ABSTRACT
Background
Resveratrol (3,4,5-trihydroxystilbene), a polyphenol derived from plant sources, despite its cardioprotective properties, has limited therapeutic impact due to its low in vivo bioavailability and rapid metabolism, which are major barriers to its effectiveness. Piperine, a nitrogenous substance, has well-established bioenhancer activity and cardioprotective potential. The objective of the study was to investigate the cardioprotective potential of resveratrol and piperine on isoproterenol-induced myocardial infarction in rats, both alone and in combination and to assess the oral bioavailability of resveratrol when co-administered with piperine.
Materials and Methods
Resveratrol, piperine, and their combinations (20 mg/kg, oral) were administered to rats as pre-treatments for 28 days. Myocardial infarction was induced by giving isoproterenol (85 mg/kg) subcutaneously. Pharmacokinetic parameters were analyzed by UFLC using rat plasma. The chromatographic separation was achieved on Eclipse plus C18Column (25 cm x 5 cm x 4.6μ) with L1 packing using mobile phase containing mixer of milli Q water and methanol (30:70% v/v) at a flow rate of 1 mL/min.
Results
The treatment group exhibited a considerable (p<0.001) improvement in heart rate, biomarker, antioxidant, and histological changes compared to the isoproterenol group, confirming the effectiveness of resveratrol. A significant improvement in pharmacokinetic parameters of resveratrol was observed in plasma samples of animals treated with a combination, as compared to those treated with resveratrol alone.
Conclusion
The combination of resveratrol and piperine significantly reduced the risk of myocardial infarction induced by isoproterenol when compared to the group that received resveratrol alone. This effect can be attributed to the cardioprotective potential of resveratrol and piperine, as well as piperine’s ability to enhance the oral bioavailability of resveratrol.
INTRODUCTION
Around one-third of all fatalities are caused by Cardiovascular Disease (CVD), a significant and rapidly rising issue. New risk factors have emerged due to changes in people’s lifestyles in developed and developing countries, increasing the risk of cardiovascular disease.1 According to the World Health Organization report, cardiovascular disease causes more than 30% of all fatalities globally, making it the leading cause of mortality. As a result, researchers are now focusing on cardiovascular disease because it affects most of the population.2
Myocardial Infarction (MI), sometimes referred to as heart attack, is brought on by inadequate blood flow to the heart, which damages the heart muscle. The leading cause of death and disability worldwide is MI.3 The use of conventional medications such as statins, beta blockers, narcotics, and ACE inhibitors to treat MI has proven successful but not without side effects. As a result, interest in using natural products has increased because they may be just as effective and more tolerable.4 The use of herbal medicines and supplements has increased tremendously over the past few decades, and many people worldwide now rely on them.5
Resveratrol (RSV) is a polyphenolic phytoalexin present in red wine, peanuts, the skin of grapes, blueberries, raspberries, mulberries, and blueberries reported to possess a number of cardioprotective mechanisms, particularly anti-inflammatory, antioxidant, and anti-fibrotic properties. RSV has cardioprotective effects by inhibiting prohypertropic signaling molecules, improving myocardial Ca2+ handling, regulating blood pressure, and reducing oxidative stress and inflammation.6,7 Having all the potential to be a potent drug with favorable physicochemical properties (log P 3.1), the use of RSV has been limited due to the poor oral bioavailability, which makes them unavailable in their optimum concentration in the systemic circulation to show therapeutic activity.8 The rapid phase II metabolism of RSV into RSV-4’-O glucuronide, RSV-3’-O-glucuronide, and RSV-3-O-sulfate is the key reason for this. Further, the gut bacteria metabolize RSV into dihydroresveratrol, 3,4’-dihydroxy-trans- stilbene, and 3,4’-dihydroxybibenzyl.9 Consequently, different approaches have been considered to improve its oral bioavailability.
Piperine (PIP), a member of the Piperaceae family of peppers, is found in black pepper (P. nigrum), white pepper (P. alba), and long pepper (P. longum). Recently, it has attracted much attention for its wide range of benefits, including anti-cancer, anti-inflammatory, anti-viral, pesticide, anti-depressant, anti-Alzheimer, cardioprotective potential and most importantly, as an oral bioavailability enhancer. The inhibition of drug-metabolizing enzymes, inhibition of the cell pump responsible for drug elimination from cells, stimulation of absorption by gut amino acid transporters, inhibition of human P-glycoprotein and cytochrome P450 3A4 which contribute to a significant amount of first-pass metabolism, and inhibition of intestinal production of glucuronic acid are the mechanisms by which PIP increases drug bioavailability.10,11 PIP could even alter the pharmacokinetic parameters when combined with other drugs such as curcumin,12 silybin,13 ginsenoside,14 celecoxib,15 quercetin,16 and emodin.17
To date, no efforts have been made to explore the collective impact of RSV and PIP on cardioprotective functions, specifically MI. As far as our knowledge goes, this is the first study to determine the potential of PIP to enhance RSV’s ability to prevent MI induced by Isoproterenol (ISO). Additionally, this investigation aims to assess the oral bioavailability of RSV when administered alone and in conjunction with PIP.
MATERIALS AND METHODS
Materials
Pure resveratrol (98%) was obtained from Yarrow Chem Products, Mumbai, India. Piperine (98.5%) was purchased from Sigma Aldrich-Merck, Bengaluru, India. Biochemical kits were procured from Agappe Diagnostic Ltd., Bangalore, India. All additional reagents and solvents were of analytical grade and obtained from reputable suppliers.
Animal studies
Literatures reported that rats were suitable for assessing the cardio protectivity of the drug. The pharmacodynamic investigations were performed on mature male albino rats with a body weight of 175-250 g obtained from Adita Biosys Private Limited, Tumakuru (1868/PO/RcBt/S/16/CPCSEA). At the Central Animal House of the Institute, rats were acclimatized in polypropylene cages and provided standardized environments (25±3°C, a 12 hr dark-light cycle), with regular access to food and water, before studies. The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India, issued criteria that were followed to in all experiments, which were authorized by the Institutional Animal Ethics Committee.
Dose selection
Experimental design
Five groups of six male albino rats were used. The normal control group (I) received 1% tween-80, the toxic control group (II) received isoproterenol (85 mg/kg subcutaneously), groups III and IV received an oral dose of RSV and PIP (20 mg/kg each), respectively, and group V received an oral dose of both RSV (20 mg/kg) and PIP (20 mg/kg) for 28 days (Figure 1A).
Experimental protocols
Animals received ISO for two days in a row, 24 hr after the intervention. Each group’s symptoms and death were noted and compared to those of the rats’ given ISO alone. Blood was taken by cardiac puncture 48 hr after the first ISO dosage. Serum was separated by centrifugation and subjected to biochemical estimations of enzymes levels such as SGOT (Aspartate aminotransferase), SGPT (Alanine aminotransferase), CK-MB (Creatine Kinase Myocardial Band), CKNAC (N-acetyl-cysteine), Alkaline Phosphatase (ALP) and Lactate Dehydrogenase (LDH). Electrocardiographic (ECG) changes were recorded for all the animals. Then the rats were sacrificed under anesthesia and autopsied. The hearts from each group were removed and weighed. Half of the isolated heart was used for the preparation of tissue homogenate, which was subjected to analysis for Glutathione (GSH), Thiobarbituric Acid Reactive Substances (TBARS), Superoxide Dismutase (SOD), and catalase, and the remaining portion was used for histopathological analysis.
Figure 1:
(A) Rats receiving treatment (RSV and PIP were orally administered via oral gavage). (B) Computerized ambulatory ECG equipment was employed to capture the electrocardiogram signal of the mouse via electrode connection method.
Electrocardiography determination
48 hr after the first dose of ISO, ECG recordings were noted using computerized ambulatory ECG equipment to collect the mouse’s electrocardiogram signal (electrode connection method). The ECG leads were inserted into the front and hind legs of the dermal layer of the animals under anesthetic conditions by intraperitoneal administration of a combination of ketamine hydrochloride and xylazine (Figure 1B).
Biochemical estimation
Blood samples were drawn after the animals were sacrificed. The serum was separated by centrifugation. Separated serum was used to estimate biochemical enzymes, i.e., SGOT, SGPT, CK-MB, CK-NAC, ALP, and LDH, using standard enzymatic kits (Agappe Diagnostic Ltd, India).
The heart tissues were isolated, wiped clean of blood and bodily fluids, weighed, and stored at -80°C until the analysis was performed. During the experiment, heart samples were homogenized using a cold buffer. The homogenate’s clear supernatant obtained after the centrifugation (10,000 rpm using a cooling centrifuge, REMI, India) was utilized to estimate lipid peroxidation, SOD, catalase, and GSH.
Histopathological analysis
Impact of piperine on the pharmacokinetics of resveratrol
Two groups of six animals each were formed from the animal population. Group I animals were given orally with RSV (20 mg/ kg). Group II animals were given orally with RSV (20 mg/kg) and PIP (20 mg/kg). Under partial ether anesthesia, blood samples (0.5 mL) were taken at regular intervals over the course of 24 hr (0, 2, 4, 8, 16, and 24 hr) and then analyzed. Immediately following the removal of blood at each time point, normal saline (0.5 mL) was administered intraperitoneally to prevent hypovolemia. Plasma was separated from the blood samples by centrifuging them at 13,000 rpm for 5 min, which were stored at -40°C for UFLC analysis. Chromatographic development was achieved on Shimadzu Ultra Flow Liquid Chromatography (UFLC) equipped with a binary pump LC-20AD and Controller SPD-M20A along with UV Detector. LC Real-time Analysis (Lab solutions) software was utilized for data integration and post-run analysis purposes. Chromatographic separation was taken place on Eclipse plus C18 Column (25cm x 5cm x 4.6μ) with L1 packing. The mobile phase containing mixer of milli Q water and methanol (30:70% v/v) was selected as an optimum composition for symmetrical peak (Peak purity index 0.9999) at the flow rate of 1 mL/min. UV detection was carried out at 306 nm and 342 nm with 30 min run time at column temperature 25°C.21
Statistical analysis
Parameters are presented as mean±standard error, and significance was analyzed using a One-way analysis of variance followed by Tukey-Karmer multiple comparison tests. p<0.05 was considered statistically significant. The statistical program utilized for data analysis was GraphPad Prism 6.01.
RESULTS
Effect of resveratrol on electrocardiographic parameters
The most important diagnostic indicators for MI are electrocardiographic measurements. The disturbance or alterations in the subject’s ECG pattern caused by ISO can be used to interpret MI. ST-segment elevation in ISO injected group is suggestive of MI. ECG determination revealed a significant decrease in the heart rate of ISO control compared to normal control. Among all other groups, the combination group with RSV-PIP was the most effective against MI (Figure 2) (Table 1).
Effect of resveratrol on enzyme and biomarkers concentrations
Each treatment group, likely RSV, PIP, and the combination of RSV and PIP, demonstrated a considerable recovery of enzyme and biomarker levels compared to the ISO group. Among the other treatment groups, the RSV and PIP combination group had the most substantial cardioprotective effect (Table 2).
Effect on TBARS, GSH, SOD, and catalase activities
Table 3 shows the concentrations of LPO, SOD, catalase, and GSH in normal control and experimental groups. Compared to the normal control, the ISO group showed a higher level of LPO and significantly decreased SOD, catalase, and GSH activity, indicating oxidative stress and cardiac tissue damage. The activities of LPO, antiperoxidative enzymes, and GSH were considerably recovered in pre-treated groups (RSV, PIP, and the combination of RSV-PIP) as compared to ISO groups. The RSV-PIP combination exhibited the most potent effect compared to RSV alone.
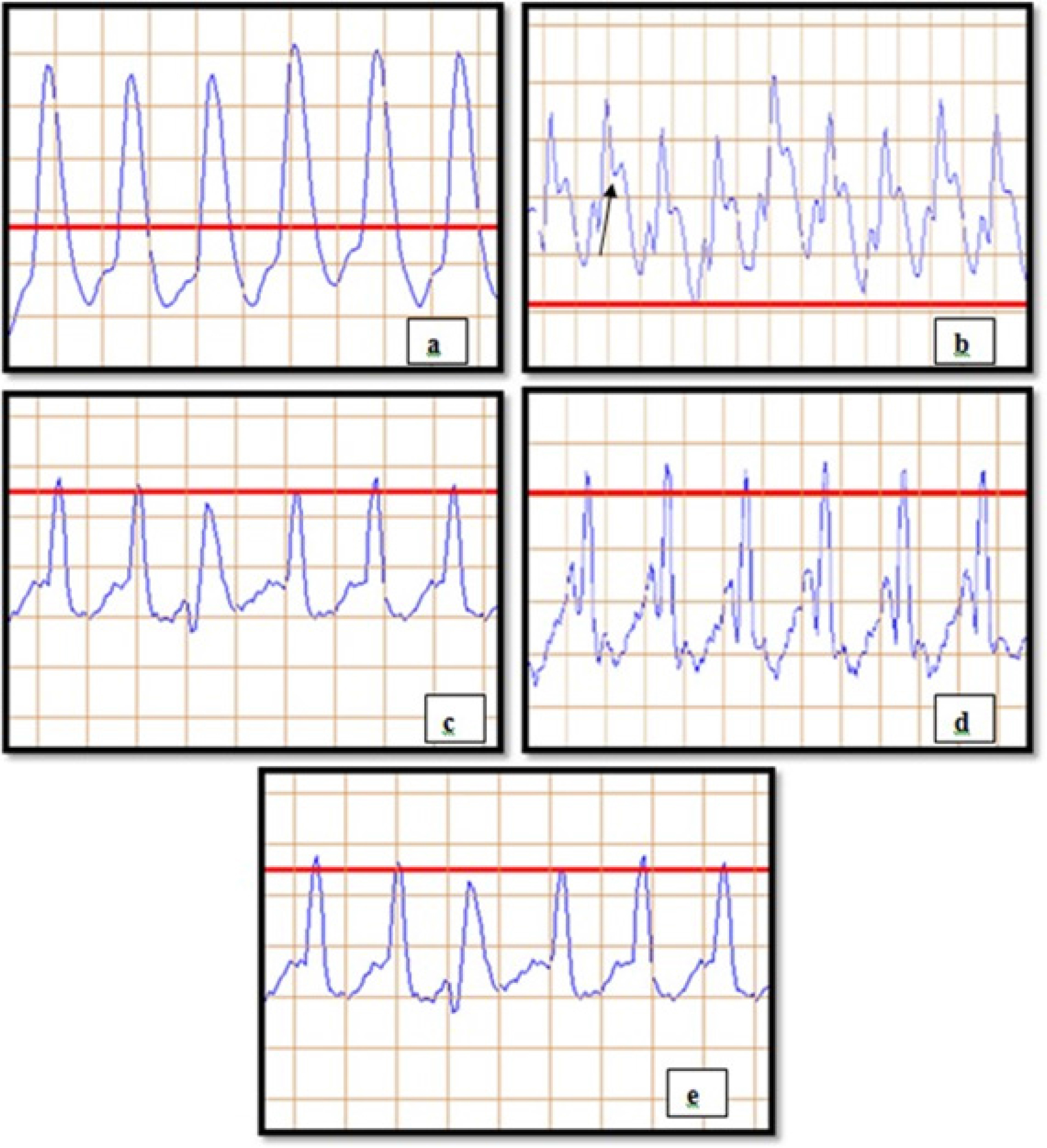
Figure 2:
Effect on electrocardiographic parameters: (a) NC (Normal control), (b) ISO (Isoproterenol), (c) RSV (Resveratrol), (d) PIP (Piperine), (e) RSV-PIP (Resveratrol- Piperine combination).
| Parameter | Heart rate (bpm) | R-R (ms) | P-R (ms) | P-Q (ms) | QT (ms) | QRS (ms) | ST (mV) |
|---|---|---|---|---|---|---|---|
| NC | 363.66±0.61 | 204.42±0.12 | 60.40±0.11 | 35.42±0.13 | 113.41±0.41 | 66.38±0.13 | 0.160±0.001 |
| ISO | 484.36±0.36* | 141.42±0.10* | 35.40±0.11* | 16.33±0.10* | 75.44±0.15* | 43.31±0.10* | 0.711±0.002* |
| RSV | 454.16±1.24a | 156.52±1.24a | 40.68±0.12a | 21.36±0.15 | 81.16±0.06a | 50.34±0.11a | 0.619±0.003a |
| PIP | 442.50±1.64a | 165.63±0.18a | 41.84±0.20a | 20.32±0.17a | 79.19±0.06a | 47.23±0.04a | 0.571±0.01a |
| RSV-PIP | 424.83±1.85ab | 173.97±1.03ab | 47.04±0.17ab | 25.12±0.06ab | 84.16±0.06ab | 55.40±0.13ab | 0.453±0.004ab |
Effect on electrocardiographic parameters.
| Parameters | SGPT | SGOT | CK-MB | CK-NAC | ALP | LDH |
|---|---|---|---|---|---|---|
| (U/L) | ||||||
| NC | 38.88±2.74 | 63.14±2.36 | 144.18±2.92 | 236.41±3.83 | 278.32±4.64 | 284.84±3.34 |
| ISO 85 mg/mL | 113.1±1.12* | 216.17±1.23* | 545.87±4.25* | 728.81±2.36* | 522.47±4.52* | 1152.09±4.09* |
| RSV | 82.95±0.78a | 133.44±0.50a | 333.92±4.7a | 428.54±2.78a | 441.34±0.52a | 714.99±2.80a |
| PIP | 95.18±1.28a | 205.89±0.50a | 508.33±3.32a | 605.17±1.40a | 489.48±2.11a | 986.94±2.77a |
| RSV-PIP | 73.89±0.92ab | 108.85±1.66ab | 292.29±2.89ab | 391.12±3.21ab | 391.49±2.70ab | 540.66±2.27ab |
Effect on serum cardiac marker enzymes.
| Parameters | LPO | GSH | SOD | Catalase |
|---|---|---|---|---|
| (μg/mg of protein) | ||||
| Normal control | 2.07±0.12 | 54.16±0.54 | 19.98±0.7 | 97.38±0.32 |
| ISO 85 mg/kg | 13.01±0.34* | 6.36±0.35* | 2.29±0.03* | 47.08±0.47* |
| RSV | 10.53±0.21# | 25.85±0.41a | 6.13±0.45# | 56.31±0.51a |
| PIP | 11.52±0.61a | 20.09±0.75a | 4.31±0.34# | 52.16±0.04a |
| RSV-PIP | 8.04±098ab | 32.06±0.86ab | 12.85±0.32ab | 62.63±0.03ab |
Effect on LPO, GSH, SOD, and catalase activities.
Histopathological observations
In response to ischemia, the separated hearts exhibited a variety of pathological alterations, including significant myocardial edema, fiber separation, and disruption of striation. The histopathological evaluations of a regular rat heart section (Group I) showed normal sized sub-circular/central nucleus, normal cardiac muscle, normal fibers with striations and no inflammatory cell infiltration (Figure 3a). However, we observed myocardial necrotic cell damage, neutrophil infiltration, interstitial edema, nuclear pyknosis, and inflammatory cells in the heart sections of ISO groups (Figure 3b). In group III and IV (RSV and PIP) sections mild inflammation induced by ischemia, focal aggregates in the form of neutrophil, lymphocyte and macrophages and few scattered aggregates of inflammatory cells were observed (Figure 3c and 3d). Compared to the RSV group, the tissue from the RSV-PIP group (group V) had a significant decline in the histopathological evaluations with slight inflammation (Figure 3e). Histopathological evaluation confirmed the restoration of cellular architecture in treatment groups compared to the disease group.
Impact of piperine on the pharmacokinetics of resveratrol
Compared to animals treated with pure RSV, the plasma samples from the RSV-PIP combination group showed significantly higher levels of RSV, as evidenced by the Cmax, Tmax, and AUC total values. Furthermore, the rats treated with RSV-PIP exhibited lower clearance and volume of distribution of RSV compared to those treated with RSV alone (Table 4). The pure RSV attained maximum plasma concentration of 7.39±0.13 μg/mL at 3 hr, whereas RSV-PIP exhibited maximum plasma concentration of 10.46±0.09 μg/mL at 3.5 hr (Figure 4). With the aid of piperine, resveratrol bioavailability may have been improved, but piperine also has the potential to enhance cardioprotection, which may have contributed to the improved cardioprotective impact.
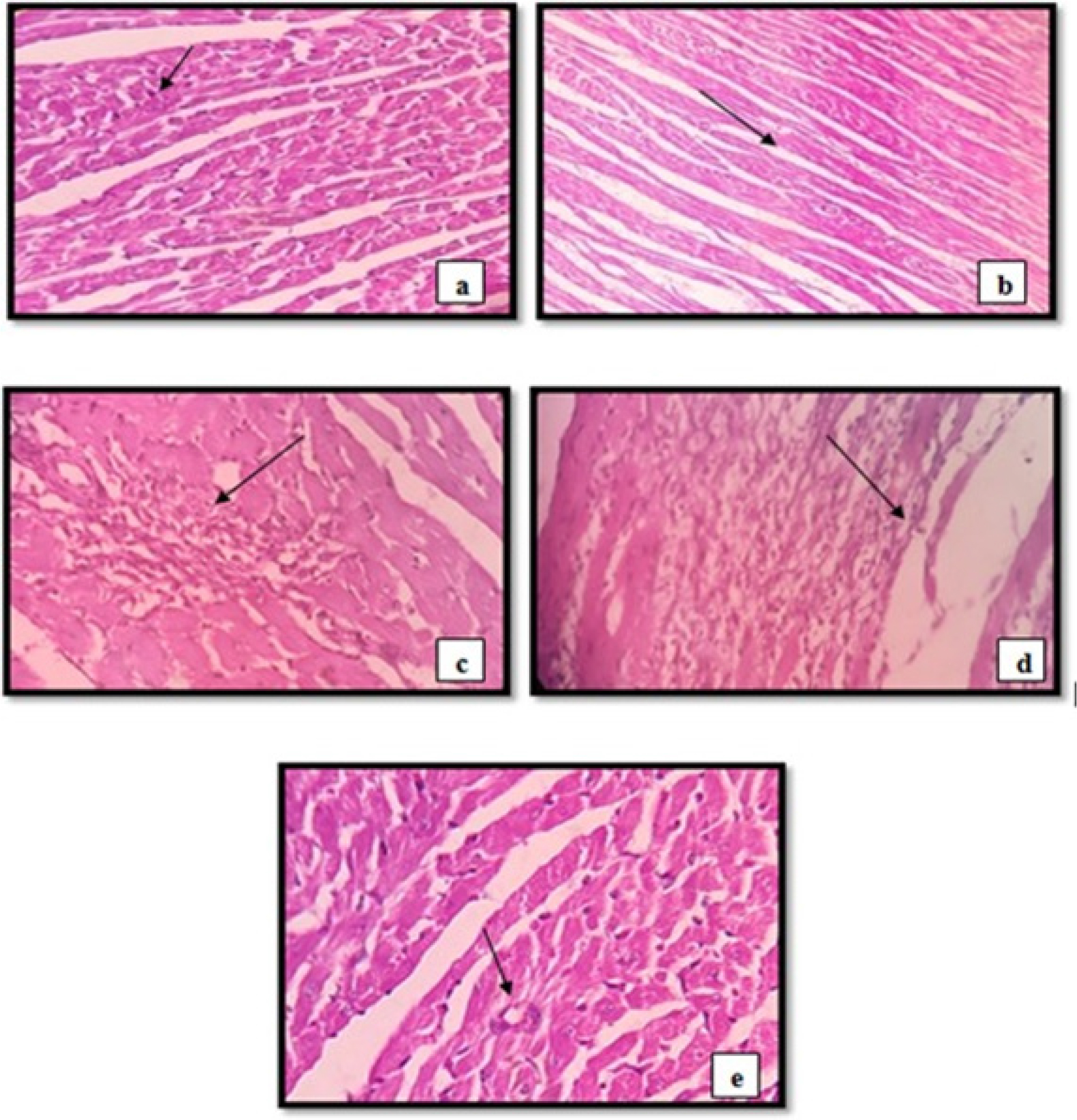
Figure 3:
Photomicrograph of hematoxylin and eosin (H&E) stained sectioned heart. (a) Normal group (NC) (Normal sized nucleus, normal cardiac muscle, sub-circular/ central nucleus), (b) ISO control group (myocardial necrotic cell damage, nuclear pyknosis, and inflammatory cells), (c) RSV group (mild inflammation induced by ischemia, focal aggregates in the form of neutrophil, lymphocyte and macrophages, no coagulative necrosis seen.), (d) PIP group (Mild myocardial edema, few scattered aggregates of inflammatory cells), (e) RSV-PIP group (Slight inflammation).
DISCUSSION
Over the last two decades, scientists and researchers have paid remarkable attention to the benefits of RSV. The predominant pharmacological actions are attributed to trans-RSV, the most common and extensively implemented isoform.22 Numerous pharmacological studies have shown that RSV has a wide range of physiological advantages, particularly antitumor activity, neuroprotective effects, cardio protection, and antidiabetic activity, all of which are directly related to its antioxidant and anti-inflammatory abilities.23
| Parameters | RSV | RSV-PIP |
|---|---|---|
| Cmax (μg/mL) | 7.39±0.13 | 10.46±0.09*** |
| Tmax (h) | 3 | 3.5 |
| AUC total (μg/mL/h) | 28.46±0.12 | 35.45±0.25*** |
| CL (L/h) | 0.96±0.01 | 0.72±0.008*** |
| Vd (L/kg) | 2.45±0.06 | 2.13±0.01** |
Pharmacokinetic parameters of pure Resveratrol (RSV) and Resveratrol-Piperine mixture (RSV-PIP).
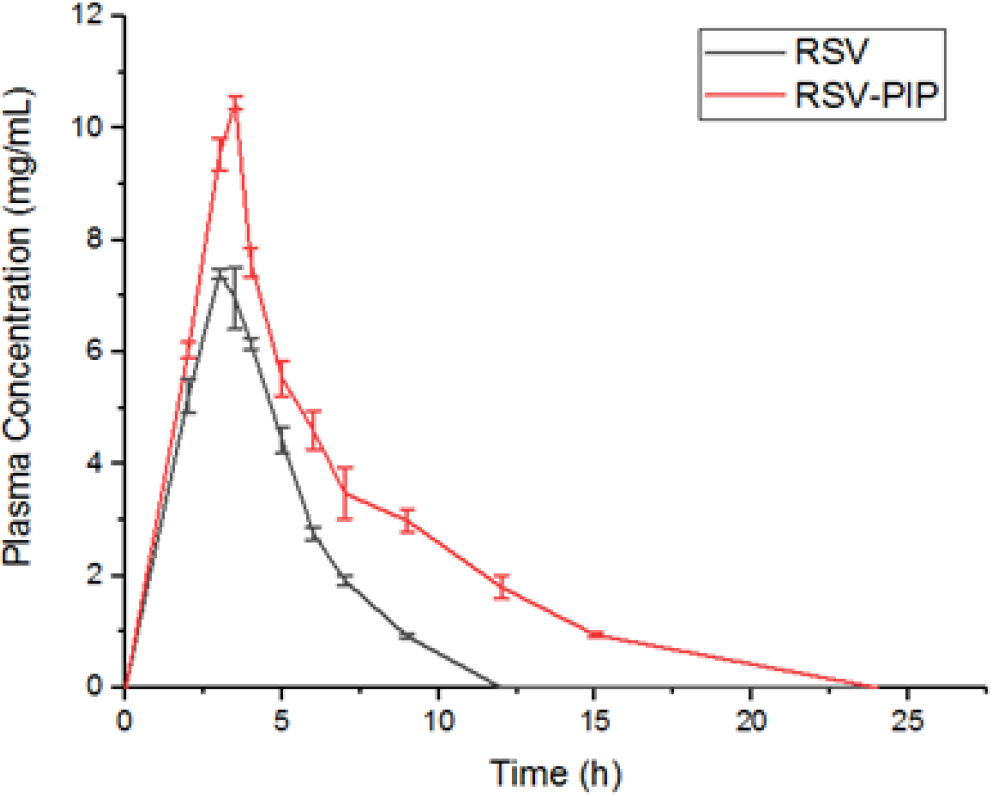
Figure 4:
Plasma concentration-time profile of RSV following administration of RSV alone and in combination with PIP (RSV-PIP) through oral route. Results are expressed as mean±SEM for 6 rats.
The effects of RSV in myocardial ischemia have been explained by a few different mechanisms, including preconditioning muscle tissue to Ischemia-Reperfusion (IR), restraint of platelet aggregation, and expected recovery of tissue in the infarcted region.24 Lin et al. investigated the effects of RSV on cardiac dysfunction after permanent coronary artery ligation in Sprague-Dawley rats. They found that when RSV was given daily intraperitoneally to rats that had had MI for four weeks, it lowered the size of the infarct and enhanced LV systolic and diastolic performance.25 Li et al. studied the preventive effect of RSV on myocardial ischemia and explored its potential mechanism. They claimed that through lowering oxidative stress and attenuating ferroptosis, RSV protects against myocardial ischemia-reperfusion damage.26 Chen et al. reported that RSV reduces ventricular arrhythmias and increases the survival rate for an extended period in rats with MI.27 Also, RSV protects against ISO-induced MI in rats through VEGF-B/AMPK/eNOS/ NO signaling pathway, as reported by Feng et al.28
Despite the advantages, RSV does have some drawbacks which restrict its use. Limitations of RSV are attributable to very low aqueous solubility, sensitivity to degradation, which lead to the isomerization of trans form to less active cis form, and fast metabolism and excretion, leading to limited oral bioavailability. A variety of techniques have been used to enhance the pharmacokinetic properties and then positive benefits of RSV.29–31
Co-administration with bioenhancer is one such approach to improve oral bioavailability. Several herbal substances, such as PIP, quercetin, naringin, curcumin, and glycyrrhizin, have been shown to enhance the drug’s pharmacokinetic parameters. Various studies on PIP have shown that it is an effective bioenhancer that boosts the oral bioavailability of medicines with subpar ADME properties. Also, it exhibits a synergistic effect when given along with a particular class of drugs.10 Furthermore, piperine has also been documented for its cardioprotective activity. Bi et al. reported that PIP enhances the oral bioavailability of silybin via the inhibition of efflux transporters BCRP and MRP2.13 Zhao-Hui et al. reported that PIP could improve the oral bioavailability and immune response of ginsenoside Rh2 when co-administered.14 However, the combined effects of RSV and PIP on cardioprotective functions, primarily MI, have not been studied till now. Therefore, we investigated the effect of PIP on the cardioprotective properties of RSV in rats.
Induction of myocardial damage was done by several techniques, such as the surgical LAD ligation method,32 infusion of β-adrenergic agonists, and aorta banding. Yet, the incidence of morbidity and mortality in these procedures was high. Consequently, induction of MI by the administration of ISO was found to be simple, rapid, and non-invasive, as reported by Wong et al.4 Therefore, we have used the ISO model to induce myocardial damage. Being a synthetic non-selective β-adrenergic agonist on high doses, ISO could cause calcium overload, myocardial oxidative stress, coronary hypotension, and energy depletion by acting on the heart’s β-adrenergic receptors.33 A few additional potential mechanisms reported are damage to the cardiac membrane caused by high ROS production from ISO injection, raising the level of lipid peroxidation,34 high levels of inducible NOS (Nitric Oxide Synthase), and low levels of endothelial NOS expression lead to endothelial dysfunction, which reduces cardiac contractility and further boosts ROS production, leading to cardiomyocyte apoptosis,35 increased cyclic adenosine monophosphate and intracellular Ca++ overload.36,37
ECG is the most important preliminary diagnostic tool for the detection of MI. MI is characterized by a number of ECG characteristics, including abnormalities in the ST segment, t-wave inversion, elevated heart rate, and QT interval.38 We observed a substantial reduction (p<0.001) in R-R, P-R, Q-T interval, and QRS complex and a significant increase (p< 0.001) in heart rate in the rats treated with ISO (85 mg/kg) when compared to the normal control group (Table 1). The altered ECG patterns are attributed to the subsequent disruption of cell membrane integrity in the ischemic heart muscle. An increase in heart rate causes an increase in oxygen consumption, which speeds up myocardial necrosis, as reported by Patel et al.39 Also, ISO administration caused elevation in the ST segment (Figure 2b), confirming MI. Besides, we observed a protective effect with restoring ECG abnormalities in the treatment groups (RSV and RSV-PIP). Our results were compatible with Patel et al., as they investigated the cardioprotective effect of melatonin against ISO-induced MI in rats. The combination group showed the most substantial effect compared to the pure drug.
ISO elevated the levels of specific and sensitive enzyme biomarkers (SGOT, SGPT, ALP, and LDH) of MI in the myocardium. These Cytosolic enzymes act as diagnostic markers. Damaged tissue causes the leakage of these contents into the extracellular fluid, indicating disrupted cellular function and increased permeability. Additionally, serum CK-MB and CK-NAC activity detection are crucial to detecting MI.40 In the current research study, compared to the control group, the serum levels of these cardiac enzyme indicators increased substantially in the ISO group (Table 2). On the contrary, pre-treatment with RSV, PIP, and a combination of RSV-PIP restored the enzyme levels compared to the ISO group indicating its protective action against MI. Thus, our biochemical evaluation proved that RSV, PIP, and RSV-PIP combination might keep membranes intact, preventing the release of these enzymes. The combination of RSV and PIP had a significant impact. The results are consistent with those of an earlier investigation of hydroalcoholic extract of Brazilian red propolis against ISO-induced MI in rats.20
The degree of Lipid Peroxidation (LPO) is enhanced by the polyunsaturated fatty acids in the myocardium being peroxided by the increased ROS production brought on by auto-oxidation as a result of ISO administration. An elevated level of LPO (TBARS) indicates the myocardium’s oxidative damage affecting the membrane function and integrity and reducing the antioxidant defense system’s activity.41,42 Present study findings reveal that LPO levels in the heart tissue have increased significantly in the ISO groups compared to NC, possibly due to the unrecoverable cardiac membrane damage seen during myocardial infarction. Nevertheless, treatment groups considerably reduced the levels of lipid peroxides in ISO-induced rats and provided some protection to the heart tissue, probably due to the ability of RSV to neutralize free radicals.
One of the potential molecular pathways by which ISO damages the myocardium is the accumulation of lipid peroxides and the formation of free radicals. Antioxidant defense enzymes (SOD and Catalase) and GSH provide the first line of defense by decomposing hydrogen peroxide (H2O2) and superoxide (O2) radicals before they combine to produce the more reactive hydroxyl radical (OH) and protecting the myocardial cell from oxidative stress.43 The fact that the quantities of these enzymes in the cardiac tissue are decreased provides additional evidence that oxidative stress causes heart injury. Our research study observed a significant decrease in SOD, Catalase, and GSH levels in the heart tissues of the ISO-administered animal group compared to the NC (Table 3). This may be due to the increased formation of ROS, reducing the activities of these enzymes.44 Overall, pre-treatment with RSV, PIP, and RSV-PIP resulted in restoration of SOD, catalase, and GSH levels, which mitigated ROS-induced oxidative stress when compared to the ISO group.
Histopathological results confirmed the biochemical findings. The myocardial cell membranes of normal control groups were clearly intact, and there was no inflammatory cell infiltration in the cardiac tissues, which revealed normal cardiac muscle fibers. However, the heart tissues of rats that had only received ISO exhibited significant myocyte death, inflammatory cell infiltration, and extensive cardiac fiber disconnection. The animal groups pre-treated with the combination of RSV-PIP exhibited almost normal heart morphology, with only slight inflammation, which suggests the synergistic activity of RSV and PIP. Moreover, this effect may also be attributed to the enhanced bioavailability of RSV resulting
RSV undergoes extensive metabolism through glucuronidation and sulfation. However, some studies suggest that PIP can prevent the glucuronidation of certain polyphenolic antioxidants.45 In our study, we observed that PIP increased both the Cmax and Tmax of RSV (Figure 4). Overall, our pharmacokinetic results demonstrate that co-administration of PIP enhanced the oral bioavailability of RSV by preventing its extensive metabolism (Table 4) from the co-administration of PIP (Figure 3).
The improved cardioprotective effect may not be only because of the improved bioavailability of resveratrol with the help of piperine, but also due to the cardioprotective potential of piperine too.
CONCLUSION
The study findings provide enough evidence from ECG, biochemical estimation, and histopathological results that subcutaneous administration of ISO causes MI in rats. The present study also offered experimental proof that RSV restored antioxidant enzyme levels with enhanced cardiac performance. Since oxidative stress has been recognized for a long time to be involved in the pathophysiology of cardiac injury, this finding may provide scientific evidence for the therapeutic benefits of RSV in cardio protection. Along with the cardioprotective potential of RSV and PIP, and PIP’s ability to enhance the oral bioavailability of RSV, a significant cardioprotective effect was observed, which was greater than that of RSV alone.
Cite this article
Salian TR, Noushida N, Gowda BHJ, Chakraborty M, Priya SES, Ahmed MG. Cardioprotective Potential of Resveratrol Alone and in Combination with Piperine on Isoproterenol-induced Myocardial Infarction in Rat: Investigation on Oral Bioavailability of Resveratrol. Int. J. Pharm. Investigation. 2024;14(1):107-16.
ACKNOWLEDGEMENT
The authors thank Yenepoya (Deemed to be University), Mangalore, India, for providing laboratory facilities to conduct this research. B.H. Jaswanth Gowda acknowledges the Yenepoya (Deemed to be University), Mangalore, India, for providing him with a Senior Research Fellowship. Graphical abstract is created with BioRender.com.
ABBREVIATIONS
| CVD | Cardiovascular disease |
|---|---|
| MI | Myocardial infarction |
| RSV | Resveratrol |
| PIP | Piperine |
| ISO | Isoproterenol |
| ECG | Electrocardiographic |
| GSH | Glutathione |
| SOD | Superoxide dismutase |
| TBARS | Thiobarbituric acid reactive substances |
| LDH | Lactate dehydrogenase |
| CK-MB | Creatine kinase myocardial band |
| LPO | Lipid peroxidation |
| H2O2 | Hydrogen peroxide |
| O2 | Superoxide |
References
- Stewart J, Manmathan G, Wilkinson P. Primary prevention of cardiovascular disease: a review of contemporary guidance and literature. JRSM Cardiovasc Dis. 2017;6:2048004016687211 [PubMed] | [CrossRef] | [Google Scholar]

- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982-3021. [PubMed] | [CrossRef] | [Google Scholar]

- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20):e618-51. [PubMed] | [CrossRef] | [Google Scholar]

- Wong ZW, Thanikachalam PV, Ramamurthy S. Molecular understanding of the protective role of natural products on isoproterenol-induced myocardial infarction: a review. Biomed Pharmacother. 2017;94:1145-66. [PubMed] | [CrossRef] | [Google Scholar]

- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177 [PubMed] | [CrossRef] | [Google Scholar]

- Burstein B, Maguy A, Clément R, Gosselin H, Poulin F, Ethier N, et al. Effects of resveratrol (trans-3,5,4’-trihydroxystilbene) treatment on cardiac remodeling following myocardial infarction. J Pharmacol Exp Ther. 2007;323(3):916-23. [PubMed] | [CrossRef] | [Google Scholar]

- Riba A, Deres L, Sumegi B, Toth K, Szabados E, Halmosi R, et al. Cardioprotective effect of resveratrol in a postinfarction heart failure model. Oxid Med Cell Longev. 2017;2017:6819281 [PubMed] | [CrossRef] | [Google Scholar]

- Sayed N, Khurana A, Godugu C. Pharmaceutical perspective on the translational hurdles of phytoconstituents and strategies to overcome. J Drug Deliv Sci Technol. 2019;53:101201 [CrossRef] | [Google Scholar]

- Smoliga JM, Vang O, Baur JA. Challenges of translating basic research into therapeutics: resveratrol as an example. J Gerontol A Biol Sci Med Sci. 2012;67(2):158-67. [PubMed] | [CrossRef] | [Google Scholar]

- Tiwari A, Mahadik KR, Gabhe SY. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Medicine in Drug Discovery. 2020;7:100027 [CrossRef] | [Google Scholar]

- Kesarwani K, Gupta R, Mukerjee A. Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed. 2013;3(4):253-66. [PubMed] | [CrossRef] | [Google Scholar]

- Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PSSR, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353-6. [PubMed] | [CrossRef] | [Google Scholar]

- Bi X, Yuan Z, Qu B, Zhou H, Liu Z, Xie Y, et al. Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2. Phytomedicine. 2019;54:98-108. [PubMed] | [CrossRef] | [Google Scholar]

- JIN ZH, QIU W, LIU H, JIANG XH, WANG L. Enhancement of oral bioavailability and immune response of ginsenoside Rh2 by co-administration with piperine. Chin J Nat Med. 2018;16(2):143-9. [PubMed] | [CrossRef] | [Google Scholar]

- Srivastava S, Dewangan J, Mishra S, Divakar A, Chaturvedi S, Wahajuddin M, et al. Piperine and celecoxib synergistically inhibit colon cancer cell proliferation via modulating Wnt/β-catenin signaling pathway. Phytomedicine. 2021;84:153484 [PubMed] | [CrossRef] | [Google Scholar]

- Singh S, Kumar P. Piperine in combination with quercetin halt 6-OHDA induced neurodegeneration in experimental rats: biochemical and neurochemical evidences. Neurosci Res. 2018;133:38-47. [PubMed] | [CrossRef] | [Google Scholar]

- Di X, Wang X, Di X, Liu Y. Effect of piperine on the bioavailability and pharmacokinetics of emodin in rats. J Pharm Biomed Anal. 2015;115:144-9. [PubMed] | [CrossRef] | [Google Scholar]

- Dudhatra GB, Mody SK, Awale MM, Patel HB, Modi CM, Kumar A, et al. A comprehensive review on pharmacotherapeutics of herbal bioenhancers. Scientific World Journal. 2012;2012:637953 [PubMed] | [CrossRef] | [Google Scholar]

- Wightman EL, Reay JL, Haskell CF, Williamson G, Dew TP, Kennedy DO, et al. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: a randomised, double-blind, placebo-controlled, cross-over investigation. Br J Nutr. 2014;112(2):203-13. [PubMed] | [CrossRef] | [Google Scholar]

- Neto JC, Paulino ET, Rodrigues AKBF, Silva JCGd, Bernardino AC, Oliveira JMdS, et al. Cardioprotective effect of hydroalcoholic extract of Brazilian red propolis against isoproterenol-induced myocardial infarction in rats. Phytomed Plus. 2022;2(1):100190 [CrossRef] | [Google Scholar]

- Chakraborty M, Bhattacharjee A, Ahmed MG, Priya S, Shahin H, Taj T, et al. Effect of naringin on myocardial potency of resveratrol against ischemia reperfusion induced myocardial toxicity in rat. Synergy. 2020;10:100062 [CrossRef] | [Google Scholar]

- Su M, Dong C, Wan J, Zhou M. Pharmacokinetics, tissue distribution and excretion study of trans-resveratrol-3-O-glucoside and its two metabolites in rats. Phytomedicine. 2019;58:152882 [PubMed] | [CrossRef] | [Google Scholar]

- Santos AC, Veiga FJ, Sequeira JAD, Fortuna A, Falcão A, Pereira I, et al. First-time oral administration of resveratrol-loaded layer-by-layer nanoparticles to rats – a pharmacokinetics study. Analyst. 2019;144(6):2062-79. [PubMed] | [CrossRef] | [Google Scholar]

- Raj P, Louis XL, Thandapilly SJ, Movahed A, Zieroth S, Netticadan T, et al. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014;95(2):63-71. [PubMed] | [CrossRef] | [Google Scholar]

- Lin JF, Lin SM, Chih CL, Nien MW, Su HH, Hu BR, et al. Resveratrol reduces infarct size and improves ventricular function after myocardial ischemia in rats. Life Sci. 2008;83(9-10):313-7. [PubMed] | [CrossRef] | [Google Scholar]

- Li T, Tan Y, Ouyang S, He J, Liu L. Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene. 2022;808:145968 [PubMed] | [CrossRef] | [Google Scholar]

- Chen YR, Yi FF, Li XY, Wang CY, Chen L, Yang XC, et al. Resveratrol attenuates ventricular arrhythmias and improves the long-term survival in rats with myocardial infarction. Cardiovasc Drugs Ther. 2008;22(6):479-85. [PubMed] | [CrossRef] | [Google Scholar]

- Feng L, Ren J, Li Y, Yang G, Kang L, Zhang S, et al. Resveratrol protects against isoproterenol induced myocardial infarction in rats through VEGF-B/AMPK/eNOS/NO signalling pathway. Free Radic Res. 2019;53(1):82-93. [PubMed] | [CrossRef] | [Google Scholar]

- Chimento A, De Amicis F, Sirianni R, Sinicropi MS, Puoci F, Casaburi I, et al. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int J Mol Sci. 2019;20(6):1381 [PubMed] | [CrossRef] | [Google Scholar]

- Peñalva R, Morales J, González-Navarro CJ, Larrañeta E, Quincoces G, Peñuelas I, et al. Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. Int J Mol Sci. 2018;19(9):2816 [PubMed] | [CrossRef] | [Google Scholar]

- Ćućuz V, Cvejić J, Gojković-Bukarica L. Clinical trials of resveratrol efficacy and safety. Vojnosanit Pregl. 2022;79(6):613-8. [CrossRef] | [Google Scholar]

- Blom JN, Lu X, Arnold P, Feng Q. Myocardial infarction in neonatal mice, A model of cardiac regeneration. J Vis Exp. 2016(111):54100 [PubMed] | [CrossRef] | [Google Scholar]

- Abdelzaher WY, Ahmed SM, Welson NN, Alsharif KF, Batiha GE, Labib DAA, et al. Dapsone Ameliorates Isoproterenol-Induced Myocardial Infarction via Nrf2/HO-1; TLR4/TNF-α Signaling Pathways and the Suppression of Oxidative Stress, Inflammation, and Apoptosis in Rats. Front Pharmacol. 2021;12:669679 [PubMed] | [CrossRef] | [Google Scholar]

- Singh BK, Pillai KK, Kohli K, Haque SE. Effect of Cissampelos pareira root extract on isoproterenol-induced cardiac dysfunction. J Nat Med. 2013;67(1):51-60. [PubMed] | [CrossRef] | [Google Scholar]

- Sithuraj S, Viswanadha VP. Berbamine protects the heart from isoproterenol induced myocardial infarction by modulating eNOS and iNOS expressions in rats. J Appl Biomed. 2018;16(4):301-10. [CrossRef] | [Google Scholar]

- cAMP activity and isoproterenol-induced myocardial injury in rats — PubMed. [Accesscited, 2023]. Available from
https://pubmed.ncbi.nlm.nih.gov/202000/
- Calcium as mediator of isoproterenol-induced myocardial necrosis — PubMed. [Accesscited, 2023]. Available from
https://pubmed.ncbi.nlm.nih.gov/5086900/
- Elasoru SE, Rhana P, de Oliveira Barreto T, Naves de Souza DL, Menezes-Filho JER, Souza DS, et al. Andrographolide protects against isoproterenol-induced myocardial infarction in rats through inhibition of L-type Ca and increase of cardiac transient outward K+ currents. Eur J Pharmacol. 2021;906:174194 [PubMed] | [CrossRef] | [Google Scholar]

- Patel V, Upaganlawar A, Zalawadia R, Balaraman R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: A biochemical, electrocardiographic and histoarchitectural evaluation. Eur J Pharmacol. 2010;644(1-3):160-8. [PubMed] | [CrossRef] | [Google Scholar]

- Thomes P, Rajendran M, Pasanban B, Rengasamy R. Cardioprotective activity of Cladosiphon okamuranus fucoidan against isoproterenol induced myocardial infarction in rats. Phytomedicine. 2010;18(1):52-7. [PubMed] | [CrossRef] | [Google Scholar]

- Khan V, Sharma S, Bhandari U, Ali SM, Haque SE. Raspberry ketone protects against isoproterenol-induced myocardial infarction in rats. Life Sci. 2018;194:205-12. [PubMed] | [CrossRef] | [Google Scholar]

- Gutteridge JMC. The role of superoxide and hydroxyl radicals in phospholipid peroxidation catalysed by iron salts. FEBS Lett. 1982;150(2):454-8. [PubMed] | [CrossRef] | [Google Scholar]

- Madhesh M, Vaiyapuri M. Effect of luteolin on lipid peroxidation and antioxidants in acute and chronic periods of isoproterenol induced myocardial infarction in rats. J Acute Med. 2012;2(3):70-6. [CrossRef] | [Google Scholar]

- Devika PT, Stanely Mainzen Prince P. Protective effect of (-)-epigallocatechin-gallate (EGCG) on lipid peroxide metabolism in isoproterenol induced myocardial infarction in male Wistar rats: A histopathological study. Biomedicine and Pharmacotherapy. 2008;62(10):701-8. [PubMed] | [CrossRef] | [Google Scholar]

- Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. Journal of Pharmacology and Experimental Therapeutics. 1985;232(1):258-62. [PubMed] | [Google Scholar]


