ABSTRACT
Background
Diabetes mellitus is the most prevalent general health worry disorder, causing a significant fatality and long-term medical problems. It is a comprehensive biochemical condition of high blood sugar, insulin resistance and insulin deficiency or insufficiency, as well as disruptions in carbohydrate, lipid, and protein metabolism. Due to their natural background and fewer complications than manufactured pharmaceuticals, medicinal herbs and their formulations are traditionally employed in a variety of antidiabetic therapy.
Objectives
Present study is designed to evaluate anti-diabetic potential of an aqueous ethanolic extract of Andrographis paniculata and Adathoda vasica against High Fat Diet (HFD)-low dose Streptozotocin (STZ) induced diabetic rats and role of trace elements in the treatment of T2DM.
Materials and Methods
Animals were induced with hyperglycemia with HFD- low dose of STZ and then examined using a variety of measures to compare diabetes development and management across all groups. The animals were given aqueous ethanolic extracts of Andrographis paniculata and Adathoda vasica in treatment groups and tested for oral glucose tolerance, serum insulin, glycosylated haemoglobin, and trace element levels.
Results
The extracts under study showed presence of trace elements (Mg, Zn, Mn, Cr and Cu). Dose dependent, substantial change in blood glucose levels were seen with diabetic rats treated with extracts of Adathoda vasica and Andrographis paniculata, significant increase in serum insulin exerted by animals treated with extract of Andrographis paniculata (300 and 400 mg/kg, b.w.). Diabetic animals treated with extracts showed significant changes in HbA1c levels also had substantial changes in serum trace elemental content.
INTRODUCTION
Diabetes mellitus a metabolic disorder, characterized by an inappropriate increase in blood glucose level due to change in metabolism of important components of living system like protein, carbohydrate and lipid. Which is also closely associated with lack of insulin secretion from β-islets of Langerhans cells or diminished sensitivity of cells for insulin (Insulin resistance) sometimes both.1 In the pathogenesis of DM following factors are involved life styles such as sedentary, obesity, stress of an individual. It is estimated that by 2030, around 7.7% of global population might affected by this metabolic disorder.2 Untreated diabetes always leads to macrovascular and microvascular complications, which expedite cost of treatment huge that cannot be afforded by patients as it involves multiple drugs and longer duration of treatment.3,4
Amongst diabetic patients majority (>90%) are recognized with Type-2 diabetes mellitus (T2DM). Currently insulin sensitizers or insulin secretagogues used alone or in combination to treat DM, still they fail to completely cure the disorder. The treatment approach for T2DM is to target multiple metabolic pathways, but it is connected with undesirable effects.5,6 Medicinal plants are an alternative medicines and are never outdated in healthcare sector. Over 1200 plants are using in the treatment of DM. It is concepted that medicinal plants act on manifold targets in human body because of presence of abundant and complex compounds in medicinal plants.7
Phytochemicals and Trace elements do have beneficial effect on human health including in the treatment of metabolic disorders.8 The focus on the entire plant instead of a single chemical component distinguishes phytotherapy from conventional medicine’s use of and artificial synthesis of plant-based active ingredients. Due to their natural background and fewer negative impacts than synthetic pharmaceuticals, medicinal herbs and their combinations are widely utilized in a variety of therapies. These pharmaceuticals are made using raw resources that help the people who grow those raw materials succeed economically. Medicinal plants are essential in both traditional and modern medical practices.9,10 For the development, physiology and proper growth of an organism, a dietary mineral trace element is needed.11 Because trace elements act as co-factors for hundreds of enzymes that catalyze vital reactions needed for proper metabolism and an anti-oxidant system, some of them have insulin-like actions.12,13 It is also evident that altered/insufficient concentration of trace elements leads to diabetes.14,15 Medicinal plants are known to have many essential and nutritional elements. Presence of various mineral elements in plants could also responsible for their hypoglycaemic activity.16–18 Both Andrographis paniculata and Adathoda vasica known for their anti-diabetic potential but on an experimemntal model of T2DM called HFD-low dose of STZ which has proved to mimic human pathophysiology of DM is not tested. By feeding experimental animals with High fat diet lipid overload and low grade inflammation occurs which results in insulin resistance, later by administering low dose of STZ (35mg/kg, b.w.) damages insulin receptor signaling pathway causes frank hyperglycaemia.19–20
Therefore, this study is focused to bridge the gap between the effect of phytochemicals present in Andrographis paniculata and Adathoda vasica, and role of trace elements against HFD-low dose of STZ induced diabetic rats.
MATERIALS AND METHODS
Evaluation of anti-diabetic activity
Plants
The aqueous ethanolic extract of aerial parts of Adathoda vasica (AEEAV) and Andrographis paniculata (AEEAP) was obtained as gift samples from Green Chem®, Herbal Extracts, and Formulations, Bengaluru which was used as study extracts for the evaluation of the antidiabetic activity.
Elemental analysis21-22
The following trace elements were estimated in extracts under study: Cr, Zn, Mn, Cu, and Mg according to the Khan et al. method by using Flame Atomic absorption spectrometry (FAAS). Standard calibaration curve obtained from standard working solutions which was further used to determine concentration of trace elements in sample. Samples were prepared as clear solution after digesting 0.25g of extracts under study with 6.5ml of acid, later diluted and filterd with Whatman filter paper no.1.
Animals
For the study Male albino Wistar rats (180–200 g) were used. All the experimental animals were maintained as per standard laboratory conditions (temperature 25±2°C with 12/12 hr dark/light cycle) in clean polypropylene cages. Water ad libitum and standard pellet diet were provided for the experimental animals. All the animals were acclimatized to experimental condition one week prior to experiment. All procedures described were reviewed and approved by the Institutional Animal Ethics Committee (IAEC).Test animals were procured from a commercial source (Sri Raghavendra Enterprises, #541/PO/Bt/04/CPCSEA) situated closer to the institution.
Acute toxicity study24
Acute oral toxicity studies of AEEAV, AEEAP according to OECD (Organization for Economic Co-operation and Development) guidelines 425 to determine the acute oral toxicity dose of the study extracts were carried out in adult female albino Wistar rats (150-180g).
Experimental design
HFD-STZ induced diabetic model:25–26 The study consists of 09 different groups, each containing 12 male albino Wistar rats weighing between 180 and 200 g. All the animals except the normal control group were fed a high-fat diet (HFD) for 4 weeks. After 4 weeks of dietary manipulation, intraperitoneal injection of STZ with a low dose (35 mg/kg) were administered except for normal control animals but they received only citrate buffer. Animals with fasting blood glucose levels greater than 200 mg/dl were selected for further study after 72hr of STZ injection.
Selected diabetic animals were subjected to a treatment protocol as follows:
Group 1: Normal control (saline treatment). Group 2: Positive control (HFD-STZ).
Group 3: Standard group; Diabetic rats treated with Pioglitazone (10mg/kg, b.w. p.o.).
Group 4: Test group; Diabetic rats treated with AEEAV (200mg/kg,b.w, p.o.).
Group 5: Test group; Diabetic rats treated with AEEAV (300mg/kg,b.w,, p.o.).
Group 6: Test group; Diabetic rats treated with AEEAV (400mg/kg,b.w,, p.o.).
Group 7: Test group; Diabetic rats treated with AEEAP (200mg/kg,b.w,, p.o.).
Group 8: Test group; Diabetic rats treated with AEEAP (300mg/kg,b.w,, p.o.).
Group 9: Test group; Diabetic rats treated with AEEAP (400mg/kg,b.w,, p.o.).
At the end of the study, animals were fasted and partially anesthetized to collect blood from retro-orbital puncture Serum and plasma samples were stored at -70°C for biochemical assays.
Oral glucose tolerance test (OGTT): On the last day of the experiment, after 60 min of drug administration, the animals from all the groups were orally treated with 2 g/kg b.w. of glucose. The blood samples were collected from retro-orbital punctures at 0, 30, 60, 90, and 120 min. The blood glucose level was determined by using a standard glucose kit following the Glucose oxidase-peroxidase (GOD-POD) method.27
Serum insulin: Enzyme-Linked Immunosorbent Assay (ELISA) method was adopted to measure serum insulin.28
Glycosylated Haemoglobin (HbA1c): A colorimetric method explained by Nayak and Pattabiraman was adopted to estimate Glycosylated Haemoglobin.29
Trace elemental analysis in the serum of animals under study30
Trace elements in serum of animals were measured by flame atomic absorption spectrometry after diluting with Triton-X 100 solution.
Statistical Analysis: One way ANOVA followed by Newman-Keuls Multiple Comparison Test for significance was selected to find out statistical difference in mean. Results with p<0.05 were considered as statistically significant. For statistical analysis GraphPad prism Version 6.0 was used.
RESULTS AND DISCUSSION
Estimation of trace elements present in extracts under the study
In Adathoda vasica Magnesium is present in highest level whereas in Andrographis paniculata Zinc and Manganese were found relatively higher. Both Chromium and Copper found to be present as lesser level in extracts under study (Table 1).
Effect of Pioglitazone, AEEAV, and AEEAP on blood glucose level of diabetic animals
Significant rise in glucose levels between 90-120 min (p<0.001) exhibited by diabetic rats in comparison with saline treated animals. Significant change in blood glucose level seen with Pioglitazone treated animals when compared with untreated diabetic animals. Animals who were administered with AEEAV showed a remarkable change in glucose levels, and AEEAP treated animals exerted dose-dependent action in lowering the glucose levels, and the results were significant when compared with positive control diabetic animals (Table 2).
Effect of Pioglitazone, AEEAV and AEEAP on serum insulin and HbA1c level of diabetic animals
There was a significant difference (p<0.001) in serum insulin levels of positive control animals when they are compared with non-diabetic negative control. But serum insulin level of diabetic animals treated with AEEAV was insignificant (p>0.05) when it was compared to diabetic positive control animals. Although diabetic animals treated with AEEAP showed dose-dependent action with serum insulin level but animals with treatment AEEAP dose 1 were significant at p<0.05 when compared to animals of positive control. Whereas Glycated Hb levels were significantly reduced in Pioglitazone, AEEAV, AEEAP treated animals in comparison with untreated diabetic animals (Figures 1 and 2).
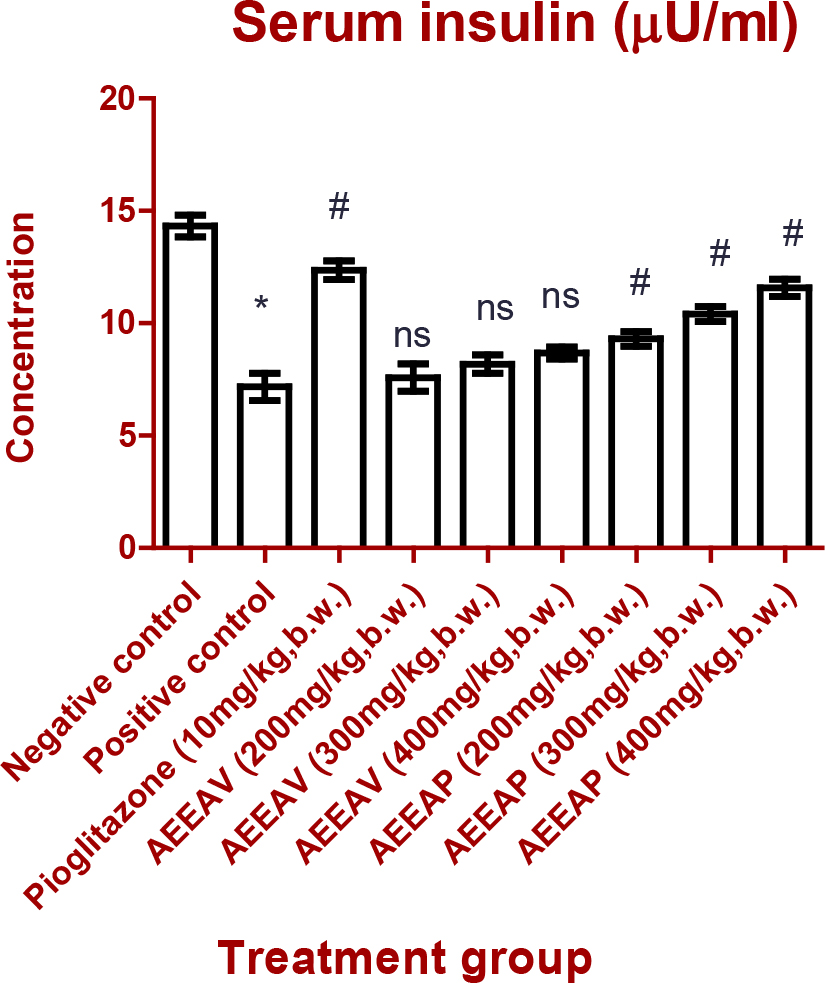
Figure 1:
Effect of Pioglitazone and different doses of AEEAV and AEEAP on Serum Insulin. (*): (p<0.05) comparison with Negative control, (#): (p<0.05) comparison with Positive control, (ns): (p>0.05) comparison with Positive control. The data are represented as mean±SEM, n=6.
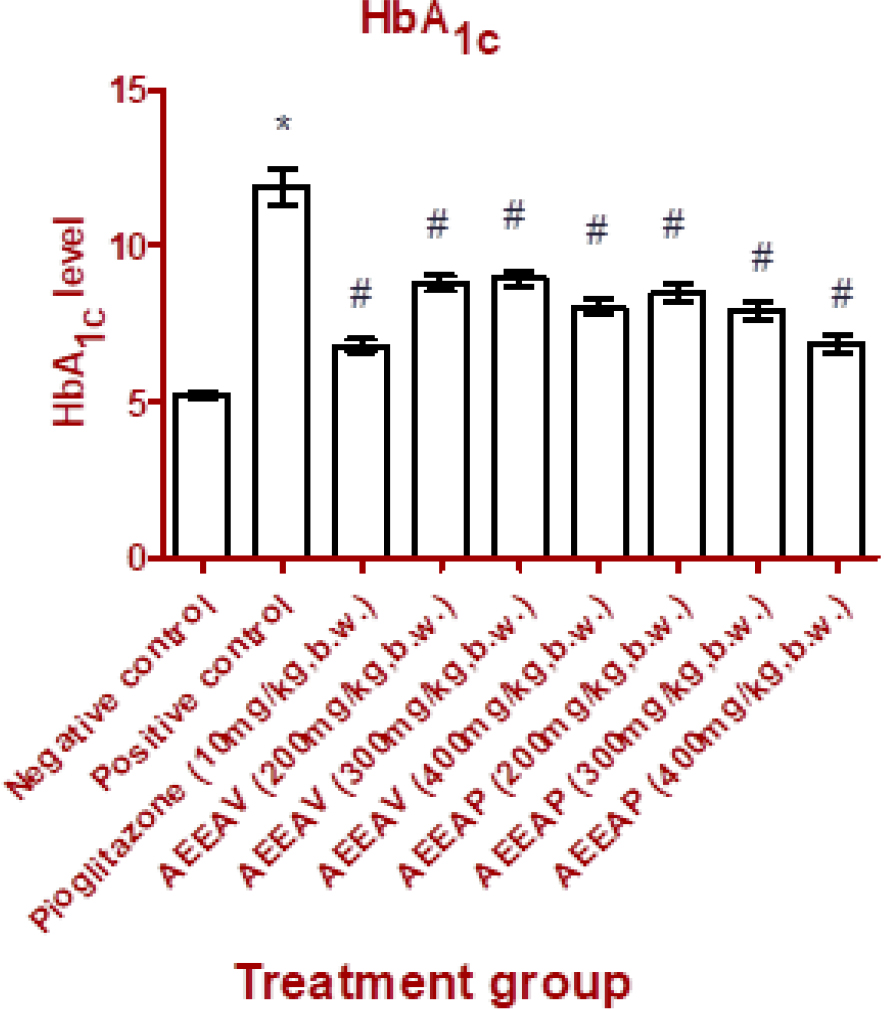
Figure 2:
Effect of Pioglitazone and different doses of AEEAV and AEEAP on HbA1c. (*): (p<0.05) comparison with Negative control, (#): (p<0.05) comparison with Positive control. The data are represented as mean±SEM, n=6.
Effect of Pioglitazone, different doses of AEEAV and AEEAP on trace elemental levels in serum of diabetic animals
Diabetic animals of standard group, animals treated with AEEAV and AEEAP have shown significant change in the trace elemental content when compared with positive control animals (p<0.001). But animals treated with AEEAV (200 and 300mg/kg,b.w.), AEEAP (200mg/kg, b.w.) shown insignificant change in trace elemental content when it is compared with positive control diabetic animals (Table 3).
| Sl.No. | Name of the Trace element | Adathoda vasica | Andrographis paniculata |
|---|---|---|---|
| 1. | Magnesium (Mg) | 2240 ppm (0.22%) | 950 ppm (0.095%) |
| 2. | Zinc (Zn) | 149 ppm (0.014%) | 176 ppm (0.017%) |
| 3. | Manganese (Mn) | 75 ppm (0.0075%) | 80 ppm (0.008%) |
| 4. | Chromium (Cr) | 3.4 ppm (0.0003%) | 5.1 ppm (0.0005%) |
| 5. | Copper (Cu) | 1.8 ppm (0.0001%) | 2.3 ppm (0.0002%) |
The trace elements with their quantities present in AEEAV and AEEAP.
| Treatment | Dose (/kg,b.w.) | Blood glucose level (mg/dl) | ||||
|---|---|---|---|---|---|---|
| 0th min | 30th min | 60th min | 90th min | 120th min | ||
| Normal saline | – | 68.67±2.21 | 133.5±1.83 | 113.7±3.20 | 82.83±2.48 | 71.67±2.53 |
| HFD-low dose of STZ | 35mg | 273.0±3.21* | 358.3±8.84* | 346.3±2.31* | 322.8±2.25* | 315.2±1.64* |
| Pioglitazone | 10mg | 225.5±3.10# | 284.5±4.10# | 272.7±2.57# | 253.3±2.66# | 235.3±1.99# |
| AEEAV | 200mg | 264.0±3.35# | 311.7±2.60# | 285.2±1.81# | 273.5±1.92# | 253.7±2.51# |
| AEEAV | 300mg | 244.0±2.67# | 322.8±1.62# | 296.2±2.57# | 279.0±2.63# | 269.5±3.41# |
| AEEAV | 400mg | 243.5±3.75# | 339.3±3.52# | 290.8±1.88# | 271.3±3.11# | 253.2±2.89# |
| AEEAP | 200mg | 268.8±3.31# | 341.3±2.06# | 314.5±2.36# | 283.7±2.78# | 262.2±1.90# |
| AEEAP | 300mg | 244.8±2.56# | 301.5±3.08# | 282.7±1.70# | 263.0±2.69# | 246.8±2.38# |
| AEEAP | 400mg | 237.2±2.46# | 304.0±2.86# | 272.5±2.69# | 251.5±2.39# | 230.5±4.50# |
Estimation of blood glucose level (Oral glucose tolerance test).
| Treatment | Dose (/kg,b.w.) | Cr | Zn | Mn | Cu | Mg |
|---|---|---|---|---|---|---|
| Normal saline | – | 16.49±0.80 | 41.04±1 | 65.13±1.22 | 14.33±0.21 | 645.2±7.67 |
| HFD-low dose of STZ | 35mg | 10.55±0.26* | 22.58±0.79* | 40.84±0.68* | 9.240±0.24* | 518.2±3.14* |
| Pioglitazone | 10mg | 13.43±0.18# | 31.86±0.88# | 52.43±0.54# | 13.31±0.12# | 625.2±4.35# |
| AEEAV | 200mg | 12.34±0.18# | 36.63±0.84# | 34.92±0.92# | 12.32±0.17# | 571.9±4.2# |
| AEEAV | 300mg | 13.18±0.12# | 43.66±0.59# | 42.77±0.64ns | 12.82±0.22# | 583.6±6.31# |
| AEEAV | 400mg | 13.56±0.14# | 43.29±0.56# | 41.17±0.73ns | 13.61±0.20# | 601.3±6.89# |
| AEEAP | 200mg | 12.80±0.33# | 42.35±1.04# | 43.96±1.08ns | 12.80±0.20# | 557.7±5.27# |
| AEEAP | 300mg | 13.09±0.17# | 43.05±0.66# | 44.63±1.03# | 13.17±0.19# | 542.1±4.14# |
| AEEAP | 400mg | 13.87±0.07# | 53.84±0.57# | 47.49±0.58# | 11.73±1.68# | 575.2±4.43# |
Effect of Pioglitazone, AEEAV and AEEAP on trace elemental levels in serum of diabetic animals.
DISCUSSION
In order to address more metabolic pathways, the paradigm of anti-diabetic medicine has changed from solitary to combination treatment. But there are negative side effects to this strategy. Medicinal plants continue to be used extensively in the treatment of human illness. Over 1200 botanicals have been listed among them as potential treatments for diabetes. Herbal medicine has always been believed to affect a number of different targets in the human body because of the richness and complexity of the substancesfound in plants.31–32
This investigation revealed that the aqueous ethanolic extract of Andrographis paniculata demonstrated a strong hypoglycemic effect against HFD-low dose STZ induced diabetic rats. According to our findings, the blood glucose levels of the diabetic animals treated with AEEAV significantly changed whereas that of diabetic animals treated with AEEAP were significantly reduced when compared to the positive control diabetic animals. However, when compared to diabetic positive control rats, the serum insulin level of diabetic animals treated with AEEAV was inconsequential. Although diabetic animals treated with AEEAP exhibited a dose-dependent effect on serum insulin levels, animals treated with AEEAP dose 1 were significantly different from animals in a positive control. It is shown from the research that a plant Adathoda vasica consisting of alkaloids in rich concentration, vacisine (a quinazoline alkaloid) also it possesses vasicinol, deoxy vasicine, l-vasicinone, maiontone.33–35 In Andrographis paniculata, lactones are known to take part in its medicinal uses and are as follows andrographolide, neoandrographolide, 14-deoxyandrographolide. In addition diterpenoid also present in Andrographis paniculata.36–38
The estimation of important micronutrient deficit comes from reputable sources like scientific findings and medical evidence from diabetes research. However, it is challenging for doctors to give nutritional recommendations for diabetics because of the numerous conflicting research. The average life span of diabetes people has increased due to advancements in therapeutics and investigation, which has coincided with a growth in the elderly population as a whole. Diabetes affects the antioxidant enzymes that are related with trace element.39
Numerous cohort studies have demonstrated that diabetes mellitus can change the homeostasis of trace elements. The disruption of insulin metabolism may be significantly influenced by early abnormalities in particular components. The bulk of cohort studies only consider one aspect or a small number of elements.39 Additionally, numerous investigations found that both diabetic humans and animals had altered mineral metabolism (Fe, Zn, and Cu).Chromium, Zinc, Manganese, Copper, and Magnesium were estimated to be trace elements in particular plants.To determine whether the provided extract changed the animals’ trace element levels and subsequently their blood glucose levels, the serum trace elemental concentration of untreated and treated diabetic rats were evaluated. It is found that Cr, Cu, Zn, Mg significantly increased in treatment groups whereas, Mn is just altered.
Therefore, overall activity of the extracts under study can be attributable for the constituents present in them which can bring antioxidant, anti-inflammatory, insulin sensitization, lipid lowering, improved metabolism effects. In this regard molecular mechanism need to be investigated.
CONCLUSION
In Present study aqueous ethanolic extract of Andrographis paniculata and Adathoda vasica showed their effectiveness against T2DM rats which induced by HFD-low dose of STZ by reducing blood glucose level, Glycated Hb, by improving trace elemental content (Cr, Zn, Mg, Cu) in an experimental animals. Though Serum insulin was not altered significantly in AEEAV treated rats but animals treated with AEEAP showed improvement with serum insulin. However, more studies are needed to find out precise mechanism of extracts under study at molecular level.
References
- Sahu U, Tiwari SP, Roy A.. Comprehensive notes on anti diabetic potential of medicinal plants and polyherbal formulation. Pharm Biosci J.. 2015;3(3):57-64. [Google Scholar]

- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4-14. [Google Scholar]

- Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, et al. Antidiabetic effects of berry extract and the identification of an effective component. Diabetes. 2002;51(6):1851-8. [Google Scholar]

- Aziz N, Wal A, Wal P, Pal RS. Preparation and evaluation of the polyherbal powder: the nature’s pharmacy for the treatment of diabetes mellitus and its complications. Pharmacophore. 2019;10(1):60-70. [Google Scholar]

- Srinivasan K, Ramarao P.. Animal model in type 2 diabetes research: an overview. Ind J Med Res. 2007;125(3):451-72. [Google Scholar]

- Chang CL, Lin Y, Bartolome AP, Chen YC, Chiu SC, Yang WC, et al. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid Based Complement Alternat Med.. 2013;378657:1-33. [Google Scholar]

- Zarshenas MM, Khademian S, Moein M. Diabetes and related remedies in medieval Persian medicine. Ind J Endocrinol Metab. 2014;18(2):142-9. [Google Scholar]

- Gunnels TA, Bloomer RJ. Increasing circulating testosterone: impact of herbal dietary supplements. J Plant Biochem Physiol. 2014;2(2):1-9. [Google Scholar]

- Wal P, Wal A, Pal RS, Singh P.. A comprehensive review on recently detected herbal phytoactives having anti-diabetic potential for various diabetes-related complications. Curr Trad Med. 2021;7(5):33-40. [Google Scholar]

- Arathy R, Murugan K, Dinesh Babu KD, Manoj GS. Assessment of antidiabetic potential of purified anthocyanin extract from floral petals of wild balsam species. J Drug Deliv Ther.. 2020;10(3):31-5. [Google Scholar]

- Wiernsperger N, Rapin J.. Trace elements in glucometabolic disorders: an update. Diabetol Metab Syndr. 2010;2(70):1-9. [Google Scholar]

- Rehman MU, Khan R, Khan A, Qamar W, Arafah A, Ahmad A, et al. Fate of arsenic in living systems: implications for sustainable and safe food chains. J Hazard Mater. 2021;417:126050 [Google Scholar]

- Dubey P, Thakur V, Chattopadhyay M.. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients. 2020;12(6):1864 [Google Scholar]

- Mahmoud AM. Hematological alterations in diabetic rats-role of adipocytokines and effect of citrus flavonoids. Excli J. 2013;12:647-57. [Google Scholar]

- Mahajan MS, Upaganlawar AB, Upasani CD. Nephroprotective Effect of coenzyme Q10 alone and in Combination with N-acetylcysteine in Diabetic Nephropathy. Eur Pharm J. 2021;68(1):30-9. [Google Scholar]

- Essawy SS, Abdel-Sater KA, Elbaz AA. Comparing the effects of inorganic nitrate and allopurinol in renovascular complications of metabolic syndrome in rats: role of nitric oxide and uric acid. Arch Med Sci. 2014;10(3):537-45. [Google Scholar]

- Koneri RB, Samaddar S, Simi SM, Rao ST. Neuroprotective effect of a triterpenoid saponin isolated from Fenzl in diabetic peripheral neuropathy. Ind J Pharmacol.. 2014;46(1):76-81. [Google Scholar]

- Bertolotto F, Massone A.. Combination of alpha lipoic acid and superoxide dismutase leads to physiological and symptomatic improvements in diabetic neuropathy. Drugs R D. 2012;12(1):29-34. [Google Scholar]

- Jubaidi FF, Zainalabidin S, Taib IS, Hamid ZA, Budin SB. The potential role of flavonoids in ameliorating diabetic cardiomyopathy via alleviation of cardiac oxidative stress, inflammation and apoptosis. Int J Mol Sci.. 2021;22(10):5094 [Google Scholar]

- Niture NT, Ansari AA, Naik SR. Anti-hyperglycemic activity of rutin in streptozotocin-induced diabetic rats: an effect mediated through cytokines, antioxidants and lipid biomarkers. Ind J Exp Biol. 2014;52(7):720-7. [Google Scholar]

- Karpiuk UV, Al Azzam KM, Abudayeh ZHM, Kislichenko V, Naddaf A, Cholak I, et al. Qualitative and quantitative content determination of macro-minor elements in L. roots using flame atomic absorption spectroscopy technique. Adv Pharm Bull.. 2016;6(2):285-91. [Google Scholar]

- Khan KY. Element content analysis of plants of genus Ficus using atomic absorption spectrometer. Afr J Pharm Pharmacol.. 2011;5(3):317-21. [Google Scholar]

- Shanker K, Naradala J, Mohan GK, Kumar GS, Pravallika PL. A sub-acute oral toxicity analysis and comparative anti-diabetic activity of zinc oxide, cerium oxide, silver nanoparticles, and in streptozotocin-induced diabetic Wistar rats. RSC Adv.. 2017;7(59):37158-67. [Google Scholar]

- OECD, Guidelines for testing of chemicals, Acute oral toxicity, Environmental Health and Safety Monograph Series on Testing and Adjustment No. 425. 2001;1(26):1-26. [Google Scholar]

- Król E, Jeszka-Skowron M, Krejpcio Z, Flaczyk E, Wójciak RW. The effects of supplementary mulberry leaf () extracts on the trace element status (Fe, Zn and Cu) in relation to diabetes management and antioxidant indices in diabetic rats. Biol Trace Elem Res. 2016;174(1):158-65. [Google Scholar]

- Pal RS, Saraswat N, Wal P, Wal A, Pal Y, Yadav R., et al. Anti-diabetic action of polyherbal ethanolic extract in alloxan-induced diabetes in Wistar rats. Curr Biotechnol. 2021;10(2):111-21. [Google Scholar]

- Sornalakshmi V, Tresinasoris P, Paulpriya K, Packialincy M, Mohan VR. Oral glucose tolerance test (OGTT) in normal control and glucose induced hyperglycemic rats with DC. Int J Tox Pharmcol Res.. 2016;8(1):59-62. [Google Scholar]

- Gandhi GR, Sasikumar P.. Antidiabetic effect of Burm.F. in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed.. 2012;2(4):281-6. [Google Scholar]

- Nayak SS, Pattabiraman TN. A new colorimetric method for the estimation of glycosylated haemoglobin. Clin Chim Acta. 1981;109(3):267-74. [Google Scholar]

- Król E, Jeszka-Skowron M, Krejpcio Z, Flaczyk E, Wójciak RW. The effects of Supplementary Mulberry leaf () Extracts on the Trace element status (Fe, Zn and Cu) in Relation to Diabetes management and Antioxidant Indices in Diabetic rats. Biol Trace Elem Res.. 2016;174(1):158-65. [Google Scholar]

- Ahmad U, Ahmad RS. Anti diabetic property of aqueous extract of Bertoni leaves in streptozotocin-induced diabetes in albino rats. BMC Complement Altern Med.. 2018;18(179):2-11. [Google Scholar]

- Vadivelan R, Dhanabal SP. Antidiabetic activity and Potential mechanism of Linn.in rats induced by High fat diet and low dose STZ. Int J Pharm Sci Res.. 2014;5(10):4170-5. [Google Scholar]

- Singh SK, Patel JR, Dangi A, Bachle D, Kataria RK. A complete over review on a traditional medicinal plants. J Med Plants Stud.. 2017;5(1):175-80. [Google Scholar]

- Gangwar AK, Ghosh AK. Medicinal uses and pharmacological activity of . Int J Herb Med.. 2014;2(1):88-91. [Google Scholar]

- Kapgate SM. Array. Int J Green Pharm.. 2017;11(04):654-62. [Google Scholar]

- Okhuarobo A, Falodun JE, Erharuyi O, Imieje V, Falodun A, Langer P., et al. Harnessing the medicinal properties of for diseases and beyond: a view of its phytochemistry and pharmacology. Asian Pac J Trop Dis.. 2014;4(3):213-22. [Google Scholar]

- Joselin J, Jeeva S.. Array. Med Aromat Plants. 2014;3(4):1-15. [Google Scholar]

- Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R. Andrographolide, a potential cancer therapeutic agent isolated from . J Exp Ther Oncol.. 2003;3(3):147-58. [Google Scholar]

- Zhang Q, Sun X, Xiao X, Zheng J, Li M, Yu M, et al. Dietary Chromium Restriction of Pregnant Mice Changes the Methylation Status of Hepatic Genes involved with Insulin Signaling in adult male off spring. PLoS One. 2017;12(1):1-16. [Google Scholar]

