ABSTRACT
Background
The bioavailability of the drug is influenced by the solubility of the drug in the biological fluids. Solubility and dissolution are the rate-limiting steps for the absorption and bioavailability of BCS class II drugs.
Materials and Methods
In the current work, Efavirenz (EFV) has been selected as a model drug for the development and assessment of Solid Dispersion (SD) by solvent evaporation and fusion methods using sugar-based carriers like xylitol, Sorbitol, lactulose, and one nonsugar carrier surplus. First, the drug was assessed for solubility in various solvents. EFV Solid Dispersions (S.D.s) have been produced by solvent evaporation and fusion at different carrier ratios (1:0.5, 1:1, 1:1.5, 1:2, and 1:3). The prepared SDs were subjected to drug content uniformity, saturation solubility studies, and in vitro dissolution studies.
Results
Preformulation investigations, such as FT-IR, proved that the excipient and drug molecules are binding with intermolecular hydrogen bonds. EFV was found to be more soluble in 7.4 pH phosphate buffer than in the other solvents. The saturation solubility of EFV was affected by carrier concentration and %yield, and in vitro drug dissolution studies were performed on the S.D.s. Pure EFV released 54.78% of the drug, whereas SDs by solvent evaporation method released 85.28± 1.84%-100.74±1.69% drug, and SDs prepared by fusion method released 76.65±0.98%- 99.28±1.95% of the drug in 60 min.
Conclusion
SDs of EFV prepared by solvent evaporation and fusion methods have improved the aqueous solubility of EFV, and, in turn, improved the in vitro drug release, and formulation with 1:1.5 drug-to-carrier ratio has been selected as an optimized formulation.
INTRODUCTION
The solubility and permeability of a molecule in an aqueous media significantly impact the drug’s absorption, bioavailability, and pharmacokinetic profile when taken orally. According to the US’s Biopharmaceutical Classification System (BCS), which is depicted in Table 1 below, drugs are categorized into four classes depending on their solubility and permeability.1
| Sl. No. | BCS Class | Solubility | Permeability |
|---|---|---|---|
| 1 | Class I | High | High |
| 2 | Class II | Low | High |
| 3 | Class III | High | Low |
| 4 | Class IV | Low | Low |
Biopharmaceutical Classification System.
The majority of lipophilic medicines with relatively poor solubility rates are used in pharmaceutical research studies of drug synthesis.2 The nonnucleoside reverse transcriptase inhibitor Efavirenz (EFV) [(S)-6-chloro-4-(cyclopropyl ethynyl)-1, 4-dihydro-4-(trifluoromethyl)-2H-3, 1-benzoxazin-2-one] is licensed for the treatment of Human Immunodeficiency Virus Type 1 infection. Efavirenz chemical structure shown in Figure 1.

Figure 1:
Chemical Structure of Efavirenz.
EFV is a class II (low solubility, high permeability) substance that frequently exhibits poor Gastrointestinal (GI) absorption as a result of insufficient drug solubility in GI fluids.3
Several methods4 have been used to increase the solubility, dissolution rate, and bioavailability of poorly soluble drugs, including micronization, cyclodextrin complexation, use of surfactants and solubilizers, solid dispersion in water-soluble and dispersible carriers, use of salts, prodrugs, and polymorphs that exhibit high solubility, microemulsions, and self-emulsifying micro and nano disperse systems. Solid dispersions in water-dispersible ingredients are a basic, industrially useful method for improving the solubility, dissolution rate, and bioavailability of low-soluble drugs among the different techniques. Solubility and bioavailability of EFV can also be enhanced by various techniques like SD by PEGylation,5 Hot melt extrusion using surfactants,6 nanocrystal formation,7 solid lipid nanoparticles,8 starch citrate SD tablets,9 complex formation by beta cyclo dextrin,10,11 Self-Nano-Emulsifying Drug Delivery System,12 co-crystallization,13 Solid Self Emulsifying Drug Delivery Systems.14
MATERIALS AND METHODS
Materials
Efavirenz (Aurobindo Pharma Ltd., Hyderabad), Sorbitol, xylitol, lactulose, and soluplus (obtained as gift samples from Spectrum labs) were selected as solid dispersion carriers. Ethanol was selected as a solvent.
Experimental
Preformulation Studies
Determination of λmax
After precisely weighing 10 mg of Efavirenz, it was added to a 10 mL volumetric flask. After dissolving in methanol and diluting it with 7.4 pH buffer to volume, a stock solution with 1000 μg/ mL was produced. To achieve a concentration of 100 μg/mL, 1 mL of the stock solution was pipetted out, put into a 10 mL volumetric flask, and brought up to the appropriate level using a 7.4 pH buffer. A volume of 1 mL was pippetted out of the stock solution and placed into a 10 mL volumetric flask. A 7.4 pH buffer was added to the flask until the desired concentration of 10 μg/mL was reached. This solution was compared to a blank for UV spectrum analysis.
Preparation of Standard Calibration Curve of Efavirenz in 7.4 pH Buffer
After precisely weighing 10 mg of Efavirenz, it was added to a 10 mL volumetric flask. It was dissolved in some amount of methanol, and the stock solution with 1000 μg/mL was obtained by diluting it to volume 7.4 pH buffer. To obtain 3 to 8 μg/mL of Efavirenz, the standard stock solution was thereafter serially diluted with 7.4 pH buffer. Using a UV-visible spectrophotometer, the absorbance of the solution was measured at 247 nm against a blank of 7.4 pH buffer.
Solubility studies of Pure Efavirenz
A solubility research was carried out to ascertain how various buffers affected the medication. Ten milliliters of distilled water, methanol, acetone, phosphate buffer solution (pH=6.8, 7.4), and 0.1N HCl were used to dilute an excess of the medication in glass stoppered tubes, in that order. To prevent solvent loss, all flasks were sealed with stoppers wrapped with cellophane membranes and left in a water bath shaker set to 37°C for a whole day. The samples were centrifuged (Hermle Z 200 A, Germany) for five minutes at 3000 rpm after they had reached equilibrium. Filtration of the supernatant was done using a 0.45 μm membrane filter. A 1 mL sample of saturated solution was diluted using appropriate solvents, and a UV-spectrophotometer (PG Instruments, T60) was used to analyse the sample at a wavelength of 247 nm.
Phase solubility studies
Studies on solubility were carried out using the Higuchi and Connors methodology. A 50 mL flask filled with distilled water and various carrier concentrations (1:1, 1:2, 1:3) was overfilled with extra medication. To prevent solvent leakage, a stopper was used to seal each flask, and a cellophane membrane was placed over them. The flasks spent seventy-two hours in the incubator shaker. Each flask’s contents were filtered using Whatman filter paper after a 72-hour period. The filtrate was diluted, and its Efavirenz level was measured at 247 nm using spectrophotometry.5
Compatibility studies
The drug and excipient compatibility study was performed using Fourier Transform Infrared Spectrophotometer analysis (FT-IR).
Fourier Transform Infrared Spectrophotometric analysis (FTIR)
The infrared spectrum of Efavirenz, lactulose, was determined on a Fourier Transform Infrared Spectrophotometer (IRAffinity-1S, Shimadzu) using the KBr dispersion method. The spectra were scanned over a frequency range of 4000-400 cm-1.
Methods
Formulation of Solid Dispersions
The formulation of EFV solid dispersions using various carriers has been shown in Table 2.
| Drug: Carrier ratio | 1:0.5 | 1:1 | 1:1.5 | 1:2 | 1:3 | |
|---|---|---|---|---|---|---|
| Formulation code (Solvent evaporation | Xylitol | EX1 | EX2 | EX3 | EX4 | EX5 |
| Sorbitol | ES1 | ES2 | ES3 | ES4 | ES5 | |
| Soluplus | ESP1 | ESP2 | ESP3 | ESP4 | ESP5 | |
| Lactulose | EL1 | EL2 | EL3 | EL4 | EL5 | |
| Formulation code (Fusion method) | Xylitol | FSX1 | FSX2 | FSX3 | FSX4 | FSX5 |
| Sorbitol | FSS1 | FSS2 | FSS3 | FSS4 | FSS5 | |
| Soluplus | FSSP1 | FSSP2 | FSSP3 | FSSP4 | FSSP5 | |
| Lactulose | FSL1 | FSL2 | FSL3 | FSL4 | FSL5 | |
Formulation of Efavirenz solid dispersions.
Preparation of Solid Dispersion (SD) of Efavirenz by solvent evaporation method
Using this technique, precisely measured amounts of the carriers in the specified ratios (1:0.5, 1:1, 1:1.5, 1:2, and 1:3) were gently put into test tubes, brought to a boil, and then dissolved in ethanol. We added precisely weighed amounts of the medication to these solutions and let it dissolve. The solvent was allowed to evaporate at room temperature after the solution was moved to a Petri dish. The dispersions were then dried for one hour at room temperature and for two hours at 45°C in a hot air oven. Each case’s mass was ground up, ground up, and sieved through sixty meshes.15–17
Preparation of Solid Dispersion (SD) of Efavirenz by Fusion Method
The carrier was heated in a water bath with a thermostat regulated to 55°C±0.5°C. The medication was distributed in the molten carrier in ratios of 1:0.5, 1:1, 1:1.5, 1:2, and 1:3 for Efavirenz to Xylitol, Sorbitol, Soluplus, and Lactulose. Using an ice-water mixture, the resultant fluid was quickly cooled to 10°C, 20°C, or 30°C. It was then kept at that temperature for two hours. After being taken out of the ice-water mixture, the hardened mass was allowed to come to room temperature. After being kept at room temperature for a full day, it was crushed with a glass crusher and pestle. The product was ground using a porcelain mortar and pestle (Dr. Scientific, Haryana, India) after being kept in a desiccator for a whole day (Singhla Scientific Industries, Haryana, India). To achieve a consistent particle size, the ground powder was run through an 80-mesh sieve (Micro Teknic, Haryana, India).18
Drug Content
A precisely weighed amount of solid dispersion equal to 10 mg was added to 10 mL standard flasks, and the volume was increased to 10 mL using methanol. Using a UV-visible spectrophotometer (T60 PG Instruments), the concentration of Efavirenz was measured spectrophotometrically at 247 nm against an appropriate blank.7
In vitro Dissolution Studies
A capsule containing 50 mg of Efavirenz’s solid dispersion was sealed and stored in a dissolution media. Using the dissolution testing USP apparatus I (basket method), the dissolving investigation of solid dispersions was carried out in 900 mL of 7.4 pH buffer at 37±0.5°C and 50 rpm. Using a UV-visible spectrophotometer (T60 PG Instruments), an aliquot of 5 mL was removed at predefined intervals, and an equal volume of new medium was added to maintain a constant volume after each sampling. The sample was then spectrophotometrically analyzed at 247 nm against a suitable blank.7,8
Characterization of EFV SDs
Pure EFV and SD formulations were characterized by X-ray diffraction studies to analyze the changes in crystalline characteristics of EFV.
RESULTS
Determination of absorption maxima and construction of calibration curve for Efavirenz
The absorption maxima for EFV were determined by using a UV-visible spectrophotometer, and the λmax of Efavirenz was found to be 247 nm. Plotting the calibration curve for Efavirenz involved producing different dilutions ranging from 3 μg/mL to 8 μg/mL. Figure 2 displays the EFV calibration plot.
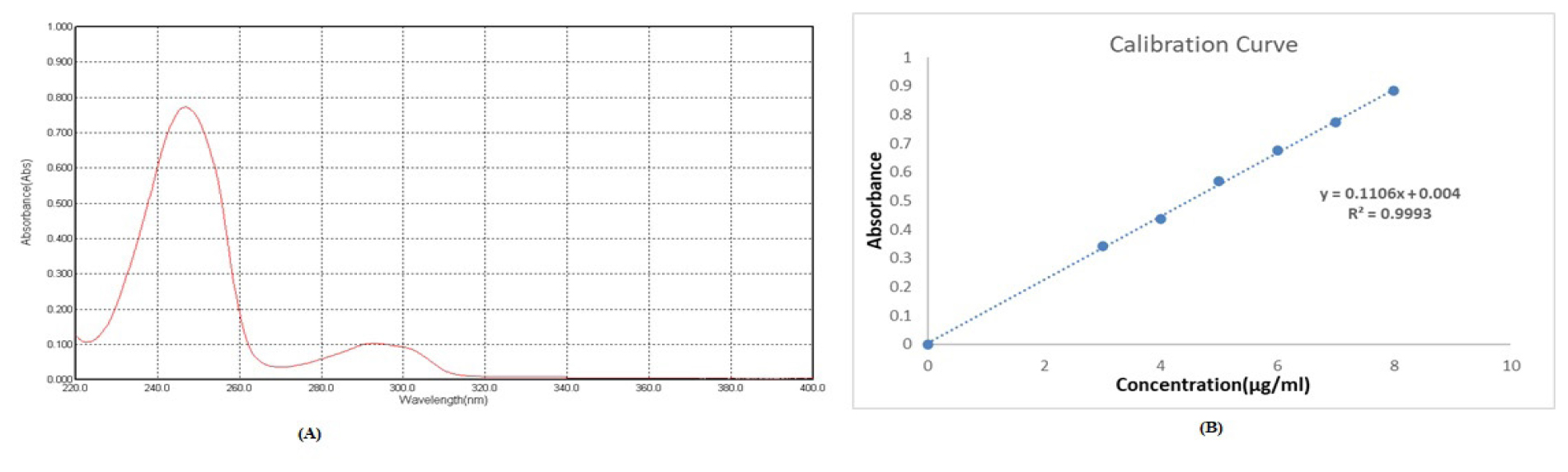
Figure 2:
(A) Absorption maxima of Efavirenz, (B) Calibration curve of Efavirenz in pH 7.4 Phosphate buffer.
Drug excipient compatibility
Drug and excipient compatibility was confirmed by comparing the spectra of the FT-IR analysis of pure drugs with that of various excipients used in the formulation. The FTIR spectrum of EFV and EFV + lactulose are shown in Figure 3.
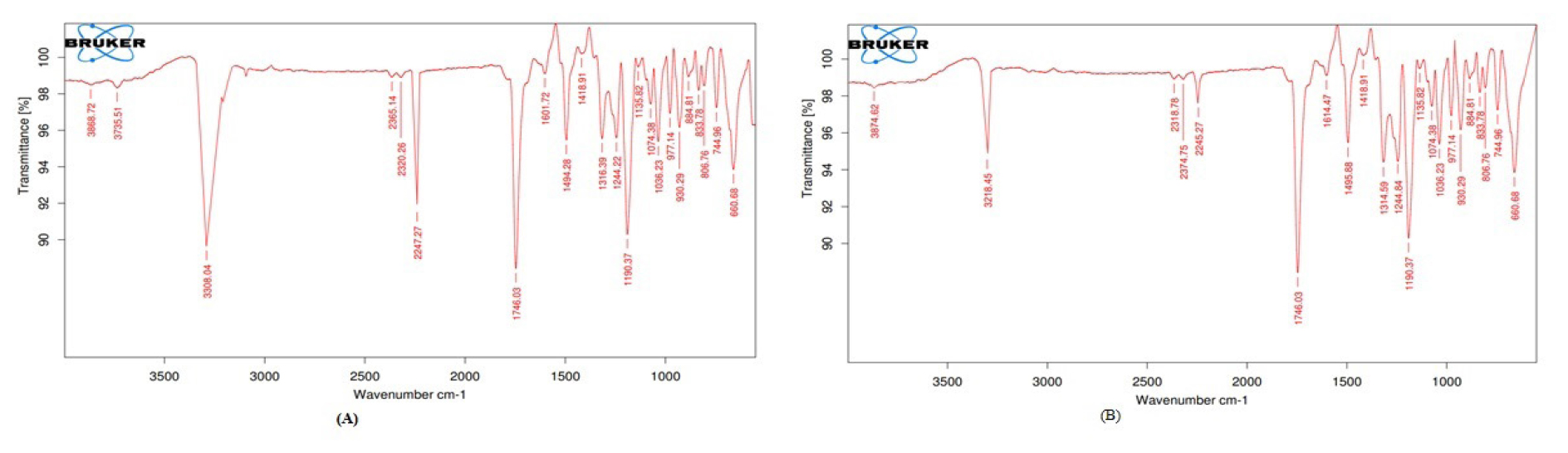
Figure 3:
(A) FT-IR Spectrum of Pure Efavirenz; (B) FT-IR spectrum of Efavirenz and Lactulose.
Phase solubility studies of Efavirenz
Efavirenz’s solubility was determined at 37°C in purified water, 0.1 N HCL, 6.8 phosphate buffer solution, and 7.4 pH buffer, ethanol, and methanol. According to EFV’s solubility studies performed in several buffer solutions, 7.4pH buffer is more soluble than other buffer solutions. It was demonstrated that Efavirenz was soluble in the following solvent systems: pH 7.4 phosphate buffer>ethanol>pH 6.8 phosphate buffer>methanol>pH 1.2 acidic buffer >water. The results are displayed in Table 3 and Figure 4.
| Solvent | Solubility (μg/mL) |
|---|---|
| 1.2 pH acidic buffer | 0.182±0.024 |
| 6.8pH phosphate buffer | 0.342±0.028 |
| 7.4pH phosphate buffer | 0.435±0.021 |
| ethanol | 0.410±0.031 |
| Methanol | 0.341±0.026 |
| Water | 0.125±0.029 |
Solubility of Efavirenz in various solvents.
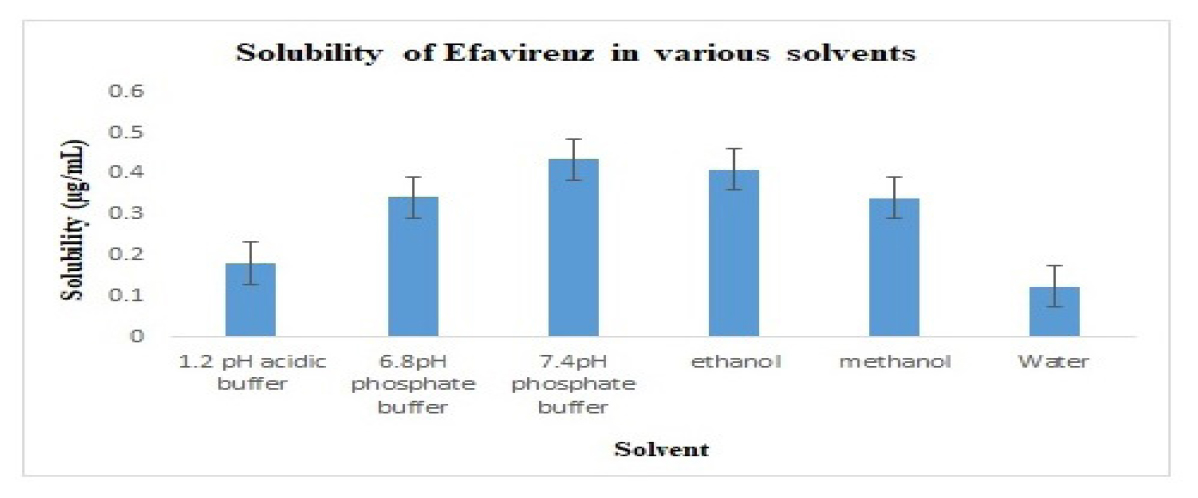
Figure 4:
Solubility of Efavirenz in various solvents.
The solubility of EFV S.D.s produced using physical mixing, solvent evaporation, and fusion techniques was assessed in distilled water to determine their saturation. The results are presented in Table 4.
| Method | Carrier | Efavirenz: Carrier Ratio | ||||
|---|---|---|---|---|---|---|
| 1:0.5 | 1:1 | 1:1.5 | 1:2 | 1:3 | ||
| Physical mixtures | Xylitol | 0.224±0.142 | 0.324±0.158 | 0.314±0.136 | 0.424±0.185 | 0.277±0.165 |
| Sorbitol | 0.203±0.187 | 0.257±0.168 | 0.298±0.185 | 0.324±0.126 | 0.302±0.159 | |
| Lactulose | 0.136±0.184 | 0.236±0.145 | 0.278±0.181 | 0.305±0.145 | 0.232±0.189 | |
| Soluplus | 0.228±0.198 | 0.288±0.169 | 0.296±0.154 | 0.330±0.195 | 0.315±0.162 | |
| Solvent evaporation | Xylitol | 0.689±0.174 | 0.748±0.175 | 0.849±0.184 | 0.892±0.187 | 0.738±0.166 |
| Sorbitol | 0.614±0.158 | 0.708±0.184 | 0.812±0.148 | 0.848±0.185 | 0.758±0.158 | |
| Lactulose | 0.569±0.128 | 0.624±0.128 | 0.656±0.195 | 0.745±0.128 | 0.728±0.113 | |
| Soluplus | 0.749±0.128 | 0.898±0.184 | 0.909±0.169 | 0.986±0.184 | 0.898±0.153 | |
| Fusion Method | Xylitol | 0.614±1.39 | 0.698±1.64 | 0.801±1.74 | 0.828±1.28 | 0.858±1.45 |
| Sorbitol | 0.598±1.28 | 0.697±1.64 | 0.801±1.87 | 0.824±1.94 | 0.841±1.26 | |
| Lactulose | 0.542±1.68 | 0.601±1.74 | 0.624±1.28 | 0.739±1.74 | 0.768±1.46 | |
| Soluplus | 0.729±1.73 | 0.854±1.57 | 0.895±1.98 | 0.961±1.21 | 0.974±1.58 | |
Saturation Solubility of Efavirenz Solid Dispersions.
Drug-to-carrier proportions ranging from 1:0.5 to 1:3 were used to yield S.Ds. The saturation solubility of EFV was influenced by carrier concentration as well. Up to a certain concentration of carrier, an increase in carrier concentration increased the drug’s solubility; beyond that point, it lowered the drug’s solubility.
Drug Content Uniformity
Various EFV solid dispersions using sugar carriers like xylitol, Sorbitol, lactulose, and nonsugar carrier soluplus at different ratios (1:1 and 1:3) were prepared by solvent evaporation and fusion technique to enhance the solubility and/or dissolution of BCS class II drug, Efavirenz. The percentage drug content in various prepared EFV SDs ranged from ranging from 92.95±1.42% to 99.71±1.87%, and the drug content in formulations made using the fusion approach, ranged from 85.36±0.45-98.89±0.36%, as reported in Table 5. This indicated that EFV was uniformly distributed in all of these prepared solid dispersions.
| Solid dispersion | Ratio | Solvent evaporation | Fusion | Solid dispersion | Ratio | Solvent evaporation | Fusion |
|---|---|---|---|---|---|---|---|
| EFV: Xyletol | 1:0.5 | 92.95±1.42 | 87.71±0.19 | EFV: Lactulose | 1:0.5 | 97.18±1.65 | 92.74±0.36 |
| 1:1 | 95.48±1.36 | 91.28±0.24 | 1:1 | 97.54±1.36 | 94.85±0.19 | ||
| 1:1.5 | 97.26±1.48 | 93.25±0.36 | 1:1.5 | 98.24±1.81 | 96.31±0.54 | ||
| 1:2 | 96.47±1.67 | 96.74±0.28 | 1:2 | 99.71±1.87 | 98.89±0.36 | ||
| 1:3 | 98.47±1.85 | 98.15±0.21 | 1:3 | 97.85±1.84 | 97.48±0.65 | ||
| EFV: Sorbitol | 1:0.5 | 93.18±1.47 | 85.36±0.45 | EFV: Soluplus | 1:0.5 | 95.48±1.99 | 92.48±0.65 |
| 1:1 | 95.36±1.25 | 89.98±0.26 | 1:1 | 99.69±1.35 | 94.37±0.42 | ||
| 1:1.5 | 96.42±1.24 | 92.78±0.64 | 1:1.5 | 98.32±1.84 | 95.37±0.61 | ||
| 1:2 | 97.28±1.21 | 96.36±0.84 | 1:2 | 98.78±1.28 | 98.31±0.52 | ||
| 1:3 | 96.47±1.24 | 98.61±0.56 | 1:3 | 97.47±1.36 | 95.81±0.47 |
Drug content Uniformity of Efavirenz Solid Dispersions.
In vitro Dissolution studies of Solid dispersions
Studies on the in vitro release of solid dispersions and pure Efavirenz were conducted.
In 60 min, the pure EFV releases 56.48±1.45% of release. A non-sugar-based carrier called Soluplus released 80.24±1.87% to 99.23±2.09% in 60 minutes, while formed solid dispersions with sugar-based carriers such as xylitol, Sorbitol, and lactulose released 83.26±1.35% to 99.22± 1.02%, 74.56±1.74% to 98.11±1.28%, and 91.85±1.98% to 100.02±1.73%, respectively. Figures 5 (A) and (B) depict the drug release patterns obtained from the solvent evaporation and fusion methods, respectively.
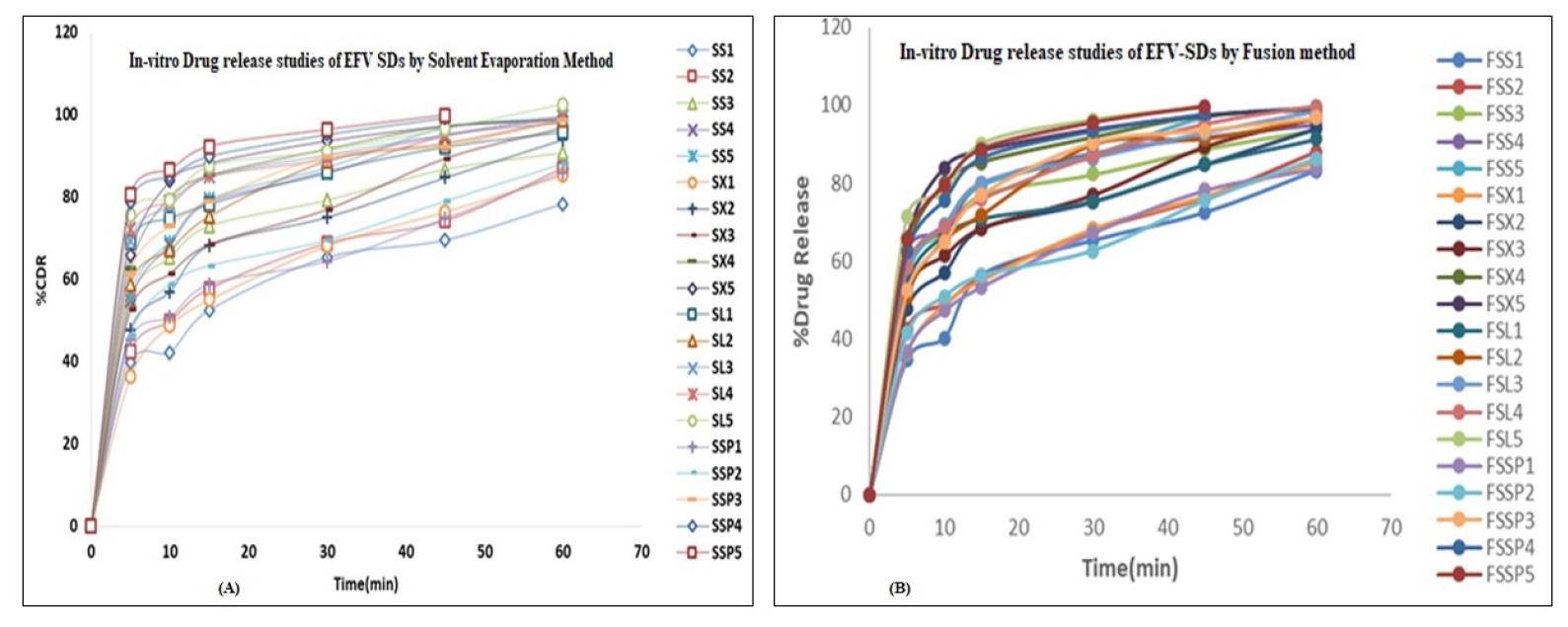
Figure 5:
In vitro drug release studies of Efavirenz SDs By (A) Solvent evaporation method, (B) Fusion method.
Characterization of EFV SDs by XRD Studies
The XRD is a well-established analytical tool for phase identification and determination of crystal and lattice parameters.
The diffractograms of pure EFV and EFV SD are shown in Figure 6.
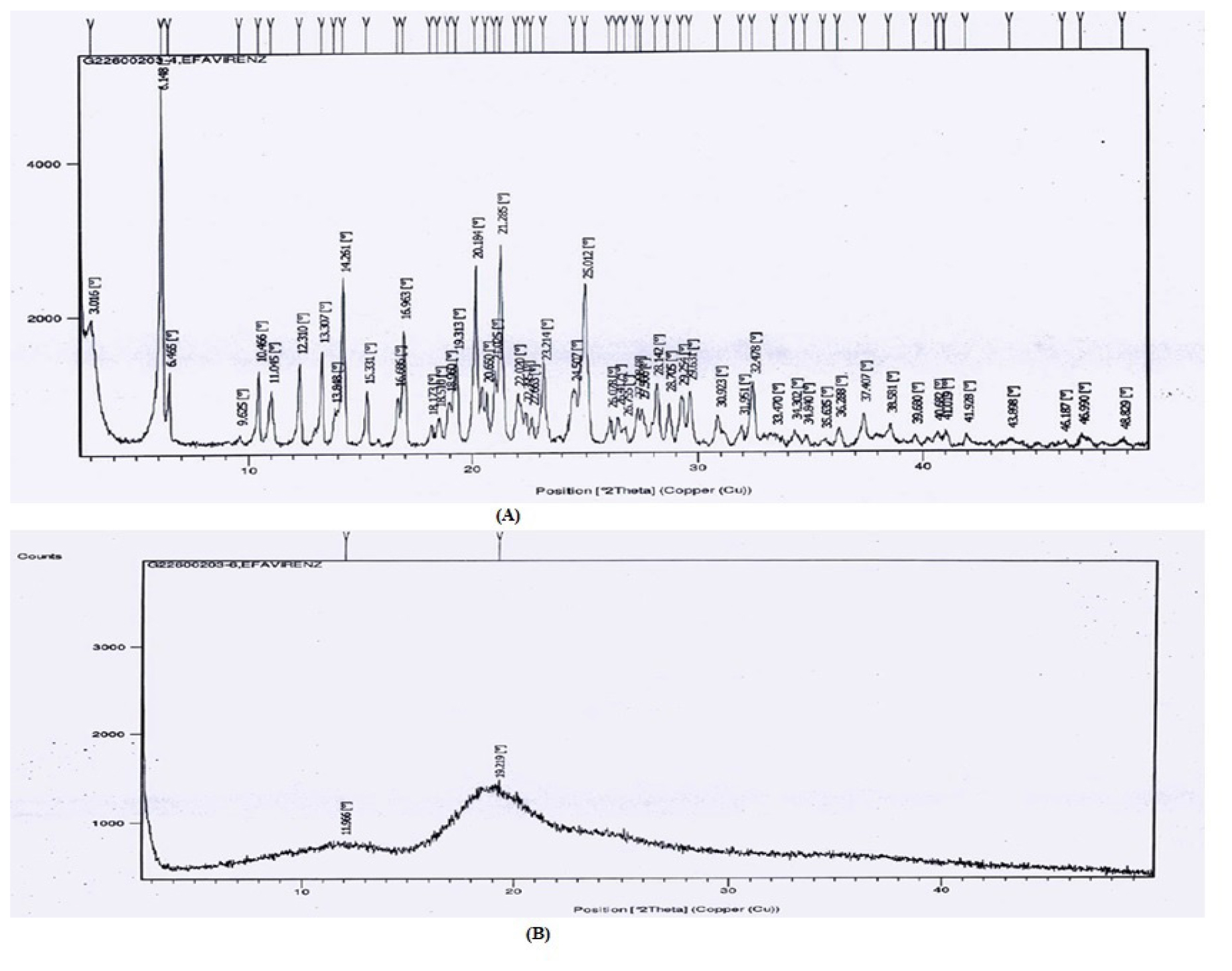
Figure 6:
(A) XRD Spectrum of Pure Efavirenz, (B) XRD spectrum of Optimized formulation containing Efavirenz and Lactulose.
DISCUSSION
Interactions between the EFV and the carrier often result in detectable differences in the I.R. profile of S.D.s. The I.R. spectra of S.D.s were compared to the standard spectrum of EFV. Pure
EFV I.R. spectra showed a peak at 3308 cm-1, which denotes the presence of a -N-H. Bond. The peak at 2247cm-1 indicates the presence of a C-C triple bond, 1746 cm-1 represents the presence of the carbonyl group, 1601 and 1494 cm-1 peaks represent the presence of Ph(C=C), and the peak at 1190 cm-1 indicates the presence of the CF3. All the characteristic peaks in EFV were not affected by the presence of lactulose, but the amine stretching characteristic peak was displaced from 3308 cm-1 to 3218 cm-1.19 The presence of a peak at 3218 cm-1 evidenced the presence of weakly hydrogen-bonded N H groups. Hydrogen bonds cause amine, amide, and hydroxyl groups to vibrate less, which lowers the strength or wavenumber of the bands associated with these functional groups.20
The saturation solubility of EFV SDs prepared using fusion, solvent evaporation, and physical mixing techniques was assessed. The solubility of EFV in water was found to be enhanced by the addition of sugar-based carriers. The drug’s saturation solubility was also impacted by the preparation technique and carrier concentration. The order of saturation solubility of EFV by various methods is as follows: Physical mixing<Fusion method<Solvent evaporation method.
Efavirenz, a crystalline drug, resulted in sharp characteristic XRD peaks. XRD diffraction patterns of SD formulations did not show any sharp peaks; instead, SDs exhibited an amorphous halo. The addition of a sugar-based carrier converted the strong crystalline form of the drug into an amorphous state. Because of this transformation of the EFV from a crystalline state to an amorphous state, the aqueous solubility might have been improved. The solubility and dissolution characteristics of EFV have been improved due to the formation of intermolecular hydrogen bonds between the amine group of efavirenz and the carrier molecule as well as the conversion of the crystalline drug into the amorphous state (an amorphous form of substance will have better solubility than that of the crystalline form).
CONCLUSION
The current study examined the phase solubility and dissolution behavior of EFV at different concentrations using a variety of sugar-based carrier types. The findings showed that the inclusion of sugar-based carriers significantly increased the solubility of EFV. The amount of the drug’s bioavailability was determined by its dissolution. The SDs of EFV with sugar carriers showed a significant increase in EFV dissolution, which suggests that the addition of sugar carriers improved EFV’s bioavailability. From the drug release studies, the carrier that is exhibiting better release at low concentrations has been selected as a better carrier for further study. i.e., EFV SDs containing lactulose showed better release properties, and a formulation with a 1:1.5 drug-to-carrier ratio was selected as an optimized formulation for further study.
Cite this article
Nakka VNJ, Gubbiyappa KS. Development and Evaluation of Efavirenz Solid Dispersion by Solvent Evaporation and Fusion Methods. Int. J. Pharm. Investigation. 2024;14(2):517-24.
ACKNOWLEDGEMENT
The authors would like to acknowledge GITAM, (Deemed to be a university), for providing the necessary facilities to carry out the research work.
ABBREVIATIONS
| BCS | Biopharmaceutical Classification System |
|---|---|
| EFV | Efavirenz |
| SD | Solid Dispersions |
| US | United States |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| USP | United States Pharmacopoeia |
| XRD | X-Ray Diffraction. |
References
- Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3-4):278-87. [PubMed] | [CrossRef] | [Google Scholar]

- Maurin MB, Rowe SM, Blom K, Pierce ME. Kinetics and mechanism of hydrolysis of efavirenz. Pharm Res. 2002;19(4):517-21. [PubMed] | [CrossRef] | [Google Scholar]

- Srivalli KM, Mishra B. Improved aqueous solubility and antihypercholesterolemic activity of ezetimibe on formulating with hydroxypropyl-β-cyclodextrin and hydrophilic auxiliary substances. AAPS PharmSciTech. 2016;17(2):272-83. [PubMed] | [CrossRef] | [Google Scholar]

- Chowdary KPR, Madhavi BLR. Novel drug delivery technologies for insoluble drugs. Indian Drugs. 2005;42(9):557-62. [PubMed] | [CrossRef] | [Google Scholar]

- Madhavi BB, Kusum B, Chatanya ChK, Madhu MN, Harsha VS, Banji D, et al. Dissolution enhancement of efavirenz by solid dispersion and pegylation techniques. Int J Pharm Investig. 2011;1(1):29-34. [PubMed] | [CrossRef] | [Google Scholar]

- Kolhe S, Chaudhari PD, More D. Dissolution and bioavailability enhancement of efavirenz by hot melt extrusion technique. IOSR J Pharm. 2014;4(5):47-53. [PubMed] | [CrossRef] | [Google Scholar]

- Sartorii GJ. D, Livia Deris Prado, helvécio Vinícius Antunes rocha. Efavirenz dissolution enhancement V – A combined top-down/bottom-up approach on nanocrystal formulation. Braz J Pharm Sci. 2022;58:1-14. [CrossRef] | [Google Scholar]

- Gaur PK, Mishra S, Bajpai M, Mishra A. Enhanced oral bioavailability of efavirenz by solid lipid nanoparticles: in vitro drug release and pharmacokinetics studies. BioMed Res Int. 2014;2014 Article ID 363404 [PubMed] | [CrossRef] | [Google Scholar]

- Chowdary KPR, Enturi V. Enhancement of dissolution Rate and formulation development of efavirenz tablets employing starch citrate-A new modified starch. J Appl Pharm Sci. 2011;1(5):119-23. [PubMed] | [CrossRef] | [Google Scholar]

- Chaitanya V, Narayan PN, Kumar S, Chowdary KPR. Enhancement of solubility dissolution Rate and formulation development of efavirenz tablets employing ?cd and Lutrol: A Factorialstudy. J Glob Trends Pharm Sci; 1360. 2014;5(1):356 [PubMed] | [CrossRef] | [Google Scholar]

- Yogananda R, Chowdary KPR. Enhancement of Solubility, Dissolution Rate and Bioavailability of efavirenz by Cyclodextrins and Solutol HS15 – A Factorial Study. Int J Drug Dev Res. 2013;5(1):135-42. [PubMed] | [CrossRef] | [Google Scholar]

- Kamble RN, Mehta PP, Kumar A. Efavirenz self-nano-emulsifying drug delivery system: in vitro and in vivo evaluation. AAPS PharmSciTech. 2016;17(5):1240-7. [PubMed] | [CrossRef] | [Google Scholar]

- Yadav D, Savjani J, Savjani K, Kumar A, Patel S. Pharmaceutical co-crystal of antiviral agent efavirenz with nicotinamide for the enhancement of solubility, physicochemical stability, and oral bioavailability. AAPS PharmSciTech. 2023;24(7):1-15. [CrossRef] | [Google Scholar]

- Sunitha Reddy M, Srikanth Reddy N, Mallikarjun Reddy S. Solubility enhancement of poorly water soluble drug efavirenz by solid self emulsifying drug delivery systems. Int J Pharm Res Rev. 2014;3(4):20-8. [CrossRef] | [Google Scholar]

- Perissutti B, Newton JM, Podczeck F, Rubessa F. Preparation of extruded carbamazepine and PEG 4000 as a potential rapid-release dosage form. Eur J Pharm Biopharm. 2002;53(1):125-32. [PubMed] | [CrossRef] | [Google Scholar]

- Vilhelmsen T, Eliasen H, Schaefer T. Effect of a melt agglomeration process on agglomerates containing solid dispersions. Int J Pharm. 2005;303(1-2):132-42. [PubMed] | [CrossRef] | [Google Scholar]

- Tsinontides SC, Rajniak P, Pham D, Hunke WA, Placek J, Reynolds SD, et al. Freeze drying principles and practice for successful scale-up to manufacturing. Int J Pharm. 2004;280(1-2):1-16. [PubMed] | [CrossRef] | [Google Scholar]

- Das A, Nayak AK, Mohanty B, Panda S. Solubility and dissolution enhancement of etoricoxib by solid dispersion technique using sugar carriers. ISRN Pharmacol 2011Sep 5. 2011;2011:819765 [PubMed] | [CrossRef] | [Google Scholar]

- Baskaran P, Vimalan M, Anandan P, Bakiyaraj G, Kirubavathi K, Selvaraju K, et al. Synthesis, growth, and characterization of a nonlinear optical crystal: aluminum perchlorate. J Taibah Univ Sci. 2017;11(1):11-7. [CrossRef] | [Google Scholar]

- Theophile T. Infrared Spectroscopy – Materials science, engineering and technology. London: InTechOpen. 2012 [CrossRef] | [Google Scholar]


