secretion by selective alpha-2 adrenoceptor antagonist receptorsGLUT-4 glucose transporter expression in adipose tissue of type II diabetic rats. Endocrinology. 1996;137:2022-7. [CrossRef] | [Google Scholar]
- Burcelin R, Printz RL, Kande J, Assan R, Granner DK, Girard J., et al. Regulation of glucose transporter and hexokinase-II expression in tissues of diabetic rats. Am J Physiol.. 1993;265(3 Pt 1):E392-401. [PubMed] | [CrossRef] | [Google Scholar]
- Maithili V, Dhanabal SP, Mahendran S, Vadivelan R.. Antidiabetic activity of ethanolic extract of tubers of in alloxan induced diabetic rats. Indian J Pharmacol. 2011;43(4):455-9. [PubMed] | [CrossRef] | [Google Scholar]
- Flechtner-Mors M, Jenkinson CP, Alt A, Biesalski HK, Adler G, Ditschuneit HH, et al. Sympathetic regulation of glucose uptake by the α1-Adrenoceptor in human obesity. Obes Res. 2004;12(4):612-20. [PubMed] | [CrossRef] | [Google Scholar]
- King GL, Loeken MR.. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122(4):333-8. [PubMed] | [CrossRef] | [Google Scholar]
- Fossati P, Prencipe L.. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28(10):2077-80. [PubMed] | [CrossRef] | [Google Scholar]
- Zlatkis A, Zak B, Boyle AJ. A direct method for the estimation of serum cholesterol. J Lab Clin Med.. 1953;41(3):486-92. [PubMed] | [Google Scholar]
