ABSTRACT
Objectives
In this work, the photo components were investigated utilising GC-MS analysis and in vitro antioxidant activity, and the anthelmintic potential of methanolic extract of aerial parts of the Lagenaria siceraria (MEALS). By using the soxhlation method.
Materials and Methods
MEALS were made from aerial components, and phytochemical analysis was carried out for a qualitative evaluation. The in vitro antioxidant activity was measured using the ABTS technique, and the current extract was utilised to analyse phytocomponents by GC-MS and study anthelmintic activity on Pheretima posthuma. The extract of Lagenaria siceraria included 59 components, according to the GC-MS study’s phytochemical analysis.
Results
Results from anthelmintic research reveal that MEALS had paralysis times between 4.76 and 2.17 min and death times between 6.18 and 2.34 min when compared to the medicine Albendazole, which is the gold standard (paralysis time 4.76 min to 2.17 min and death time 5.87 min to 1.96 min on Peritima posthuma in increasing order of concentration). The percentage of scavenging ranged from 33.31% to 82.33% (IC50 143) and by standard medicine (ascorbic acid) from 49.47% to 94.73% (IC50 103) using the ABTS technique in the evaluation of an antioxidant activity.
Conclusion
Due to the presence of physiologically active phytocomponents, aerial sections of the Lagenaria siceraria (MEALS) were shown to be potent anthelmintic and antioxidant agents.
INTRODUCTION
Traditional medical systems have historically been crucial in meeting the world’s healthcare demands. They continue to do so today and will do so in the future. The term “Indian Systems of Medicine” refers to medical systems that have been assimilated into Indian culture and are believed to have Indian origins or to have come to India from other nations (ISM). Ayurveda, Yoga, and Naturopathy all have variations, as do Unani, Siddha, and homoeopathy. India has six recognised medical systems, which is a distinction in this area1. In order to develop the best new plant-based medications, it is crucial to use a rigorous and scientific method for biologically evaluating plant products based on their use in conventional medicine. Lagenaria siceraria (Molina) standley (LS) is one such plant (Family: Cucurbitaceae). This huge, annular, softly pubescent, climbing or trailing herb is native to India.2 Lagenaria siceraria, also referred to as bottle gourd, is a significant food plant that contributes between 5 and 6 per cent of all vegetables produced globally [Figure 1]. The fruits of this plant species are frequently used in cooking in India and its subcontinents. The fruit possesses benefits that are cardioprotective, antihyperlipidemic, and antihyperglycemic. The primary bioactive components of fruit are phenolic glycosides, phenolic acids, flavonoids, flavonoids, flavonoids, flavon-C- glycosides like isovitexin, isoorientin, saponarin, and sterols such fucosterol, campesterol, cucurbitacin B, cucurbitacin D, and cucurbitacin E. The Lagenaria siceraria, family Cucurbitaceae, a literature review found that only a small number of studies have used the areal sections for diverse activities including anti-inflammatory, analgesic, antibacterial,3 antihyperlipidemic, antidiabetic,4 antioxidants,5 anticancer,6 and anthelmintic7 action. Researchers are becoming more interested in this plant because of its medicinal effects on conditions like diabetes, obesity, and related metabolic disorders. According to the results of the ethnobotanical study, raw leaf juice has been used to cure helminthiasis by removing worms from the gastrointestinal tract. The current research effort has been created to carry out the photochemical study, GC-MS study, and anthelmintic activity of the methanolic extract of the Lagenaria siceraria aerial parts after gathering the aforementioned scientific data. Future applications of this work include HPTLC analysis employing potential markers, quantitative estimate of phytoconstituent presence, isolating the active ingredients from various extracts, and other pharmacological activities.
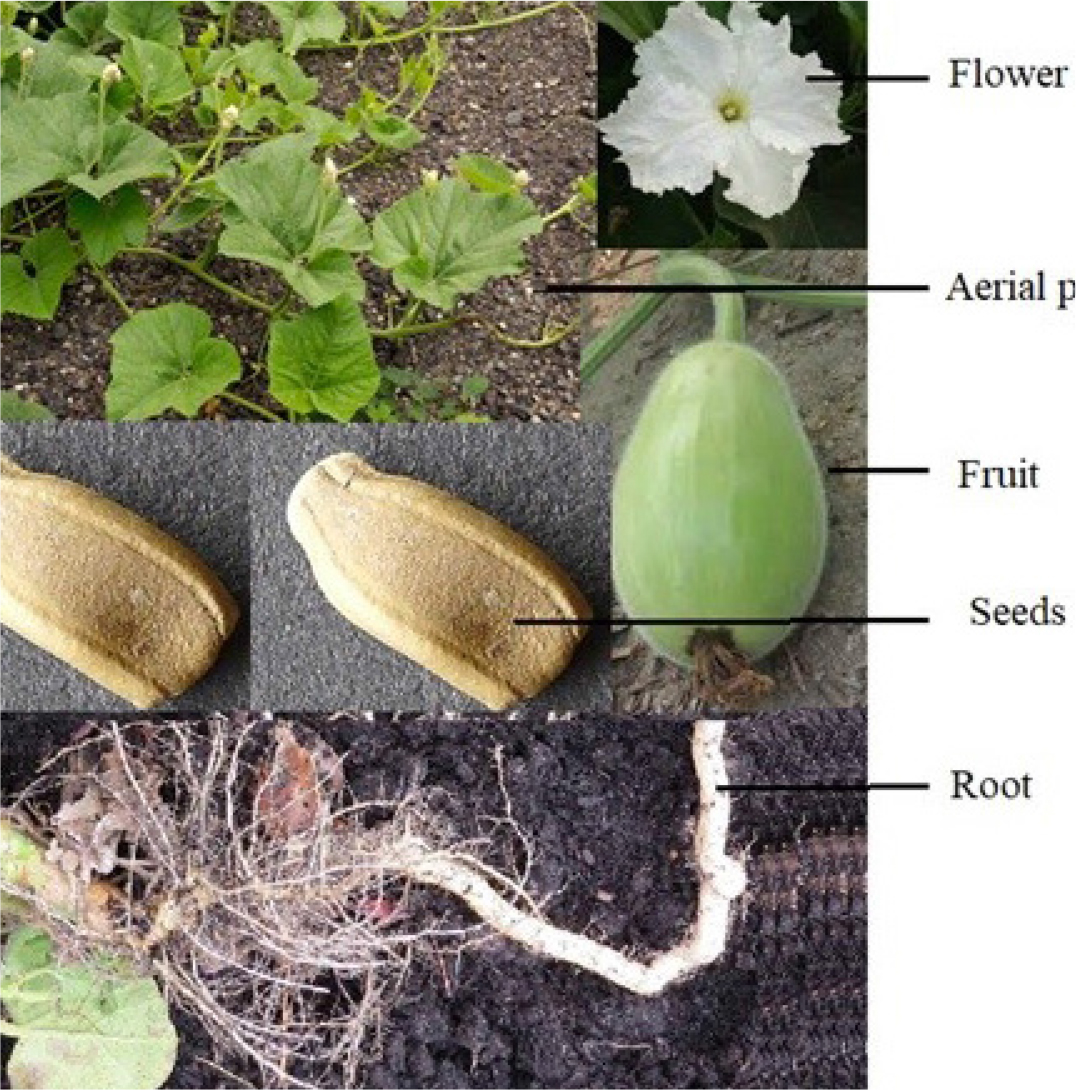
Figure 1:
Different parts of the Lagenaria sciceraria Plant.
MATERIALS AND METHODS
Plant materials
Dr. Suchandra Samanta Mandal, a botanist from Krishnanagar B.Ed College in Nadia, West Bengal, India, authenticated the disease-free area parts of Lagenaria siceraria (excluding fruits and flowers) in the month of March from a local area at Barasat, Kolkata, and assigned the reference number Cert/02/2022. The various components of a fresh plant are thoroughly cleaned before being dried in the shade until all water content has been removed. The plant’s dried aerial parts are collected and ground till a gritty texture is produced. The plant specimen in the herbarium according to protocol.
Preparation of methanolic extract
Lagenaria siceraria’s aerial components were removed using the hot soxhlation process. The aerial components underwent shade drying. For 72 hr, shade drying was done in an environment with natural airflow and a temperature of 25°C. The arial components were manually chopped into small pieces and processed into a coarse powder. For the extraction, 1200 ml of 100% methanol (Merck, Mumbai, India) was combined with 200 g of powdered Lagenaria siceraria Arial sections. The mixture was filtered, the filtrate was heated to between 45 and 50 degrees Celsius in a rotary evaporator, and the residue was kept in a refrigerator until it was needed.
Chemicals and instruments
The Soxhlet apparatus and distillation unit (Borosil) was used, for GC-MS analysis of the studied extract GCMS-QP 2010 plus a single quadrupole gas chromatograph-mass spectrometer (Shimadzu, Japan) was used. The UV-visible spectrophotometer UV-1900, manufactured by Shimadzu in Japan, was employed. The chemicals are from Mumbai’s Sigma Aldrich.
Phytochemical evaluation
Glycosides, alkaloids, volatile oils, saponins, and many more chemicals with physiological effects can be found in plants and can be thought of as a biosynthetic laboratory. These chemical compounds include those used as food by humans, such as carbohydrates, proteins, and lipids. Secondary metabolites are typically the substances in charge of therapeutic effects. A thorough investigation of crude medicine includes both main and secondary metabolites produced by plant metabolism. The methanolic extract of the Lagenaria siceraria aerial parts was subjected to preliminary phytochemical screening8 in accordance with the published technique for the detection of a number of phytoconstituents.
GC-MS analysis of phytoconstituents
The optimised conditions for the GC-MS instrument during the analysis of a methanolic extract of the aerial parts of the Lagenaria siceraria were performed on GCMS-QP 2010 plus single quadrupole gas chromatograph-mass spectrometer (Shimadzu, Japan). The following were the GC-MS analysis’s experimental conditions: TR 5-MS standard non-polar capillary column, 30 Mts, 0.25 mm ID, 0.25 m Film thickness. Helium (He), the mobile phase carrier gas, was pumped at a rate of 1.0 mL/min. The temperature programme (oven temperature) for gas chromatography was set at an injection temperature of 250°C and an increased temperature of 60°C. The ion source temperature was maintained 220oC. The column flow rate was maintained at 3mL/min. The injection volume was 1 µl. Samples dissolved in chloroform were run at a range of 50–650 m/z and the results were compared by using Wiley Spectral library search programme.
Anthelmintic activity
The assessment of the anthelmintic activity of methanolic extracts of the aerial portions of Lagenaria siceraria was conducted on adult Indian earthworms (Pheretima posthuma) for the examination of this activity.9,10 (Molina). The earthworms were gathered from the wet soil of Banamalipur, Barasat, in West Bengal’s north 24 pargana. Worms were initially kept in Tyrode solution, after being cleansed in saline water to remove the faeces, composed of sodium chloride 8g, potassium chloride 0.2g, calcium chloride 0.2g, sodium-di-hydrogen phosphate 0.1g, magnesium chloride 0.1g, glucose 1g and sodium bicarbonate 1g in 1000 ml distilled water. For the experiment, worms with measurements of 9 cm in length and 0.2–0.3 cm in width were used. According to the recognised protocol, the adult Indian earthworm Pheretima posthuma was utilised to test the anthelmintic activity. After being diluted with sterile saline solution, three quantities of the standard drug sample albendazole (99.60%, bought from Cadila Pharmaceutical Ltd., Ahmedabad, India) were applied to Petri plates. To produce concentrations of 25, 50, and 100 mg/mL, the methanolic extracts of the aerial portions of Lagenaria siceraria (Molina) were diluted with saline solution (0.90% w/v of NaCl). As the negative control, saline solution (0.9% NaCl) was utilised only. For testing, all dilutions were added to the Petri dishes. We took seven petri dish sets and numbered them. Each petri dish contained six earthworms (n=6) that were the same size (8 cm), and they were kept at ambient temperature. The paralysis and death (lethal) times were then seen, reported, and recorded in minutes for each petri dish. The tests were carried out three times. The earthworm’s paralysis (lack of movement) and death (no movement) times were observed to determine the anthelmintic property. Using a regular saline solution will allow you to verify that the worm has lost its ability to move. Dip the worm in warm water that is 50°C to determine when it will die. Additionally, the colour was assessed, and any fading of the worm’s colour was noted.
In vitro antioxidant activity
The 2, 2’ azino bis (3- ethylbenzthiazoline- 6- sulfonic acid) diammonium salt radical is scavenged using the ABTS radical scavenging method.11. The reaction between ABTS and potassium persulfate that produces the ABTS radical, a blue-green chromogen. In the presence of an antioxidant reductant, the coloured radical is converted back into colourless ABTS, and the absorbance was measured at 734 nm. A stock solution containing 1 mg of dried extract per millilitre was generated by mixing 100 mg of a dried extract with 100 ml of ethanol or distilled water to make the test sample. To get the necessary concentration, aliquots from this stock solution were then further diluted with ethanol or distilled water. Accurately 0.238 grammes of disodium hydrogen phosphate, 0.019 grammes of potassium di-hydrogen phosphate, and 0.8 grammes of sodium chloride were dissolved in 100 millilitres of distilled water to create the phosphate buffer, which has a pH of 7.4. ABTS 2mM (0.0274g in 25ml) was created in distilled water. 70 mM of potassium per sulphate (0.018g in 1 ml) was generated in distilled water. After two hours, 25ml of ABTS and 0.1ml of potassium per sulphate were mixed and used. It was necessary to use this test solution, also known as ABTS radical cation. 1 ml of extracts was combined with 3.4 ml of phosphate buffer pH 7.4 and 0.6 ml of ABTS radical cation at several concentrations (100-200 g/ml). Instead of the test, ethanol was used as the control. The absorbance was measured at 734 nm. The experiment was performed in triplicate.12
% Scavenging = (Control-Test/Control) × 100
RESULTS
Preliminary phytochemical test
Lagenaria siceraria’s aerial parts were subjected to preliminary phytochemical analysis, which revealed the presence of phytoconstituents in the classes of carbohydrates, polyphenols, flavonoids, and glycosides but the lack of alkaloids, proteins, and amino acids.
Molecular weight and molecular formula determination of the phytocomponents by GC-MS
The various compounds discovered in the methanolic extract of Lagenaria siceraria’s aerial parts were identified using mass spectrometry and GC (GC-MS). The GC-MS chromatogram (Figure 2) demonstrates the presence of a variety of components with various retention times. The mass spectrometer’s determination of the components’ various elution times indicates that the components’ structures and physiochemical properties differ. Peaks may appear at different m/z ratios as a result of a large chemical disintegrating into smaller components (as shown in Figures 3A and B). The compounds that matched the peaks obtained from the components were identified using the data library. The molecular weight and formula of 59 biomolecules were detected in the aerial portions of Lagenaria siceraria extracts. Table 1 summarises the list of phytoconstituents, and Figures 3A and 3B depict the chemical make-up.
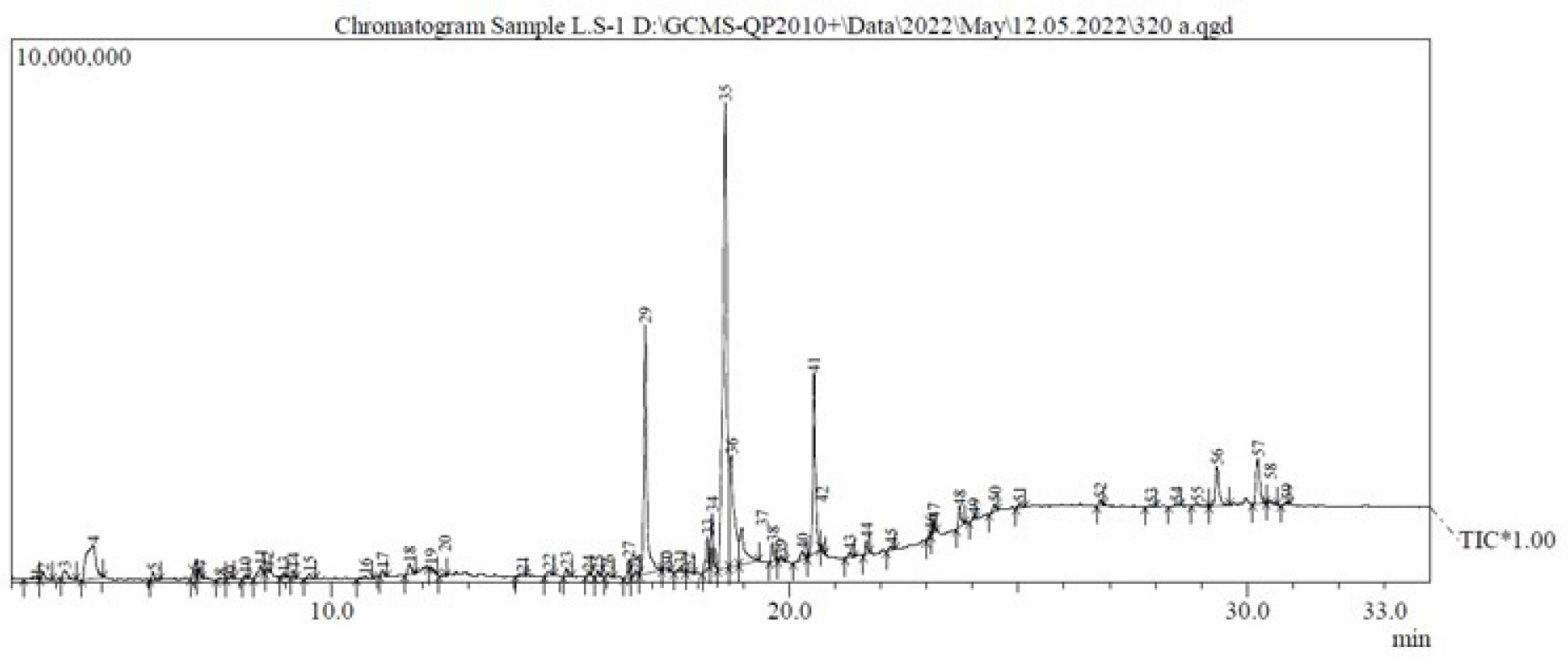
Figure 2:
GC-MS chromatogram of the methanolic extracts of the aerial parts of the Lagenaria sciceraria.
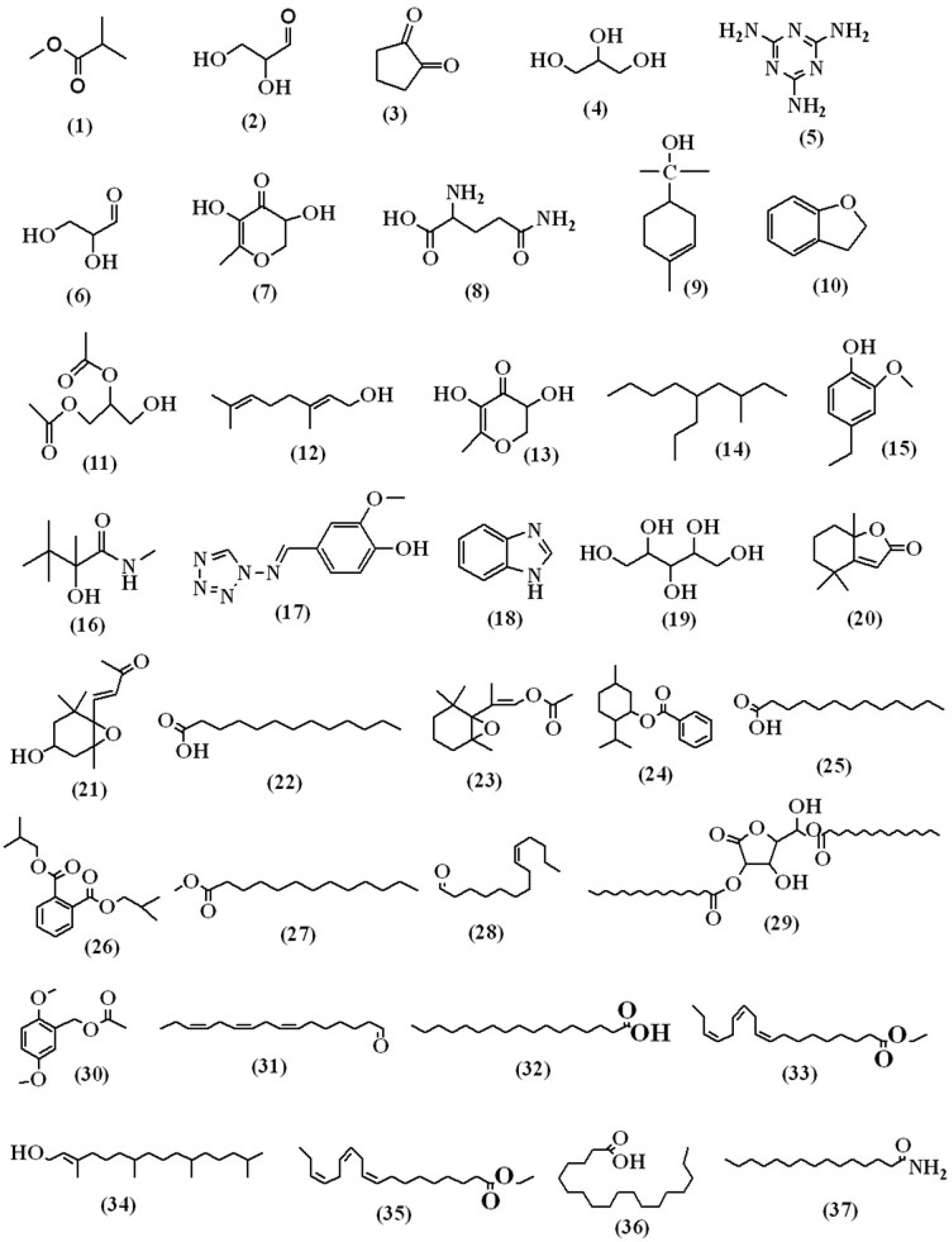
Figure 3A:
Structural representation of the compounds identified in the MEALS.
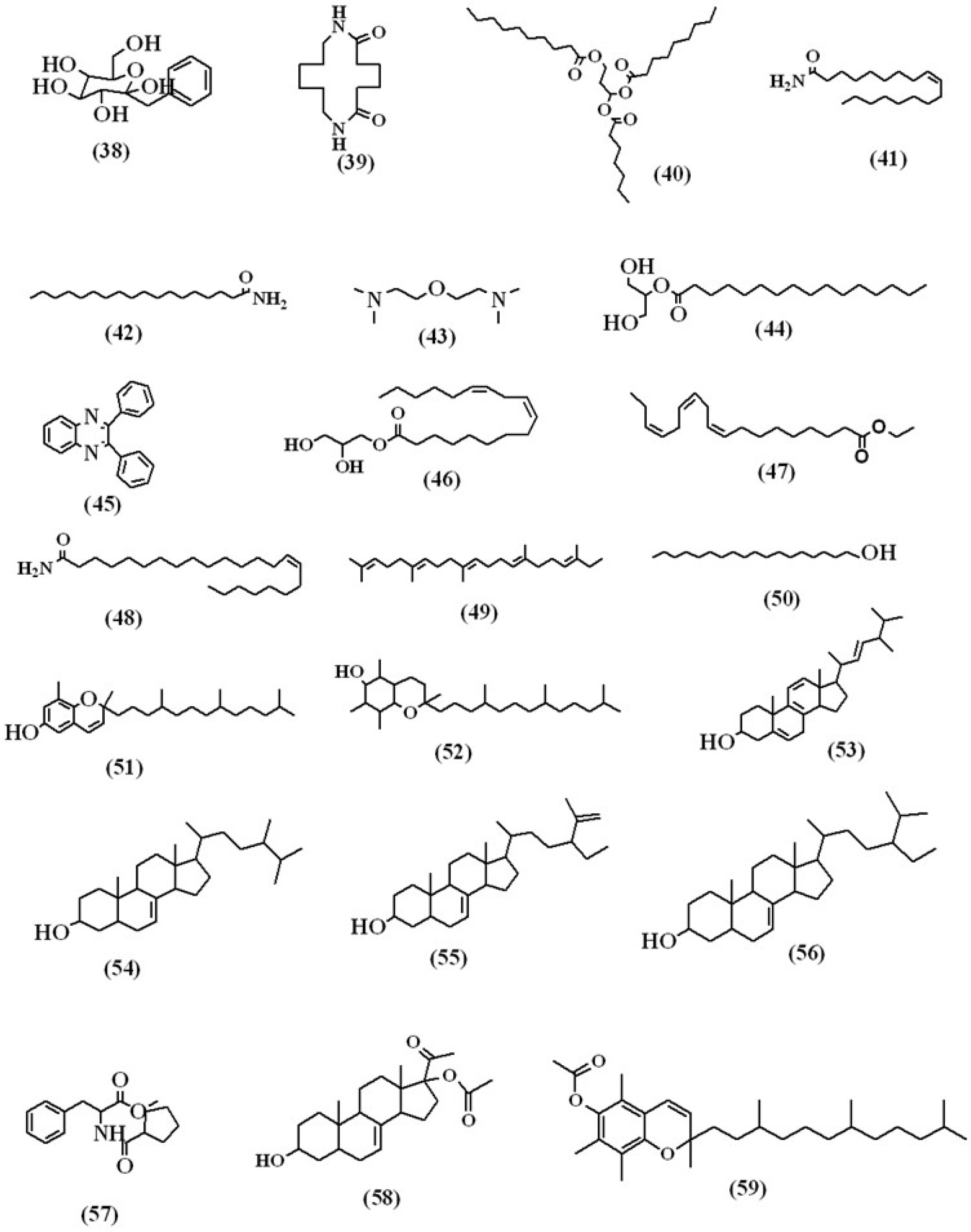
Figure 3B:
Structural representation of the compounds identified in the MEALS.
| Sl. No | Compound Name | Molecular Formula | Molecular weight | Retention time (minute) | Peak area (%) | Compound Nature |
|---|---|---|---|---|---|---|
| 1. | Propanoic acid, 2-methyl-, methyl ester | C5H10O2 | 102 | 3.540 | 0.25 | Ester |
| 2. | dl-Glyceraldehyde dimer | C6H12O6 | 180 | 3.699 | 0.37 | Aldehyde |
| 3. | 1,2-Cyclopentanedione | C5H6O2 | 98 | 4.162 | 0.62 | Ketone |
| 4. | Glycerin | C3H8O3 | 92 | 4.791 | 5.72 | Polyphenols |
| 5. | 1,3,5-Triazine-2,4,6-triamine | C3H6N6 | 126 | 6.100 | 0.36 | Amines |
| 6. | dl-Glyceraldehyde dimer | C6H12O6 | 180 | 6.985 | 0.27 | Aldehyde |
| 7. | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 7.118 | 0.34 | Polyphenols |
| 8. | Glutamine | C5H10N2O3 | 146 | 7.568 | 0.11 | Amines |
| 9. | 3-Cyclohexene-1-methanol,. alpha.,.alpha.4-trimethyl- | C10H18O | 154 | 7.805 | 0.16 | Non aromatic |
| 10. | Benzofuran, 2,3-dihydro- | C8H8O | 120 | 8.103 | 0.15 | Aromatic |
| 11. | 1,2,3-Propanetriol, diacetate | C7H12O5 | 176 | 8.445 | 0.63 | Polyphenols |
| 12. | 2,6-Octadien-1-ol, 3,7-dimethyl-, | C10H18O | 154 | 8.598 | 0.12 | polyphenols |
| 13. | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 8.945 | 0.17 | Aromatic polyphenls |
| 14. | Nonane, 3-methyl-5-propyl- | C13H28 | 184 | 9.164 | 0.27 | Alkane |
| 15. | 2-Methoxy-4-vinylphenol | C9H10O2 | 150 | 9.516 | 0.29 | Aliphatic alcohol |
| 16. | Butanamide, 2-hydroxy-N,2,3,3-tetramethyl- | C8H17NO2 | 159 | 10.722 | 0.24 | Amide |
| 17. | Phenol, 2-methoxy-4-(tetrazol-1- yliminomethyl)- | C9H9N5O2 | 219 | 11.105 | 0.28 | Heterocyclic aromatic |
| 18. | 1H-Benzimidazole | C7H6N2 | 118 | 11.698 | 0.64 | Heterocyclic aromatic |
| 19. | Ribitol | C5H12O5 | 152 | 12.155 | 0.22 | Polyphenols |
| 20. | 2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl-, (R)- | C11H16O2 | 180 | 12.455 | 0.14 | Ketone |
| 21. | 3-Buten-2-one, 4-(4-hydroxy- 2,2,6-trimethyl-7-oxa bicyclo[4.1.0]hept-1-yl)- | C13H20O3 | 224 | 14.154 | 0.21 | Polyphenols |
| 22. | Tetradecanoic acid | C14H28O2 | 228 | 14.740 | 0.31 | Carboxylic acid |
| 23. | Acetic acid, 2-(2,2,6-trimethyl-7-oxa- bicyclo[4.1.0]hept-1-yl)-propenyl ester | C14H22O3 | 238 | 15.130 | 0.38 | Ester |
| 24. | Cyclohexanol, 5-methyl-2- (1-methylethyl)-, benzoate, [1R(1. alpha.,2.beta.,5.alpha.)]- | C17H24O2 | 260 | 15.606 | 0.12 | Alcohol |
| 25. | Pentadecanoic acid | C15H30O2 | 242 | 15.805 | 0.24 | Carboxylic acid |
| 26. | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | C16H22O4 | 278 | 16.028 | 0.19 | Ester |
| 27. | Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 16.484 | 0.73 | Ester |
| 28. | 9-Tetradecenal, (Z)- | C14H26O | 210 | 16.659 | 0.21 | Aldehye |
| 29. | l-(+)-Ascorbic acid 2,6-dihexadecanoate | C38H68O8 | 652 | 16.848 | 13.93 | Ester |
| 30. | 4’-(.beta.-Chloroethyl)acetophenone $$ 1-[4-(2-Chloroethyl)phenyl]ethanone | C11H14O4 | 210 | 17.308 | 0.21 | Ketone |
| 31. | 7,10,13-Hexadecatrienal | C16H26O | 234 | 17.617 | 0.36 | Aldehyde |
| 32. | Heptadecanoic acid | C17H34O2 | 270 | 17.787 | 0.11 | Heptadecanoic acid |
| 33. | 9,12,15-Octadecatrienoic acid, ethyl ester, | C20H34O2 | 306 | 18.212 | 1.52 | Ester |
| 34. | Phytol | C20H40O | 296 | 18.312 | 1.88 | Alcohols |
| 35. | 9,12,15-Octadecatrienoic acid, ethyl ester, | C20H34O2 | 306 | 18.598 | 33.94 | Ester |
| 36. | Octadecanoic acid | C18H36O2 | 284 | 18.722 | 9.32 | Octadecanoic acid |
| 37. | Hexadecanamide | C16H33NO | 255 | 18.953 | 3.42 | Amide |
| 38. | Benzyl.beta.-d-glucoside | C13H18O6 | 270 | 19.629 | 0.93 | Glycoside |
| 39. | 1,8-Diazacyclotetradecane-2,7-dione | C12H22N2O2 | 226 | 19.813 | 0.22 | Ketone |
| 40. | 9-Octadecenoic acid, 1,2,3-propanetriyl ester, | C57H104O6 | 884 | 20.281 | 0.64 | Ester |
| 41. | 9-Octadecenamide, | C18H35NO | 281 | 20.537 | 9.26 | Amide |
| 42. | Octadecanamide | C18H37NO | 283 | 20.713 | 0.16 | Amide |
| 43. | Ethanamine, 2,2’-oxybis[N,N-dimethy | C8H20N2O | 160 | 21.316 | 0.23 | Amine |
| 44. | Hexadecanoic acid, 2-hydroxy-1- (hydroxymethyl)ethyl ester | C19H38O4 | 330 | 21.682 | 0.51 | Carboxylic acid |
| 45. | Quinoxaline, 2,3-diphenyl- | C20H14N2 | 282 | 22.216 | 0.27 | Heterocyclic |
| 46. | 9,12-Octadecadienoic acid (Z,Z)-, 2,3-dihydroxypropyl ester | C21H38O4 | 354 | 23.077 | 0.24 | Carboxylic acid |
| 47. | 9,12,15-Octadecatrienoic acid, ethyl ester, (Z,Z,Z)- | C20H34O2 | 306 | 23.149 | 0.50 | Carboxylic acid |
| 48. | 13-Docosenamide, (Z)- | C22H43NO | 337 | 23.720 | 0.94 | Amide |
| 49. | 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-E)- | C30H50 | 410 | 24.025 | 0.18 | Alkene |
| 50. | 1-Pentacosanol | C25H52O | 368 | 24.479 | 0.37 | Alcohol |
| 51. | 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,8-dimethyl-2- (4,8,12-trimethyltridecyl)-, [2R-[2R*] | C27H46O2 | 402 | 25.021 | 0.19 | Polyphenol |
| 52. | Vitamin E | C29H50O2 | 430 | 26.778 | 0.25 | Phenolic |
| 53. | Ergosterol $$ Ergosta-5,7,22-trien-3-ol, (3.beta.,22E)- Ergosterin | C28H44O | 396 | 27.894 | 0.14 | Steroid |
| 54. | L-Phenylalanine, N-cyclopentylcarbonyl-, methyl ester | C16H21NO3 | 275 | 28.449 | 0.16 | Ester |
| 55. | .gamma.-Ergostenol | C28H48O | 400 | 28.904 | 0.34 | Steroid |
| 56. | Stigmasta-7,25-dien-3-ol, (3.beta.,5. alpha.) | C29H48O | 412 | 29.340 | 2.67 | Steroid |
| 57. | Stigmast-7-en-3-ol, (3.beta.,5. alpha.,24S)- | C29H50O | 414 | 30.229 | 2.99 | Steroid |
| 58. | 17-.alpha.-Acetoxypregnenolone | C23H34O4 | 374 | 30.491 | 0.23 | Steroid |
| 59. | Vitamin E acetate | C31H52O3 | 472 | 30.859 | 0.16 | Ester |
Results of anthelmintic potential
On Pheritima posthuma (adult Indian earthworms), three different doses of the methanolic extract of Lagenaria siceraria’s aerial parts were evaluated for in vitro anthelmintic action. The relationship between the duration of paralysis and the duration of death and conventional medicine albendazole The paralysis and death periods of earthworms were 3.02 and 3.49 min, respectively, when aerial portions of Lagenaria siceraria were present in a solution at a concentration of 100 mg/mL. When the extract concentration is increased to 150 mg/mL, earthworm paralysis and death periods are revealed to be 2.17 and 2.34 min, respectively, as opposed to 1.25 and 1.96 min for normal albendazole. The anthelminthic potential of the investigated plant was evaluated using various doses of the methanolic extracts. Table 2 presents the outcomes. In Figure 4, it was depicted visually.
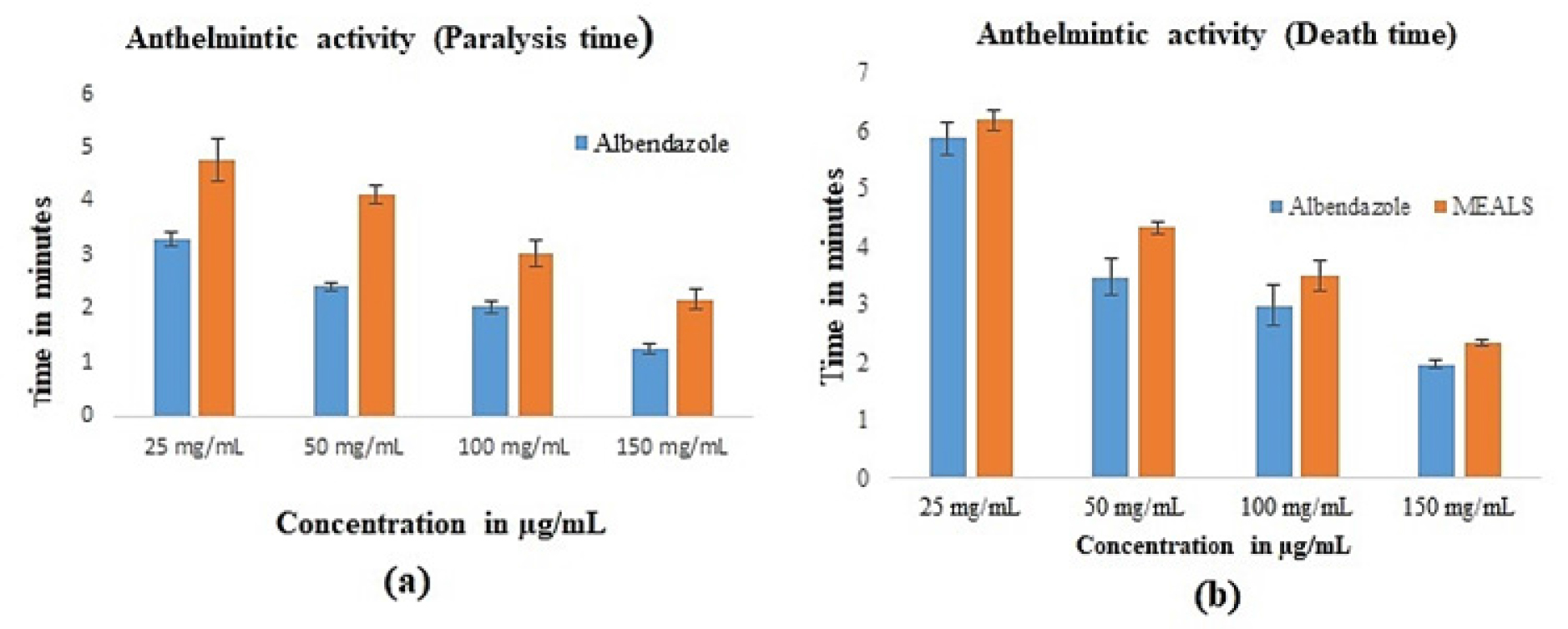
Figure 4:
Graphical representation of the anthelminthic potential of the MEALS.
| Treatment | Concentration (mg/ml) | Paralysis time (min) | Death Time (min) |
|---|---|---|---|
| Albendazole | 25 | 3.28±0.34 | 5.87±0.78 |
| (Standard) | 50 | 2.41± 0.65 | 3.47±0.47 |
| 100 | 2.03±1.32 | 2.98±1.30 | |
| 150 | 1.25±0.32 | 1.96±1.32 | |
| methanolic extract of aerial parts | 25 | 4.76±1.64* | 6.18±0.29* |
| of Lagenaria siceraria | |||
| 50 | 4.13±0.96ns | 4.32±0.98* | |
| 100 | 3.02±0.29*** | 3.49±0.48** | |
| 150 | 2.17±0.37*** | 2.34±1.32*** | |
| Control (Saline solution) | 0.9% sodium chloride | No paralysis | No death |
Results of In vitro antioxidant activity
At two distinct concentration levels, the methanolic extract of Lagenaria siceraria’s aerial portions was tested for its in vitro antioxidant activity. The concentration range of the produced extract was evaluated at 100 µg/ml, 200 µg/ml, and 500 µg/ml in order to determine the antioxidant activity by percentage scavenging. Following are the percentages of scavenging: The IC50 value for the standard was 103 ±2.17, and the percentage of scavenging ranged from 33.31 to 82.33% for the 100 µg/mL to 500 µg/ml range (with the IC50 value of 143±1.32). Rapidly occurring free radical scavengers in the test sample cause ABTS+ to react with them. This reaction is measured by watching for a drop in sample absorbance at 734 nm. The findings are displayed visually in Figure 5 and tabulated in Table 3.
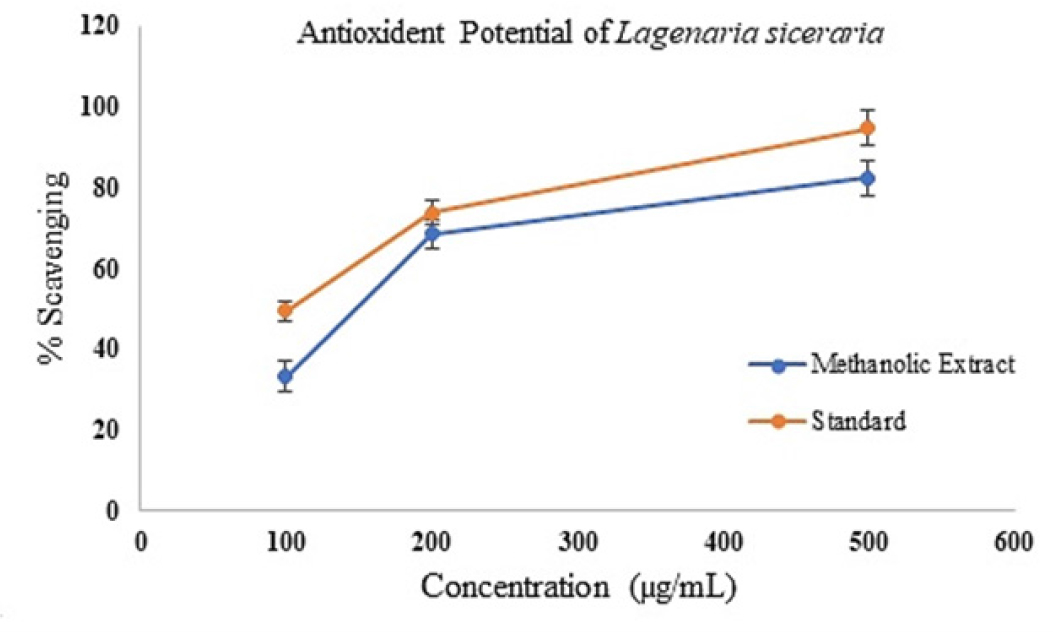
Figure 5:
Graphical representation of the antioxidant potential of the MEALS.
| Concentration (µg/ml) | % Scavenging of the methanolic extract | % Scavenging of Standard (ascorbic acid) |
|---|---|---|
| 100 | 33.31±0.58 | 49.47±1.42 |
| 200 | 68.43±1.57 | 73.64±1.01 |
| 500 | 82.33±1.16 | 94.73±1.21 |
| IC50 | 143±1.32 | 103±2.17 |
DISCUSSION
In addition to therapeutic substances, medicinal plants are a great source of knowledge for a wide range of chemical components that might be created as medications with perfect selectivity. These are the sources of perhaps valuable chemical compounds that might provide fresh leads and hints for contemporary medication design.13 The methanolic extracts of Lagenaria siceraria’s aerial parts claim to include carbohydrates, polyphenols, flavonoids, and glycosides; this important information may afterwards help in the discovery and development of new medications. In the study of the GC-MS analysis 59 compounds were obtained from the methanolic extracts of the aerial parts of Lagenaria siceraria. The obtained compounds were in the classes of polyphenols, carbohydrates, glycosides, and flavonoids as it was initially detected during the phytochemical extract’s percent scavenging activity (82.33%) was deemed satisfactory. The methanolic extract shows a strong antioxidant activity, which may be related to the abundance of phenolics, according to the obtained IC50 value for the investigated extract when compared with the standard ascorbic acid. Common plant metabolites include flavonoids, tannins, and other phenolics. The studied extract, when tested for the anthelmintic activity, shows effective dose dependent potential with the death time 2.34 min, and paralysis time, 2.17 min, in comparison with the standard albendazole 1.96 min and 1.25 min as a death and paralysis time. As the concentration of the extracts and the common medicine albendazole were increased against Pheretima posthuma, it was noticed that the values of paralysis time and death times studies. In the class of polyphenols the detected compounds were 1,2,3-Propanetrio(3), dl-Glyceraldehyde dimer(6), 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl(7) 1,2,3-Propanetriol, diacetate(11), Ascorbic acid 2, 6- dihexadecanoate(29), 1, 2, 3, 4, 5- Pentanepentol(19). Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester(44), 9,12-Octadecadienoic acid (Z,Z)-, 2,3-dihydroxypropyl ester(46). In the class of flavonoids the detected compounds were 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,8-dimethyl- 2-(4,8,12-trimethyltridecyl)(51), 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl) (52), 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl- 2-(4,8,12-trimethyltridecyl)-, acetate (59). Therefore it was quite justified to test the sample for its antioxidant potential and the empirical evidence on antioxidant activity also proves the same. The chemical structures of the other types of components like steroids, glycosides, carbohydrates obtained were cited in the figure and table. Other investigations may result in the extraction of additional phytocomponents, and understanding their structures will aid in future medication development. When compared to the standard ascorbic acid (94.73%), the methanolic significantly decreased.
CONCLUSION
Due to the undesirable side effects of synthetic pharmaceuticals, the phytocomponents of medicinal plants that are extracted in the form of extract may be useful for drug discovery and development against various diseases. The presence of phytoconstituents in the plant extract was confirmed by GC-MS analysis. Due to the presence of a substantial number of polyphenols and nitrogenous components, the methanolic extracts of Lagenaria siceraria’s aerial portions demonstrated high anthelmintic activity and antioxidant activity. As a result, the anthelmintic activity may be regarded as a phytopharmaceutical benefit of a natural antioxidant and anthelmintic agent, and there is room to conduct a number of other studies, such as HPTLC fingerprint analysis, for the further confirmation of phytoconstituents before taking the next step to isolate and Characterize them.
References
- Pullaiah T.. The encyclopedia of world medicinal plants. Regency publishers.. 2006;3:1206-7. [Google Scholar]
- Nalwade AR, Shinde SS, Bhor GL, Admuthe NM, Shinde SD, Gawade VV, et al. Rapid biosynthesis of silver nanoparticles using bottle gourd fruit extract and potential application as a bactericide. Res Pharm. 2013;3(3):22-8. [Google Scholar]
- Katare C, Saxena S, Agrawal S, Prasad GBKS. Alleviation of diabetes induced dyslipidemia by fruit extract in human type 2 diabetes. J Herb Med.. 2013;3(1):1-8. [CrossRef] | [Google Scholar]
- Satvir S, Gill NS, Arora R. Study the antioxidant activity of seeds. Int J Nar. Prod Sci.. 2012;1:224-7. [CrossRef] | [Google Scholar]
- Thakkar JH, Patel CA, Santani DD, Jani GK. Evaluation of antimutagenic potential of using Ame’s test. Am J Cancer Biol. 2013;1:2-5. [CrossRef] | [Google Scholar]
- Prajapati RP, Kalariya M, Parmar SK, Sheth NR. Phytochemical and pharmacological review of . J Ayurveda Integr Med.. 2010;1(4):266-72. [PubMed] | [CrossRef] | [Google Scholar]
- Mondal S, Panigrahi N, Sancheti P, Tirkey R, Mondal P, Almas S, et al. Evaluation of toxicological, diuretic, and laxative properties of ethanol extract from (Gaudich) aerial parts with docking studies of polyphenolic compounds on carbonic anhydrase II: an enzyme target for diuretic activity. Phcog Res.. 2018;10(4):408-16. [CrossRef] | [Google Scholar]
- Kanthal LK, Mondal P, Thakur R, Maity S, Kavi P, Srinu PB, et al. GC-MS analysis and assessment of antioxidant and anthelmintic potential of the ethanolic root extract of Linn. Pharmcog.Commn. 2020;10(4):150-5. [CrossRef] | [Google Scholar]
- Mondal P, Jana S, Balaji A, Ramakrishna R, Kanthal LK. Synthesis of some new isoxazoline derivatives of chalconised indoline 2-one as a potential analgesic, antibacterial and anthelmintic agents. J Young Pharm. 2012;4(1):38-41. [PubMed] | [CrossRef] | [Google Scholar]
- Mondal P, Mondal S. Synthesis, characterization and SAR studies of Novel Series of Spiro β-lactam of 5-methyl-indole-2,3-dione derivatives as a potential antibacterial and anthelmintic agent. Curr Chem Lett.. 2022;11(4):403-14. [CrossRef] | [Google Scholar]
- Raberta R, Nicoletta P.. Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free Radic Biol Med. 1996;26(9-10):1231-7. [CrossRef] | [Google Scholar]
- Vijayalakshmi R, Ravindran R.. Preliminary comparative phytochemical screening of root extracts of (Wild.) Bakh and (L.) Juss. Ex Schultes. Asian J Plant Sci Res. 2012;2:581-7. [CrossRef] | [Google Scholar]
