ABSTRACT
Background
Diabetes is an endocrine disorder characterized by an abnormal increase in blood sugar level. Vildagliptin is a Dipeptidyl Peptidase-4 (DPP-4) inhibitor used in the treatment of type 2 diabetes mellitus.
Purpose
The present work deals with the Quality by Design (QbD) approach based formulation and optimization of Vildagliptin-Eudragit RL 100 coated nanoparticles (VLD-EudNPs).
Materials and Methods
VLD-Eud NPs were developed by combined technique of solvent evaporation and nano-precipitation. Optimization was done by 32 factorial designs. Prepared nanoformulations were characterized for percentage yield and morphology. Further in vitro drug release and kinetics were studied. The accelerated stability study of the optimized formulation was carried out for 3 months.
Results
VLD-EudNPs ranged from 58.5±2.02 nm to 425.4±4.71 nm with a PDI of 0.27±0.036 to 0.52±0.086. Entrapment efficiency (% EE) of nanoformulations F1 to F9 ranged between 32.29±1.16 to 75.26±1.63%. The particles were found to be cylindrical-shaped, with a percentage yield of 54.7±3.45 to 84.4±1.51%. During the in vitro drug release study, at acidic pH, <5% drug release, while delayed release at basic pH, at the end of 24 hr, all formulations have released >68% of the drug following zero order kinetics. The optimized formulation has proven its stability during stability study.
Conclusion
The present work concludes that prepared and optimized VLD-Eud NPs could be a better approach for sustained delivery of Vildagliptin for the management of diabetes mellitus as compared to traditional drug delivery.
INTRODUCTION
Diabetes, an endocrine disorder characterized by an abnormal increase in blood sugar level, is one of the most prevalent illnesses throughout the world. According to a World Health Organization (WHO) estimate, diabetes affects 422 million people worldwide, predominantly in low- and middle-income nations. By 2045, it is expected to affect 693 million adults, a >50% increase from 2017. Among the Diabetes Mellitus (DM) population globally, ~95% of people suffer from type 2 DM due to a lack of physical activity and obesity.1,2 Existing medications are associated with side effects like hypoglycemic effects, gastric intolerance, and increased body weight. Hence, there is a need to focus on newer therapeutic agents to treat type 2 DM.3
Endogenous incretion hormones like Gastric Inhibitory Polypeptide (GIP) and Glucagon-Like Peptide-1 (GLP-1) are important in the pathophysiology of type 2 DM and in the physiology of glucose homeostasis by inducing the release of insulin from beta cells. GLP-1 is concentrated primarily on its ability to reduce blood sugar and body weight. Dipeptidyl Peptidase 4 (DPP-4) enzymes are found in our body and quickly inactivate incretin hormones. Hence, there is a necessity for DPP-4 inhibitors (gliptins) to manage the treatment of type 2 DM.4,5 Gliptins are attractive second-line anti-diabetic agents with good efficacy, favorable safety, and better tolerability. They are available orally, either as monotherapy or with various combination therapies. Nowadays, gliptins are gaining importance and increasing patient adherence over GLP-1 analogues, which are available as subcutaneous injections.6 Vildagliptin is a potential DPP-4 inhibitor that has a great molecular interaction with the DDP-4 enzyme.7 The major drawback of Vildagliptin is its short half-life of 2-3 hr and rapid metabolism.8 Due to the short half-life of Vildagliptin, it is recommended to take 50 mg twice a day, and patients need to adhere strictly to the dose intervals.9 Therefore, there is a need to develop a novel dosage form of Vildagliptin that will improve oral bioavailability.
Nowadays, Nanoparticulate Drug Delivery Systems (NDDSs) are most popular in the field of pharmaceutical research for the oral administration of drugs due to their capacity to deliver drugs to the proper site, dose, and timing.10 There are various NDDSs, like nanoemulsions, liposomes, niosomes, and nanoparticles, designed for oral delivery of various drugs.11 In comparison to other novel delivery systems as aforementioned, polymeric nanoparticles have a number of advantages, including site-specific targeting, prevention of dose dumping through controlled and sustained release, and high surface-to-volume ratio. Additionally, the surface characteristics of nanoparticles offer mucoadhesive, cellular uptake, immune system interaction and cell targeting properties. These qualities assist in decreasing dose and frequency of administration in an indirect manner, which enhances patient compliance.12,13 For improving the oral bioavailability of numerous drugs, polymeric coated nanoparticles are the most promising of these strategies.
Synthetic, semisynthetic, or natural polymers are used to prepare polymeric nanoparticles that enhance the circulation half-life, reduce the phagocytic uptake, and inactivation of drug, by which improves delivery and targeting of drugs.14
Quality by Design (QbD) is initiated by the FDA for pharmaceutical product development (FDA guidance of industry, 2006). It is a design effort to deliberate product conceptualization for commercialization.15 The QbD concept is a systematic approach for pharmaceutical formulation development with specific predefined objectives that provides improved process and product understanding along with a limited investment of cost, effort, and time. Designs of Experiments (DoE) are taken into consideration as an integral part of QbD, which helps in understanding a relationship between cause and effect. Many literatures reported that effective utilization of QbD and DoE concepts for formulation development to attain robustness in product performance.16 Various formulation and processing parameters may affect the physicochemical properties of nanoparticles, which may affect the product quality. Hence, there is a necessity for the QbD concept to assure the quality of nanoparticles.17
In present work nanoformulations were prepared by solvent evaporation and nanoprecipitation technique using the QbD concept for oral delivery of Vildagliptin. This method of preparation is simple, reliable and not requires any sophisticated equipment. In this context, in the present study, an attempt is made to formulate and optimize VLD-Eud NPs using the QbD concept.
MATERIALS AND METHODS
Vildagliptin and Eudragit RL 100 were received as free gift sample from Ajanta Pharma Ltd., Mumbai, India, and Centaur Pharmaceuticals Pvt. Ltd., Pune, respectively. Poloxamer 188 and other solvents and excipients were obtained from authenticated sources.
Preparation of Vildagliptin nanoformulation
VLD-Eud NPs were formulated by the combined technique of solvent evaporation and nanoprecipitation with slight modification.18 Briefly, different drug: polymer (Eudragit RL 100) ratios, viz., 1:2, 1:4 and 1:6 were dissolved in the solvent mixture of dichloromethane (DCM) and methanol at three different levels, viz., 1:3, 1:1.66 and 1:1. The above organic solution was then added drop-wise with a 22-gauge needle to 10 mL of poloxamer 188 aqueous solution (0.5% w/v) on a magnetic stirrer at 500 rpm. The organic solution was then evaporated for 2 hr at room temperature to obtain an aqueous dispersion, which was finally centrifuged (KUBOTA 7000, Japan) at 35,000 rpm at 4°C for 60 min to collect the nanoparticles. The sediment obtained was washed by re-suspending in distilled water and centrifuged with the same conditions in triplicate. The final product was dried by using a freeze dryer.
Statistical experimental design
VLD-Eud NPs were formulated by adopting 32 statistical experimental designs (Design Expert 13). In this design, two independent variables, viz., the content of (X1) Eudragit RL 100 (mg) and (X2) the composition of the organic solvent, i.e., DCM+methanol (mL), were selected. Three levels for each independent variable were coded as: (-1) low, (0) medium, and (+1) high, characterizing the 32 factorial designs consisting of 9 different formulations as shown in Table 1. The effect of two factors at their three levels on dependent variables (Y1) particle size (nm), and (Y2) entrapment efficiency (%), were studied as shown in Table 2.
| Levels | Independent variables | Dependent variables | ||
|---|---|---|---|---|
| X1=Eudragit RL 100 (mg) | X2=Volume of DCM and methanol (mL) | Y1=Particle size (nm) | Y2=Entrapment Efficiency (%) | |
| -1 | 200 | 0.5+1.5 | Goal to minimize | Goal to maximize |
| 0 | 400 | 0.75+1.25 | ||
| + 1 | 600 | 1+1 | ||
Experimental design with process variables and their levels.
| Runs | Independent variables | Dependent variables | ||||||
|---|---|---|---|---|---|---|---|---|
| Y1 (nm) | Y2 (%) | |||||||
| X1 | X2 | Actual | Predicted | Residual | Actual | Predicted | Residual | |
| 1 | 200 | 0.5+1.5 | 112.1±2.21 | 112.63 | -0.5339 | 32.29±1.16 | 32.37 | -0.0756 |
| 2 | 400 | 0.5+1.5 | 165.9±1.87 | 164.83 | 1.07 | 55.31±3.16 | 55.16 | 0.1511 |
| 3 | 600 | 0.5+1.5 | 195.6±3.73 | 196.13 | -0.5339 | 65.61±0.87 | 65.69 | -0.0756 |
| 4 | 200 | 0.75+1.25 | 58.5±2.09 | 58 | 0.4994 | 42.45±0.98 | 42.34 | 0.1061 |
| 5 | 400 | 0.75+1.25 | 106.9±3.48 | 107.9 | -0.9989 | 64.67±2.01 | 64.88 | -0.2122 |
| 6 | 600 | 0.75+1.25 | 137.4±3.59 | 136.9 | 0.4994 | 75.26±1.63 | 75.15 | 0.1061 |
| 7 | 200 | 1+1 | 268.3±3.17 | 268.29 | 0.0344 | 40.67±1.59 | 40.7 | -0.0306 |
| 8 | 400 | 1+1 | 357.2±2.78 | 357.28 | -0.0689 | 62.43±1.86 | 62.37 | 0.0611 |
| 9 | 600 | 1+1 | 425.4±4.71 | 425.38 | 0.0344 | 71.74±1.38 | 71.77 | -0.0306 |
Experimental runs and their observed values of dependent variables.
Characterization of Vildagliptin nanoparticles
Particle size (PS) and Polydispersity index (PDI)
PS and PDI of formulated nanoformulations were analyzed using Zetasizer (Nano ZS, Malvern Instruments Ltd., Worcestershire, WR14 1XZ, United Kingdom). All the samples were suitably diluted with Millipore water. The refractive index was set to 1.33, and samples were analyzed at 25°C.19
Entrapment Efficiency (EE)
The percentage EE of the formulated Vildagliptin nanoformulations was determined by the indirect method, i.e., the amount of unentrapped drug was determined.20 A known volume of Vildagliptin nanoformulations was transferred into 2 mL of centrifuge tubes and centrifuged (KUBOTA 7000, Japan) at 35,000 rpm at 4°C for 60 min. The supernatant was collected, and a suitable dilution was made with 0.1 N NaOH. The samples were analyzed using a UV-spectrophotometer (Shimadzu 1900, Japan) at 216 nm. Consequently, the % EE was calculated by the following formula:
Percentage yield determination
The percentage yield of formulated nanoformulations was determined by taking the dry weight of the nanoformulation21 and using the following formula:
FTIR Spectroscopy
A FTIR spectrophotometer (DRS 8000, Shimadzu IR Affinity-1) was used to obtain the FTIR spectra of VLD, Eudragit RL 100, and VLD-Eud NPs. The spectra were recorded in the wavelength range of 4000-400 cm-1. Further, the compatibility between drug and polymer was determined.22 Transmission Electron Microscopy
High-Resolution Transmission Electron Microscopy was used to study the morphology of optimized nanoparticles (F6). A drop of nanoformulation was deposited on a 200-mesh carbon-coated copper grid and stained with a 2% w/v phosphotungstic acid solution for 2 min. The grid was then air-dried and examined with a TEM (200 kV and 40,000 X) to capture images from a randomly selected area.23
In vitro drug release studies
In vitro drug release from Vildagliptin nanoformulations was studied using the USP dissolution apparatus (ELETROLAB TDT-06L). Throughout the study, the stirring rate (50 rpm), volume of dissolution medium (500 mL), and temperature (37±0.2°C) were maintained. The 20 mg equivalent weight of Vildagliptin nanoformulation was placed into a dialysis bag (previously soaked in dissolution media). The initial 2 hr dissolution experiment was conducted in 0.1 N HCl and then continued with pH-7.4 phosphate buffer. A 5 mL sample was withdrawn at predetermined time intervals up to 24 hr. The sink condition was maintained by adding an equal volume of fresh dissolution medium. The sample was appropriately diluted and filtered before being subjected to a UV spectrophotometer analysis at 208 nm.24
Release kinetics
To comprehend the mechanism of drug release, in vitro release data were fitted into various kinetic models, such as the zero order, first order, Higuchi, and Korsmeyer-Peppas models. When the mechanism is not well understood or when multiple types of release phenomena are present, these chosen models are frequently utilized to represent the drug release data from the polymeric drug delivery system. The accuracy of the fit was assessed for each model using the coefficient of correlation (R2).25
Stability study
The optimized formulation (F6) was stored for 3 months in three different tightly sealed, amber-colored glass bottles. According to International Council for Harmonization (ICH) recommendations, the stability tests of the nanoparticles were carried out at 25°C±2°C and RH 65%. Formulation (F6) was examined for % EE and particle size over a 3-month period with a one month sampling interval.26
RESULTS
Optimization of formulated nanoformulations
Optimization of formulated VLD-Eud NPs was done by using two factors and three levels with the help of DoE software. The nanoparticles were prepared by combined technique of solvent evaporation and nanoprecipitation method using polymer eudragit and poloxamer 188 as stabilizer. To determine how independent factors (X1, and X2) affect dependent variables (Y1 and Y2), a 32 response surface methodology was used. Figures 1a and 2a of 3D response surface plots and Figures 2b and 2b of 2D counter plots were created to investigate the impacts of an independent variable. The results of particle size and EE for 9 experimental runs are listed in Table 2 and are in the ranges of 58.5±2.02 nm to 425.4±4.71 nm and 32.29±1.16 to 75.26±1.63% respectively. Polynomial equations and counterplots are derived to study the mathematical correlation between independent and dependent variables. The correlation coefficients (R2) value for the quadratic model for the Y1 and Y2 responses was 0.9999 and 0.9998 respectively and is presented in Table 3. The responses for particle size (Y1) and EE (Y2) were calculated using the following formulae. ANOVA models for Y1 and Y2 are presented in Table 4.
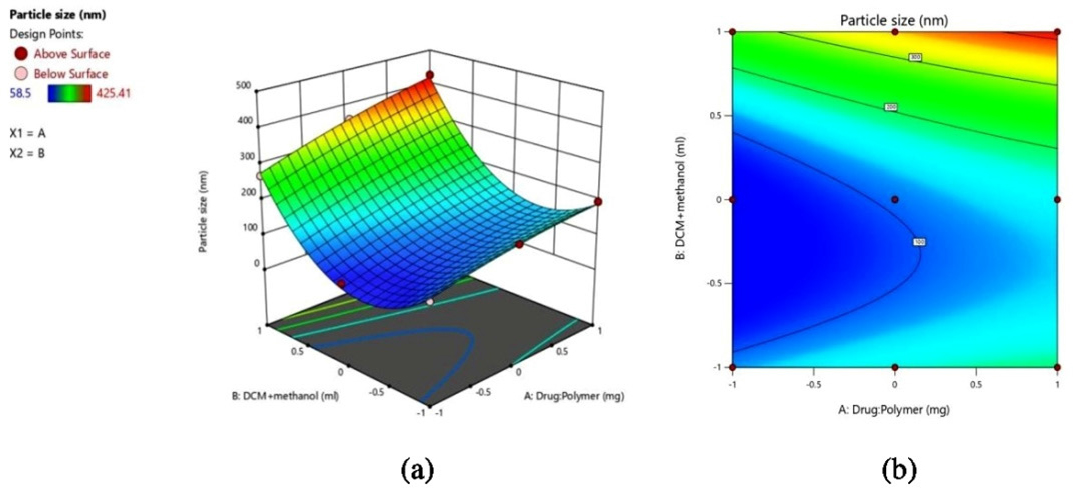
Figure 1.
(a) 3D response surface plot and (b) 2D contour plot of particle size.
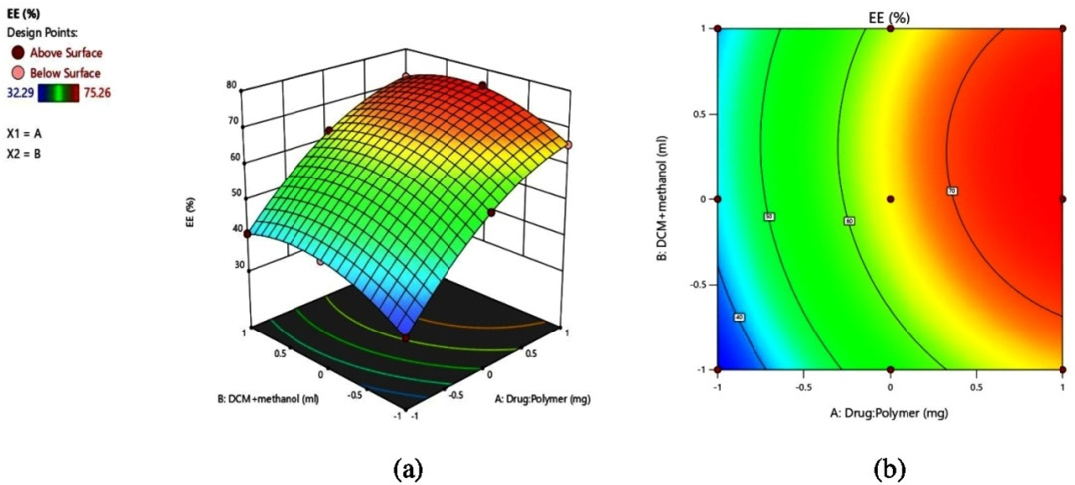
Figure 2.
(a) 3D response surface plot and (b) 2D contour plot of % EE.
| Models | R2 | Adjusted R2 | Predicted R2 | Std dev. | Press | Remarks |
|---|---|---|---|---|---|---|
| Response Y 1 Particle size | ||||||
| Linear | 20.72 | 0.9824 | 0.9718 | 0.9315 | 8327.94 | |
| 2FI | 8.59 | 0.9982 | 0.9951 | 0.9754 | 2990.92 | |
| Quadratic | 1.27 | 1 | 0.9999 | 0.9992 | 97.63 | Suggested |
| Response Y 2 EE | ||||||
| Linear | 3.92 | 0.9575 | 0.932 | 0.8786 | 219.04 | |
| 2FI | 5.01 | 0.9582 | 0.8886 | 0.4362 | 1017.13 | |
| Quadratic | 0.2317 | 0.9999 | 0.9998 | 0.9982 | 3.26 | Suggested |
Summary of results of regression analysis for responses Y1 and Y2.
| Response Y1: Particle size | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-value | p-value | Remarks |
| Model | 1.22E+05 | 6 | 20270.53 | 12613.85 | <0.0001 | Significant |
| X1-Polymer | 17012.31 | 1 | 17012.31 | 10586.34 | <0.0001 | |
| X2-DCM+Methanol | 1.03E+05 | 2 | 51233.75 | 31881.5 | <0.0001 | |
| X1 X2 | 1925.05 | 2 | 962.53 | 598.96 | 0.0017 | |
| X12 | 218.34 | 1 | 218.34 | 135.86 | 0.0073 | |
| Response Y 2 : EE | ||||||
| Model | 1804.11 | 6 | 300.69 | 5598.77 | 0.0002 | Significant |
| X1-Polymer | 1574.64 | 1 | 1574.64 | 29319.87 | <0.0001 | |
| X2-DCM+Methanol | 152.84 | 2 | 76.42 | 1422.98 | 0.0007 | |
| X1 X2 | 1.39 | 2 | 0.6959 | 12.96 | 0.0716 | |
| X12 | 75.24 | 1 | 75.24 | 1400.89 | 0.0007 | |
ANOVA models for Y1 and Y2.
Characterization of nanoformulations
Particle size (PS) and Polydispersity index (PDI)
The PS and PDI of the formulated Vildagliptin nanoformulations F1 to F9 were analyzed by using a Zeta nano ZS (Malvern instrument Ltd). All the nanoformulations were found PS and PDI in the range between 58.5±2.02 nm to 425.4±4.71 nm and 0.27±0.036 to 0.52±0.086 respectively.
Entrapment Efficiency (EE)
The EE of the formulated nanoformulations was found in the range between 32.29±1.16 to 75.26±1.63 as presented in Table 2. The effects of the VLD to Eud ratio on the EE are predicted by the 3D counter-plot from Design-Expert Figure 2b. The formulation F6 shows 75.26±1.63 EE at higher drug: polymer (1:6) ratio.
Percentage yield determination
The % production yield of all the nanoformulations was calculated, ranging from 54.7±3.45 to 84.4±1.51% at constant polymer concentration.
FTIR Spectroscopy
The FTIR spectra of (a) VLD, (b) Eudragit RL 100, and (c) VLD-EudNPs are shown in Figure 3. In the spectrum of VLD-Eud peaks, were appeared without any significant deviation from the VLD peaks.
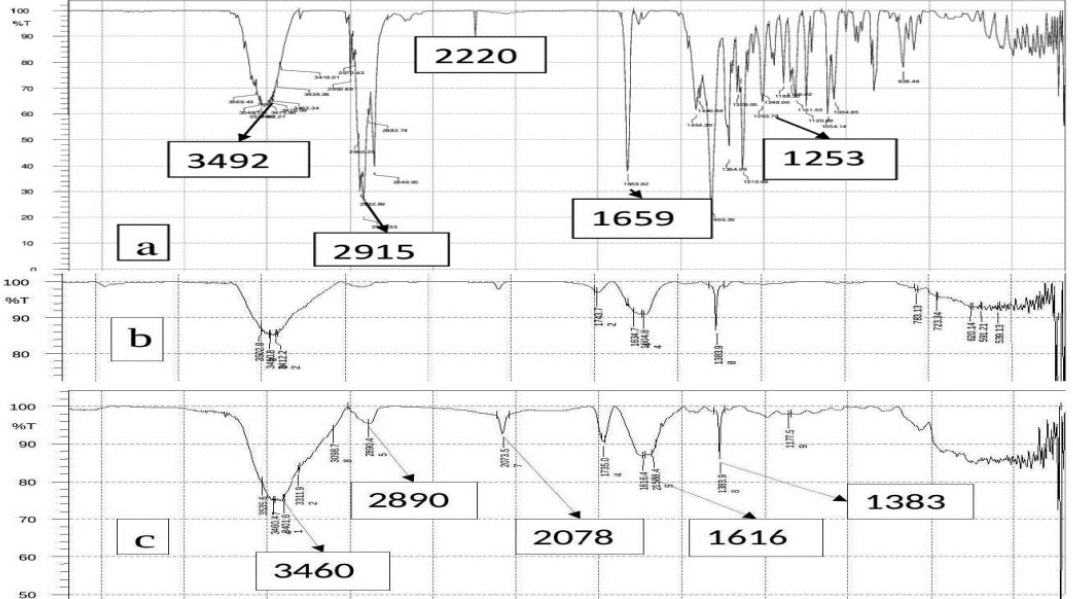
Figure 3.
The FTIR spectra of (a) VLD, (b) Eud, (c) VLD-Eud NPs.
Transmission Electron Microscopy
High-Resolution Transmission Electron Microscopy was used to study the morphology of optimized nanoparticles (F6), and its TEM image is shown in Figure 4.
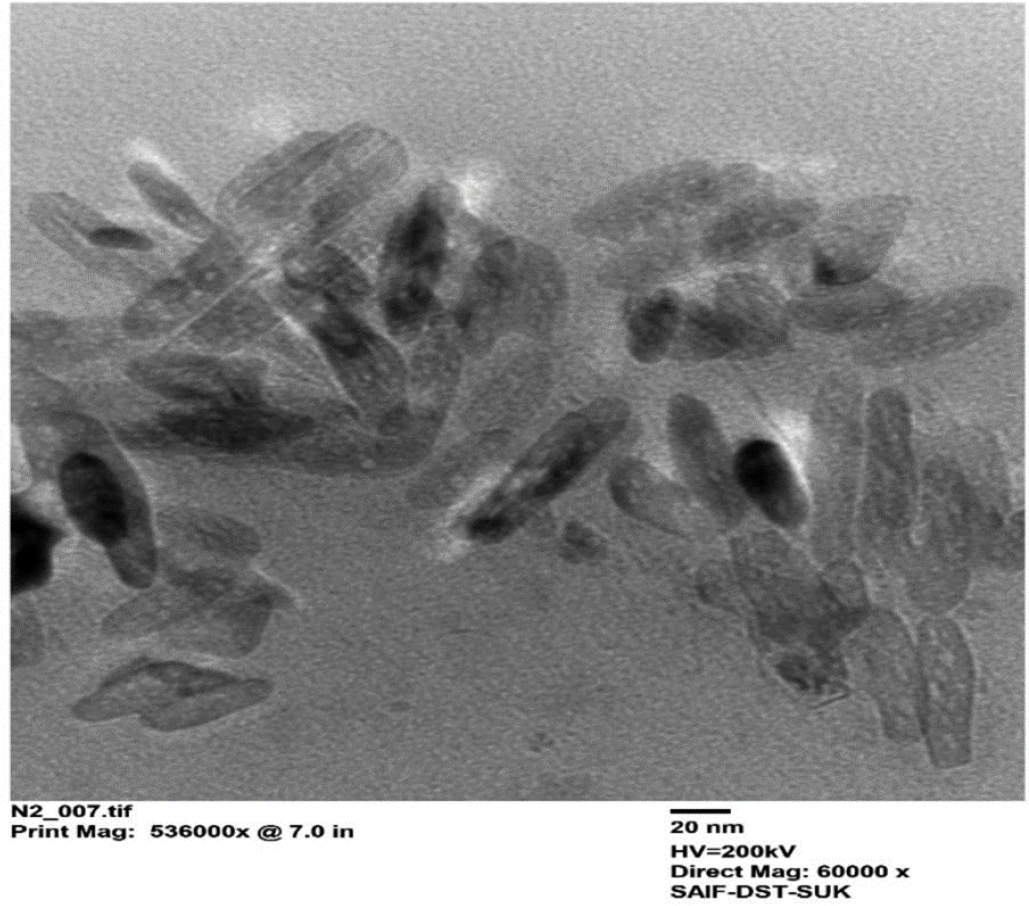
Figure 4.
TEM image of optimized Vildagliptin nanoformulation.
In vitro drug release studies
In vitro drug release of formulated nanoformulations of the VLD-Eud NPs was studied for 24 hr. The dissolution profiles of pure VLD and all formulated VLD-Eud NPs are shown in Figure 5. Each batch of the developed VLD-Eud NPs exhibits a sustained, delayed release action over the course of 24 hr. The nanoparticles exhibited prolonged drug release patterns, with around 15-25% of the drug release during the first 6 h, followed by about 68-89% of the drug release in 24 hr, and from the free drug solution >90% of the drug release was found within 2 hr.
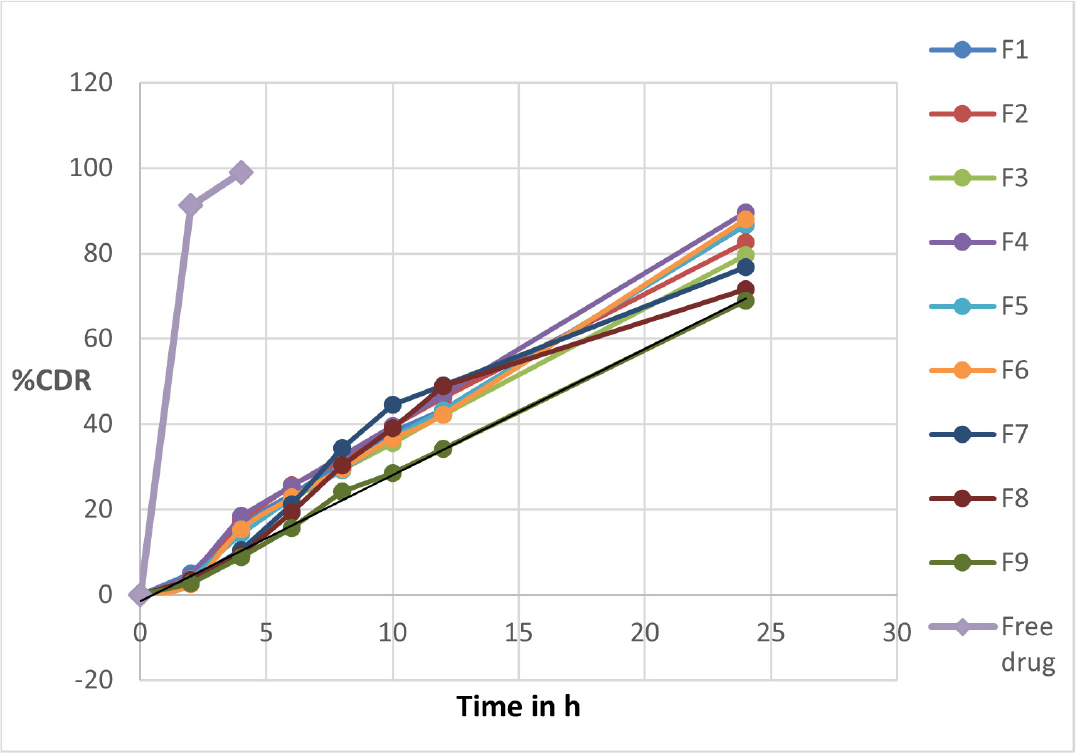
Figure 5.
Dissolution profiles of pure VLD and all formulated VLD-Eud NPs.
Release kinetics
To determine the drug release kinetics, release data were fitted to various kinetic models. The drug release mechanism for each batch is presented in Table 5.
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
|---|---|---|---|---|---|---|---|---|---|
| Zero order | 0.936 | 0.956 | 0.998 | 0.996 | 0.985 | 0.992 | 0.949 | 0.949 | 0.996 |
| First order | 0.992 | 0.933 | 0.959 | 0.937 | 0.969 | 0.966 | 0.990 | 0.987 | 0.972 |
| Higuchi | 0.924 | 0.918 | 0.878 | 0.896 | 0.916 | 0.901 | 0.905 | 0.899 | 0.870 |
| Korsmeyer-Peppas | 0.950 | 0.961 | 0.974 | 0.952 | 0.948 | 0.957 | 0.967 | 0.972 | 0.987 |
List of the drug release mechanism.
Stability study
Stability studies were conducted for optimized VLD-Eud NPs (F6) using particle size and EE% as the main parameters.
DISCUSSION
The present study deals with the formulation of Vildagliptin-loaded nanoformulations and their evaluation. The nanoformulations were optimized by a 32 factorial design. The data obtained is subjected to analytical treatment by Design Expert 13 software. Further, equations were drawn for responses. In order to comprehend the primary effects and interactions of the independent variables, 3D response surface graphs were obtained (Figure 1a and 2a of 3D).25, 27
According to the 2D and 3D plots for particle size, it was observed that particle size increased when the content of eudragit was increased. This is obvious as the increased polymer concentration leads to the deposition of more polymer molecules on each other, which results in an increase in size. In the case of the composition of the solvent system (X2), as the DCM content increased from 25% to 37.5% (respective to overall composition of mobile phase), the size of the nanoparticle decreased. The probable reason for this may be the steric hindrance provided by the solvent system for the deposition of polymer molecules. Further increase in its concentration to 50% has resulted into sharp rise in particle size. The result of EE has witnessed that, as the content of eudragit increases, the EE increases up to certain extent, further rise in content of eudragit (400 mg to 600 mg) do not increase the EE significantly. The increased concentration of polymer has increased the space for entrapment of drug which resulted into higher EE. The insignificant change in EE after certain level may be attributable to excessive denser polymer network which hinder the entry of drug molecules into polymeric network. Further, it has been observed that the composition of the solvent system (X2) does not play any significant role in the EE. This synergistic effect of polymer concentration on particle size and EE is also witnessed by higher positive values of coefficients for X1 in polynomial equations. Further, the small value of the coefficient of X2 in the EE equation is an indication of its insignificant role in EE. The model fit study has suggested that the quadratic model is the best fit model to obtain the results in design domain space with a correlation coefficient (R2) value >0.998. A further ANOVA study has highlighted the significance of the model with p<0.0002.
The heterogeneity of particle size in NPs is measured by PDI. The PDI values of all the nanoformulations were found in the range of 0.27±0.036 to 0.52±0.086, showing the narrow size distribution.28 For the formulations to have a uniform size distribution, a lower value of PDI is extremely desirable. The % production yield of all the nanoformulations was found to increase with an increase in polymer concentration.
VLD-Eud nanoparticle spectra revealed the presence of characteristic peaks of the functional groups in the polymer and pure drug, indicating the absence of molecular interactions between drug and polymer, which witnessed the compatibility between polymer and drug.29 The TEM study has shown the cylindrical shaped nanoparticles that were present as individual moiety.
The in vitro drug release study was conducted for all the formulated nanoformulations, and for the initial 2 hr in the 0.1 N HCl, it found only a small amount of drug release (<5%) and >90% of the drug released from the free drug solution. This may be attributed to the pH dependent solubility behavior of the polymer used. The eudragit RL 100 is insoluble at acidic pH, so the small quantity of drug release may be associated with an unentrapped or adsorbed drug. In the basic medium (phosphate buffer pH 7.4), prolonged drug release may be attributed by swelling of the polymeric chain. The findings also showed that when the polymer concentration increased, there was a significant decrease in the amount of VLD released from nanoparticles. These outcomes might be the consequence of an increase in the polymer concentration causing an increase in the polymer thickness of the nanoparticles, which in turn caused an increase in the length of the polymer membrane pathway.30
The kinetic treatment for the release data has depicted that the regression coefficient value (R2) was maximum for zero order, which indicated the concentration independent release characteristic of prepared nanoformulations.
During 3 months of accelerated stability study, the particle size and EE% of an optimized formulation (F6) was not shown any significant change, which depicts its stability of prepared formulations.
CONCLUSION
The VLD-Eud NPs were successfully prepared with solvent evaporation and nanoprecipitation method. The QbD approach was effectively utilized for the optimization of the nanoparticles. The study’s findings indicate that the innovative formulations prepared exhibit sustained releasing activity for 24 hr; additionally, they solve the issue of frequent dosing of VLD for efficient diabetic therapy. Thus, the present work concludes that prepared and optimized VLD-Eud NPs could be a better approach for sustained delivery of Vildagliptin for the management of diabetes mellitus as compared to traditional drug delivery.
Cite this article:
Ammanage AS, Mastiholimath VS. A Systematic Approach to Vildagliptin-Eudragit RL 100 Coated Nanoparticles by Combined Techniques of Solvent Evaporation and Nano-precipitation Using Quality by Design Concept. Int. J. Pharm. Investigation. 2024;14(1):250-7.
ACKNOWLEDGEMENT
The authors are thankful to Ajanta Pharma Ltd., Mumbai, India, and Centaur Pharmaceuticals Pvt. Ltd., Pune, for providing free gift samples of Vildagliptin and Eudragit RL 100 respectively.
ABBREVIATIONS
| ANOVA | Analysis of variance |
|---|---|
| DCM | Dichloromethane |
| DoE | Designs of Experiments |
| DM | Diabetes mellitus |
| DPP-4 | Dipeptidyl peptidase-4 |
| EE | Entrapment efficiency |
| GIP | Gastric inhibitory polypeptide |
| GLP-1 | Glucagon-like peptide-1 |
| mg | Milligram |
| mL | Milliliter |
| nm | Nanometer |
| NDDSs | Nanoparticulate drug delivery systems |
| QbD | Quality by design |
| R2 | Coefficient of correlation |
| RH | Relative humidity |
| SD | Standard deviation |
| TEM | Transmission electron microscopy |
| USP | United State Pharmacopeia |
| VLD-Eud NPs | Vildagliptin-Eudragit RL 100 coated nanoparticles |
| X1 | Eudragit RL 100 (mg) |
| X2 | The composition of the organic solvent |
| Y1 | Particle size (nm) |
| Y2 | Entrapment efficiency (%) |
References
- Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377-90. [PubMed] | [CrossRef] | [Google Scholar]

- Nair AB, Sreeharsha N, Al-Dhubiab BE, Hiremath JG, Shinu P, Attimarad M, et al. HPMC- and PLGA-based nanoparticles for the mucoadhesive delivery of sitagliptin: optimization and evaluation in rats. Materials (Basel). 2019;12(24):4239-55.
https://doi:10.3390/ma12244239
[PubMed] | [CrossRef] | [Google Scholar]
- Aschner P. Recent advances in understanding/managing type 2 diabetes mellitus. F1000Res. 2017:6 [PubMed] | [CrossRef] | [Google Scholar]

- Nagaraja SH, Dhubiab BE, Tekade RK, Venugopala KN, Ghorpade RV, Meravanige G, et al. Novel preparation and effective delivery of mucoadeshive nanoparticles containing anti-diabetic drug. Indian J Pharm Educ Res. 2019;53(2s) [CrossRef] | [Google Scholar]

- Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(1) [PubMed] | [CrossRef] | [Google Scholar]

- Heo CU, Choi CI. Current progress in pharmacogenetics of second-line antidiabetic medications: towards precision medicine for type 2 diabetes. J Clin Med. 2019;8(3):393-420. [PubMed] | [CrossRef] | [Google Scholar]

- Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35(6):992-1019.
https://doi:10.1210/er.2014-1035
[PubMed] | [CrossRef] | [Google Scholar]
- Fayyaz S, Ahmed D, Khalid S, Khan SN, Shah MR, Choudhary MI, et al. Synthesis of vildagliptin conjugated metal nanoparticles for type II diabetes control: targeting the DPP-IV enzyme. New J Chem. 2020;44(47):20853-60. [CrossRef] | [Google Scholar]

- Keating GM. Vildagliptin: a review of its use in type 2 diabetes mellitus. Drugs. 2010;70(16):2089-112. [PubMed] | [CrossRef] | [Google Scholar]

- Patil GB, Patil ND, Deshmukh PK, Patil PO, Bari SB. Nanostructured lipid carriers as a potential vehicle for carvedilol delivery: application of factorial design approach. Artif Cells Nanomed Biotechnol. 2016;44(1):12-9. [PubMed] | [CrossRef] | [Google Scholar]

- Younis N, Shaheen MA, Abdallah MH. Silymarin-loaded Eudragit(®) RS100 nanoparticles improved the ability of silymarin to resolve hepatic fibrosis in bile duct ligated rats. Biomed Pharmacother. 2016;81:93-103. [PubMed] | [CrossRef] | [Google Scholar]

- Wilson B, Samanta MK, Santhi K, Kumar KPS, Paramakrishnan N, Suresh B, et al. Targeted delivery of tacrine into the brain with polysorbate 80-coated poly(n-butylcyanoacrylate) nanoparticles. Eur J Pharm Biopharm. 2008;70(1):75-84. [PubMed] | [CrossRef] | [Google Scholar]

- Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557-70. [PubMed] | [CrossRef] | [Google Scholar]

- Prasanthi D, Kumari JK, S Hymavathi H. Formulation and evaluation of floating polymeric nanoparticles of linagliptin in capsules. J Young Pharm. 2020;12(2s) [CrossRef] | [Google Scholar]

- Rahman Z, Zidan AS, Habib MJ, Khan MA. Understanding the quality of protein loaded PLGA nanoparticles variability by Plackett-Burman design. Int J Pharm. 2010;389(1-2):186-94.
https://doi:10.1016/j.ijpharm.2009.12.040
[PubMed] | [CrossRef] | [Google Scholar]
- Bonthagarala B, Dasari V, Kotra V, Swain S, Beg S. Quality-by-Design based development and characterization of pioglitazone loaded liquisolid compact tablets with improved biopharmaceutical attributes. J Drug Deliv Sci Technol. 2019;51:345-55. [CrossRef] | [Google Scholar]

- Ismail R, Sovány T, Gácsi A, Ambrus R, Katona G, Imre N, et al. Synthesis and statistical optimization of poly (lactic-co-glycolic acid) nanoparticles encapsulating GLP1 analog designed for oral delivery. Pharm Res. 2019;36(7):99 [PubMed] | [CrossRef] | [Google Scholar]

- Melo CM, Cardoso JF, Perassoli FB, de Oliveira Neto AS, Pinto LM, de Freitas Marques MB, et al. Amphotericin B-loaded Eudragit RL100 nanoparticles coated with hyaluronic acid for the treatment of vulvovaginal candidiasis. Carbohydr Polym. 2020;230:115608 [PubMed] | [CrossRef] | [Google Scholar]

- Alva C, Vidakovic I, Lorber B, Schachner-Nedherer AL, Zettl M, Khinast J, et al. Can liposomes survive inkjet printing? The effect of jetting on key liposome attributes for drug delivery applications. J Pharm Innov. 2022:1-9. [PubMed] | [CrossRef] | [Google Scholar]

- Khaira R, Sharma J, Saini V. Development and characterization of nanoparticles for the delivery of gemcitabine hydrochloride. Scientific World Journal. 2014;2014:560962 [PubMed] | [CrossRef] | [Google Scholar]

- Pandey AP, More MP, Karande KP, Chitalkar RV, Patil PO, Deshmukh PK, et al. Optimization of desolvation process for fabrication of lactoferrin nanoparticles using quality by design approach. Artif Cells Nanomed Biotechnol. 2017;45(6):1-14. [PubMed] | [CrossRef] | [Google Scholar]

- Shirsath NR, Goswami AK. Vildagliptin-loaded gellan gum mucoadhesive beads for sustained drug delivery: design, optimisation and evaluation. Mater Technol. 2021;36(11):647-59. [CrossRef] | [Google Scholar]

- Malatesta M. Transmission electron microscopy for nanomedicine: novel applications for long-established techniques. Eur J Histochem. 2016;60(4):2751 [PubMed] | [CrossRef] | [Google Scholar]

- Waghulde M, Rajput R, Mujumdar A, Naik J. Production and evaluation of vildagliptin-loaded poly(dllactide) and poly(dllactide-glycolide) micro-/ nanoparticles: response surface methodology approach. Drying Technol. 2019;37(10):1265-76. [CrossRef] | [Google Scholar]

- Deshmukh RK, Naik JB. Optimization of sustained release aceclofenac microspheres using response surface methodology. Mater Sci Eng C Mater Biol Appl. 2015;48:197-204. [PubMed] | [CrossRef] | [Google Scholar]

- Hernandez-Patlan D, Solis-Cruz B, Cano-Vega MA, Beyssac E, Garrait G, Hernandez-Velasco X, et al. Development of chitosan and alginate nanocapsules to increase the solubility, permeability and stability of curcumin. J Pharm Innov. 2019;14(2):132-40. [CrossRef] | [Google Scholar]

- Nayak AK, Pal D, Santra K. Array. Int J Biol Macromol. 2014;65:329-39. [PubMed] | [CrossRef] | [Google Scholar]

- Alam S, Aslam M, Khan A, Imam SS, Aqil M, Sultana Y, et al. Nanostructured lipid carriers of pioglitazone for transdermal application: from experimental design to bioactivity detail. Drug Deliv. 2016;23(2):601-9. [PubMed] | [CrossRef] | [Google Scholar]

- Waghulde MR, Naik JB. Comparative study of encapsulated vildagliptin microparticles produced by spray drying and solvent evaporation technique. Drying Technol. 2017;35(13):1644-54. [CrossRef] | [Google Scholar]

- Naik JB, Waghulde MR. Development of vildagliptin loaded Eudragit® microspheres by screening design: evaluation. J Pharm Investig. 2018;48(6):627-37. [CrossRef] | [Google Scholar]


