ABSTRACT
Background
The primary objective of this research was to establish a spectrophotometric technique to quantitatively analyze the concentration of favipiravir in both its pure form and pharmaceutical preparations using bromothymol blue reagent.
Materials and Methods
In this method, a yellow-colored chromagen was developed when favipiravir reacted with bromothymol blue reagent. Acetonitrile was selected as a solvent and the colored complex was detected at a wavelength of 475 nm.
Results
The validation of the developed method was conducted following the guidelines set by the International Council for Harmonisation (ICH). The results demonstrated a strong linear relationship within the concentration range of 10-50 μg/ mL, exhibiting a correlation coefficient of 0.9995. Moreover, the developed method exhibited excellent accuracy, precision, specificity, and sensitivity
Conclusion
For routine analysis purposes, this method can be readily utilized to determine the concentration of favipiravir in both bulk samples and pharmaceutical dosage forms.
INTRODUCTION
Favipiravir (Figure 1) is an anti-viral drug developed for the treatment of various viral infections like influenza, and COVID-19.1 Chemically it is designated as 6-fluoro- 3-hydroxypyrazine-2-carboxamide, with molecular formula C5H4FN3O2 and molecular weight 157.104 g/mol. It is a colorless powder, soluble in organic solvents and slightly soluble in water, and has a pKa value of 5.1. It is an organic compound belonging to the pyrazine carboxamides class.2 Favipiravir belongs to the anti-viral category where it acts by inhibiting RNA-dependent RNA polymerase enzyme which prevents viral transcription and replication.3,4
Figure 1:
Chemical Structure of Favipiravir.
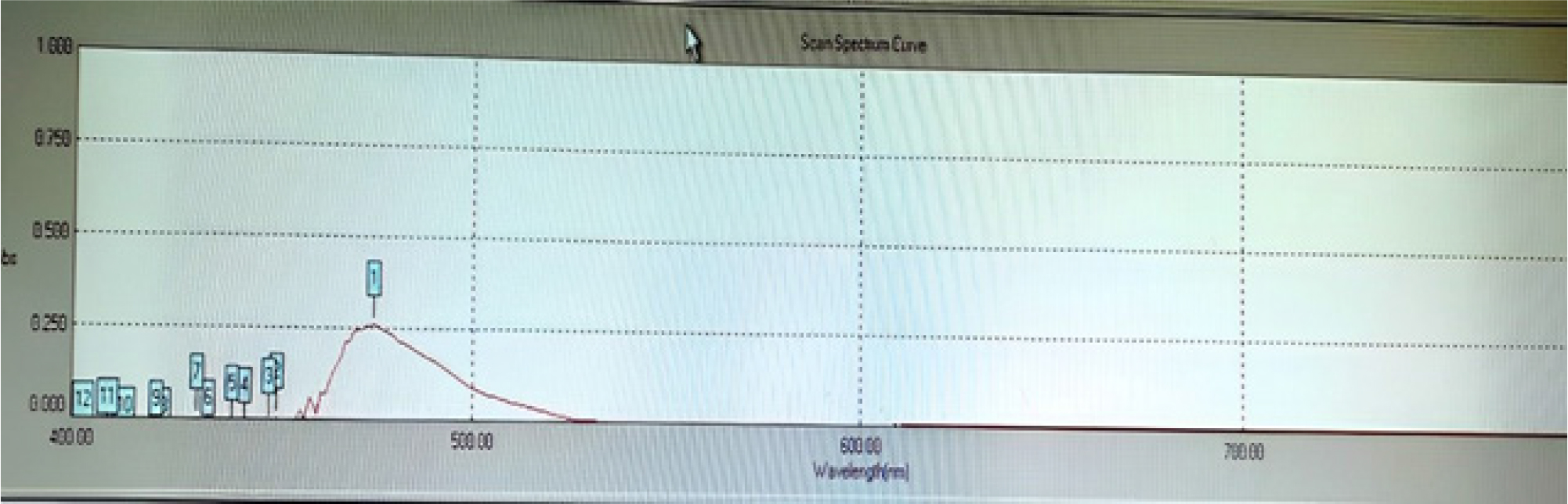
Figure 2:
UV-visible spectrum of favipiravir.
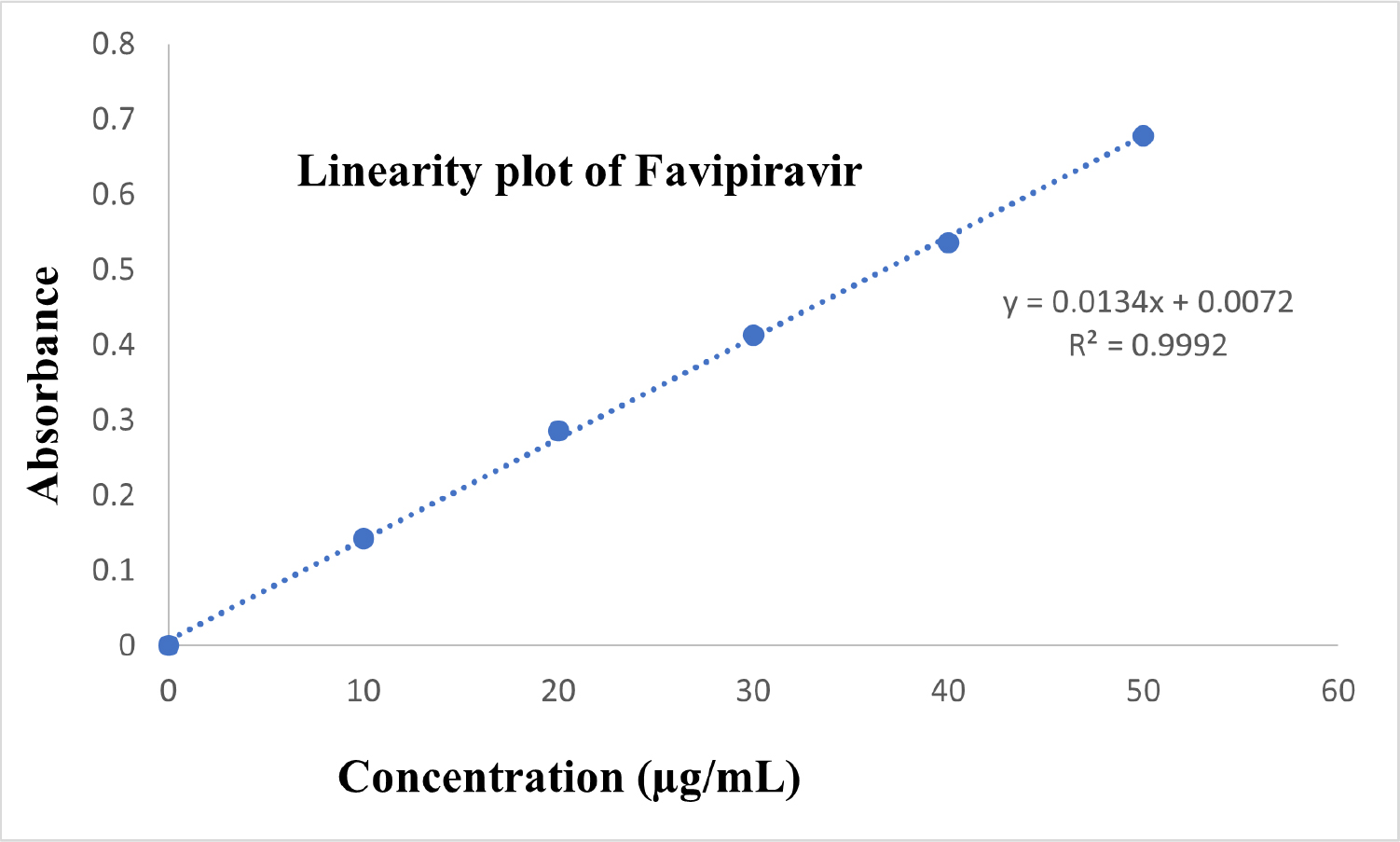
Figure 3:
Linearity plot of favipiravir.
As per the literature survey, it was known that analytical methods were developed for the estimation of favipiravir formulations. The developed methods included spectroscopic methods such as UV spectroscopic methods,5–10 Visible spectrophotometric methods,11 Fourier Transform Infrared Spectroscopic (FTIR) method,12 spectrofluorimetric method,13,14 chromatographic methods such as RP-HPLC methods,15–34 thin layer chromatography (TLC)35 and hyphenated techniques such as UPLC-MS/MS methods,36,37 LC-MS/MS methods38–42 and electrical methods such as voltammetric methods.43–45 But it was evident that only one method was developed for the estimation of favipiravir in pharmaceutical formulations using methyl orange and methyl red reagents in spectroscopy. Hence, this current study targets to validate a method using spectrophotometry for the estimation of favipiravir in bulk and in pharmaceutical formulation using bromothymol blue reagent.
MATERIALS AND METHODS
Reagents and chemicals
Favipiravir working standard was obtained as gift sample from the Hetero Labs, Hyderabad. The favipiravir tablets (fabiflu) were bought at a nearby pharmacy. All of the solvents required for the method’s development came from Merck in Mumbai, India. Additionally, all of the chemicals used for the method’s development were of the AR grade and came from Sigma Aldrich in Bangalore, India.
Instruments
The estimate of favipiravir in pharmaceutical formulations utilized a T60V UV-vis double-beam spectrophotometer. UV Win software was used to regulate every parameter. Other instruments utilized in the study included weighing digital balance, and ultrasonic bath sonicator.
Preparation of standard and sample solutions
An accurately weighed 100 mg of favipiravir working standard was made to dissolve in 100 mL of acetonitrile solvent constituting to a concentration of 1000 μg/mL. A volume of 10 mL was taken from the stock solution and diluted with 100 mL of distilled water (concentration 100 μg/mL). A mixture of 3 mL of the above solution and 1 mL of bromothymol blue reagent was diluted to 10 mL with distilled water to obtain a concentration of 30 μg/mL.
20 tablets of fabiflu were accurately weighed and an average weight was calculated. Weight equivalent to 100 mg of favipiravir was made to dissolve in 100 mL of acetonitrile solvent. The stock solution was subjected to sonication using an ultrasonic bath sonicator for 30 min. Later the solution was filtered and 10 mL of filtrate was diluted to 100 mL with distilled water. Finally, 3 mL of the above solution along with 1 mL of bromothymol blue reagent was diluted to 10 mL with distilled water.
Method validation46
Linearity
The linearity of this method was determined by preparing a serial dilution in the concentration ranges of 10-50 μg/mL and their absorbance was measured. A graph was plotted between concentration and absorbance values.
Precision
%RSD calculation was done by performing intraday and interday precision studies. Six replicates of 30 μg/mL concentration solution were produced, and their absorbance was assessed within the day (intraday precision studies) and for two days (interday precision studies).
Accuracy
Solutions in three levels 50%, 100% and 150% were prepared by std addition method and their absorbance was noted. From these values, % recovery was calculated at three levels.
Specificity
For the determination of specificity of this method, a blank solution was prepared and observed.
RESULTS
UV-visible spectrum for standard drug was shown in Figure 2.
The prepared standard solutions and sample solutions were placed in UV-visible spectrophotometer and their respective absorbance was measured and noted as presented in Table 1.
As illustrated in Figure 3, a linearity graph was created by plotting the concentration on the x-axis and the absorbance values on the y-axis. The results are reported in Table 2.
DISCUSSION
The present study aimed to develop and validate a simple, novel spectrophotometric method for favipiravir in bulk and pharmaceutical formulations using bromothymol blue as a reagent.
| Sl. No. | Parameters | Results |
|---|---|---|
| 1. | Absorption maximum | 475 nm |
| 2. | Linearity range | 10-50 μg/mL |
| 3. | Regression equation | y=0.0134x+0.0072 |
| 4. | Slope | 0.0134 |
| 5. | Intercept | 0.0072 |
| 6. | Correlation coefficient (r) | 0.9995 |
| 7. | Molar extinction coefficient (L.mol-1 cm-1) | 2245 |
| 8. | Sandell’s sensitivity (μg/cm2-0.001 absorbance units | 0.069 |
| 9. | Accuracy (% recovery) | 99.84%-100.32% |
| 10. | Precision (Intra-day) % RSD (Inter-day) % RSD | 0.34 0.28 |
| 11. | LOD | 1.60 |
| 12. | LOQ | 4.85 |
| 13. | Standard error | 0.0065 |
Optical Characteristics.
Solubility studies
Initially, for the development of this method, the standard drug favipiravir was subjected to solubility studies where the drug was made to dissolve in various solvents like methanol, acetonitrile, water, 0.1 N HCl, and 0.1 N NaOH.
Selection of solvent
From the above solubility assays, it was found that the drug was readily soluble in methanol and acetonitrile. For further study, acetonitrile solvent was used as a diluent for the preparation of solutions. The medication was discovered to be easily soluble in methanol from the aforementioned solubility assays.
Selection of ion pair reagent
Different reagents were used such as phenol red, bromothymol blue, and thymol blue. When these reagents were individually mixed with a standard solution, a clear solution was found with bromothymol blue. Hence, it was selected as ion pair reagent in this study.
| Sl. No. | Concentration (μg/mL) | Absorbance |
|---|---|---|
| 1 | 10 | 0.143 |
| 2 | 20 | 0.286 |
| 3 | 30 | 0.413 |
| 4 | 40 | 0.536 |
| 5 | 50 | 0.678 |
| Regression coefficient (r2) | 0.9992 | |
| Correlation coefficient (r) | 0.9995 |
Results of linearity.
| Sl. No. | Sample absorbance | % Assay |
|---|---|---|
| 1 | 0.413 | 99.60 |
| 2 | 0.414 | 99.84 |
| 3 | 0.411 | 99.12 |
| 4 | 0.415 | 100.08 |
| 5 | 0.413 | 99.60 |
| 6 | 0.412 | 99.36 |
| Average | 0.413 | 99.60 |
| %RSD | 0.34 | 0.34 |
Intra-day precision results.
| Sl. No. | Sample absorbance | % assay |
|---|---|---|
| 1 | 0.416 | 99.60 |
| 2 | 0.415 | 99.36 |
| 3 | 0.418 | 100.08 |
| 4 | 0.417 | 99.84 |
| 5 | 0.417 | 99.84 |
| 6 | 0.418 | 100.08 |
| Average | 0.417 | 99.80 |
| % RSD | 0.28 | 0.28 |
Inter-day precision results.
Selection of detection wavelength
In order to detect the wavelength for the measurement, a standard solution of concentration 10 μg/mL was prepared and scanned in the visible range of 400-800 nm in UV-visible spectrophotometer. Maximum absorbance was observed at a wavelength of 475 nm and it was utilised for further investigation.
For the determination of linearity of the method, serial dilutions in the range 10-50 μg/mL were prepared and absorbance was measured. Later, a graph between the concentration and absorbance values was established. From the graph, correlation coefficient values was determined and it was found to be 0.9995. The %RSD for intraday precision studies was 0.34 and for interday precision studies, it was 0.28, indicating the method to precision.
Accuracy of the method was determined by calculating % recovery. The % recovery was found to be 99.84%-100.32% which indicates that the method was accurate.
The UV-visible spectrum of the standard solution when compared with that of the blank solution, there was no interference observed in the blank spectrum, indicating that the method was specific.
The approach was found to be sensitive with a limit of detection of 1.60 g/mL and a limit of quantification of 4.85 g/mL.
| Sl. No. | Level (in %) | Amount of Favipiravir added (mg) | Amount of Favipiravir found (mg) | % Recovery | Mean % Recovery |
|---|---|---|---|---|---|
| 1 | 50 | 50.00 | 49.92 | 99.84 | 99.84 |
| 2 | 50 | 50.00 | 50.16 | 100.32 | |
| 3 | 50 | 50.00 | 49.68 | 99.36 | |
| 1 | 100 | 100.00 | 100.08 | 100.08 | 100.08 |
| 2 | 100 | 100.00 | 99.84 | 99.84 | |
| 3 | 100 | 100.00 | 100.32 | 100.32 | |
| 1 | 150 | 150.00 | 150.49 | 100.32 | 100.32 |
| 2 | 150 | 150.00 | 150.73 | 100.48 | |
| 3 | 150 | 150.00 | 150.24 | 100.16 |
Accuracy results.
CONCLUSION
A simple, novel spectrophotometric method was developed for the determination of favipiravir in bulk and pharmaceutical dosage form using bromothymol blue reagent and validated in accordance with ICH guidelines. The developed method was found to be accurate, precise, linear, specific, and sensitive. This developed method can be easily applied for the estimation of favipiravir for routine analysis or for quality control in formulations.
Cite this article
Nandini R, Mahithavani S, Gayatri S, Lakshman MVS, Bandla J. Spectrophotometric Determination of Favipiravir in Bulk and Pharmaceutical Formulation Using Bromothymol Blue Reagent. Int. J. Pharm. Investigation. 2024;14(1):101-6.
ACKNOWLEDGEMENT
The authors are thankful to the management of the Vishnu Institute of Pharmaceutical Education and Research for providing the required facilities to complete this research article.
ABBREVIATIONS
| RNA | Ribonucleic Acid |
|---|---|
| UV | Ultra Violet |
| FTIR | Fourier Transform Infrared Spectroscopy |
| RP-HPLC | Reverse Phase High-Performance Liquid Chromatography |
| TLC | Thin Layer Chromatography |
| UPLC-MS/MS | Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry |
| LC-MS/MS | Liquid Chromatography Tandem Mass Spectrometry |
| AR | Analytical Reagent |
| RSD | Relative standard deviation |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| ICH | International Conference on Harmonization |
References
- Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life threatening RNA virus infections. Pharmacol Ther. 2020;209:107512 [PubMed] | [CrossRef] | [Google Scholar]

- Du YX, Chen XP. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. 2020;108(2):242-7. [PubMed] | [CrossRef] | [Google Scholar]

- Pilkington V, Toby P, Andrew H. A review of the safety of favipiravir-a potential treatment in the COVID-19 pandemic. J Virus Erad. 2020;6(2):45-51. [PubMed] | [CrossRef] | [Google Scholar]

- Hayden FG, Shindo N. Influenza virus polymerase inhibitors in clinical development. Curr Opin Infect Dis. 2019;32(2):176-86. [PubMed] | [CrossRef] | [Google Scholar]

- Pritee RA, Baig MS. Analytical method development and validation of favipiravir in bulk and tablet formulation. Int J Pharm Pharm Res. 2022;26(1):52-64. [PubMed] | [CrossRef] | [Google Scholar]

- Bulduk İ. Comparison of HPLC and UV spectrophotometric methods for quantification of favipiravir in pharmaceutical formulations. Iran J Pharm Res. 2021;20(3):57-65. [PubMed] | [CrossRef] | [Google Scholar]

- Ali SNS, Mobina L, Mehfuza M, Seema P, Ahmed A, Khan GJ, et al. Analytical method development and validation and forced degradation stability indicating studies of favipiravir by RP-HPLC and UV in bulk and pharmaceutical dosage form. J Pharm Res Int. 2021;33(48B):254-71. [CrossRef] | [Google Scholar]

- Santosh VG, Hanmant AB. Development and validation of UV-spectrophotometric method for estimation of favipiravir. Int J Pharm Pharm Res. 2022;24(4):172-9. [CrossRef] | [Google Scholar]

- Jeevana Jyothi B, Venkata Kavya R. Ultraviolet spectrophotometric method development for estimation of new antiviral repurposing drug favipiravir. Asian J Pharm Clin Res. 2021;14(7):67-9. [CrossRef] | [Google Scholar]

- Itigimatha N, Chadchan KS, Yallur BC, Hadagali MD. New analytical methods for the determination of new anti-viral drug favipiravir: A potential therapeutic drug against Covid-19 virus, in bulk and dosage forms. Pharm Chem J. 2023;56(10):1419-25. [PubMed] | [CrossRef] | [Google Scholar]

- Panchale WA, Bisen SB, Manwar JV, Bakal RL, Tidke TV, Paithankar MN, et al. Development of visible spectrophotometric methods for the analysis of favipiravir in pure drug and tablet formulation. GSC Biol Pharm Sci. 2022;20(2):184-95. [CrossRef] | [Google Scholar]

- Nithila P, Raghavendrababu N, Padmavathi Y, Neena G, Sushma K, Poojitha A, et al. New FTIR method development and validation for quantitative analysis of favipiravir in bulk and pharmaceutical dosage forms. Int J Curr Pharm Res. 2022;14(5):25-9. [CrossRef] | [Google Scholar]

- Megahed SM, Habib AA, Hammad SF, Kamal AH. Experimental design approach for development of spectrofluorimetric method for determination of favipiravir; a potential therapeutic agent against COVID-19 virus: application to spiked human plasma. Spectrochim Acta A Mol Biomol Spectrosc. 2021;249:119241 [PubMed] | [CrossRef] | [Google Scholar]

- Mikhail IE, Elmansi H, Belal F, Ehab Ibrahim A. Green micellar solvent free HPLC and spectrofluorimetric determination of favipiravir as one of COVID-19 antiviral regimens. Microchem J. 2021;165:106189 [PubMed] | [CrossRef] | [Google Scholar]

- Pallavi VD, Kamalkishor GB. Bioanalytical method development and validation for the determination of favipiravir in spiked human plasma by using RP-HPLC. J Pharm Res Int. 2021;33(47A):275-81. [PubMed] | [CrossRef] | [Google Scholar]

- Taşkin D. Development and validation of a rapid HPLC-DAD method for determination of favipiravir in pharmaceutical formulation. Clin Exp Health Sci. 2022;12(3):648-52. [CrossRef] | [Google Scholar]

- Komarov TN, Karnakova PK, Archakova OA, Shchelgacheva DS, Bagaeva NS, Shohin IE, et al. Determination of favipiravir in human plasma by HPLC-UV. Drug Dev Regist. 2022;11(3):220-9. [CrossRef] | [Google Scholar]

- Hailat M, Al-Ani I, Zakareia Z, Al-Shdefat R, Al-Meanazel O, Anwer MK, et al. Development and validation of HPLC-DAD method for the determination of favipiravir and studying the impact of vitamin C on the pharmacokinetics of COVID-19 antiviral drug favipiravir. Separations. 2022;9(10):303 [CrossRef] | [Google Scholar]

- Hailat M, Al-Ani I, Hamad M, Zakareia Z, Abu Dayyih W. Development and validation of a method for quantification of favipiravir as COVID-19 management in spiked human plasma. Molecules. 2021;26(13):3789 [PubMed] | [CrossRef] | [Google Scholar]

- Ghune VB, Tapkir AS. Method development and validation of favipiravir by RP-HPLC. Innov J Sci. 2022;6(2):13-9. [PubMed] | [CrossRef] | [Google Scholar]

- Bulduk İ. HPLC-UV method for quantification of favipiravir in pharmaceutical formulations. Acta Chromatogr. 2021;33(3):209-15. [CrossRef] | [Google Scholar]

- Shinde J, Sharma PK, Sharma J. Development and validation of RP-HPLC method for estimation of favipiravir API and its tablet dosage form using quality by design approach. J Pharm Negat Results. 2022;13(8):4115-26. [CrossRef] | [Google Scholar]

- Ramarao N, Abhinandana P. A validated high performance liquid chromatographic method for the quantification of favipiravir by PDA detector. Int J Life Sci Pharm Res. 2021;11:181-8. [CrossRef] | [Google Scholar]

- Kalshetti MS, Adlinge SG. Development and validation of HPLC method for quantification of favipiravir in tablet. Res J Pharm Technol. 2022;15(3):1319-22. [CrossRef] | [Google Scholar]

- L570 quantitative analysis of favipiravir spiked in plasma using by HPLC. Shimadzu Corporation. 2020 [CrossRef] | [Google Scholar]

- Yamani NS, Annapurna MM. Stability indicating RP-HPLC method for the estimation of favipiravir in API and Pharmaceutical dosage forms (tablets). Res J Pharm Technol. 2022;15(12):5700-6. [CrossRef] | [Google Scholar]

- Megahed SM, Habİb AA, Hammad SF, Kamal AH. Chemometric approach based on factorial and Box-Behnken designs for determination of anti-coronavirus drug; favipiravir in bulk and spiked human plasma by green HPLC method. Turk J Anal Chem. 2021;3(2):70-8. [CrossRef] | [Google Scholar]

- Aniket J, Tandale Prashant WSK, Kolhe SD. Simple validation and estimation HPLC method for favipiravir. Int Res J Mod Eng Technol Sci. 2022;4(12):1100-6. [CrossRef] | [Google Scholar]

- Varma SA, Yadav P. Stability indicating HPLC method development and validation for estimation of favipiravir in pharmaceutical dosage form. World J Pharm Pharm Sci. 2021;10(5):1749-60. [CrossRef] | [Google Scholar]

- Balu PA, Paresh MS. Stability indicating RP-HPLC method development for estimation of favipiravir in bulk and pharmaceutical dosage form. World J Pharm Res. 2021;10(14):1444-65. [CrossRef] | [Google Scholar]

- Abdallah IA, Hammad SF, Bedair A, Elshafeey AH, Mansour FR. Determination of favipiravir in human plasma using homogeneous liquid-liquid microextraction followed by HPLC/UV. Bioanalysis. 2022;14(4):205-16. [PubMed] | [CrossRef] | [Google Scholar]

- Abdallah IA, Hammad SF, Bedair A, Mansour FR. Menthol-assisted homogenous liquid-liquid microextraction for HPLC/UV determination of favipiravir as an antiviral for COVID-19 in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2022;1189:123087 [PubMed] | [CrossRef] | [Google Scholar]

- Abdallah IA, Hammad SF, Bedair A, Abdelaziz MA, Danielson ND, Elshafeey AH, et al. A gadolinium-based magnetic ionic liquid for supramolecular dispersive liquid-liquid microextraction followed by HPLC/UV for the determination of favipiravir in human plasma. Biomed Chromatogr. 2022;36(6):e5365 [PubMed] | [CrossRef] | [Google Scholar]

- Vemuri DK, Gundla R, Konduru N, Mallavarapu R, Katari NK. Favipiravir (SARS-CoV-2) degradation impurities: identification and route of degradation mechanism in the finished solid dosage form using LC/LC-MS method. Biomed Chromatogr. 2022;36(6):e5363 [PubMed] | [CrossRef] | [Google Scholar]

- Jain B, Jain R, Jaiswal PK, Zughaibi T, Sharma T, Kabir A, et al. A non-instrumental green analytical method based on surfactant-assisted dispersive liquid-liquid microextraction-thin-layer chromatography-smartphone-based digital image colorimetry (SA-DLLME-TLC-SDIC) for determining favipiravir in biological samples. Molecules. 2023;28(2):529 [PubMed] | [CrossRef] | [Google Scholar]

- Suleyman Goökce S, Aysen Hoöl A, Ibrahim Bulduk I. Development and validation of UPLC-MS/MS method for obtaining favipiravir tablet dosage form and evaluation of its behavior under forced conditions. J Pharm Res Int. 2021;33(56A):130-140. [CrossRef] | [Google Scholar]

- Eryavuz O, Duygu A, Abusoglu S, Onmaz M, Yerlikaya F, Unlu A, et al. Development and validation of a sensitive, fast and simple LC-MS /MS method for the quantitation of favipiravir in human serum. Chromatogr B Analyt Technol Biomed Life Sci. 2021;1176:122768 [CrossRef] | [Google Scholar]

- Rezk MR, Badr KA, Abdel-Naby NS, Ayyad MM. A Novel, rapid and simple UPLC-MS/MS Method for Quantification of Favipiravir in Human Plasma: Aapplication to a Bioequivalence study. Biomed Chromatogr. 2021;35(7):e5098 [PubMed] | [CrossRef] | [Google Scholar]

- Itigimath N, Ashoka H, Yallur BC, Hadagali MD. LC-MS/MS Method Ddevelopment and Validation for the Determination of Favipiravir in Pure and Tablet Ddosage Forms. Turk J Pharm Sci. 2022 [PubMed] | [CrossRef] | [Google Scholar]

- Morsy MI, Nouman EG, Abdallah YM, Zainelabdeen MA, Darwish MM, Hassan AY, et al. A Novel LC-MS/MS method for Determination of the Potential antiviral candidate favipiravir for the emergency treatment of SARS-CoV-2 virus in human Plasma; Application to a Bioequivalence study in Egyptian human Volunteers. J Pharm Biomed Anal. 2021;199:114057 [PubMed] | [CrossRef] | [Google Scholar]

- Dominic F, Lisa J, David M. LC-MS/MS analysis of small molecule anti-viral and anti-inflammatory drugs in plasma in clinical research. Waters Corporation. 2020 [PubMed] | [CrossRef] | [Google Scholar]

- Curley P, Neary M, Arshad U, Tatham L, Pertinez H, Box H, et al. Development of a highly sensitive bioanalytical assay for the quantification of favipiravir. Biorxiv. 2021.02.03.429628 [PubMed] | [CrossRef] | [Google Scholar]

- Erşan T, Dilgin DG, Kumrulu E, Kumrulu U, Dilgin Y. Voltammetric determination of favipiravir used as an antiviral drug for the treatment of COVID-19 at pencil graphite electrode. Electroanalysis. 2022 10.1002/elan.202200295 [PubMed] | [CrossRef] | [Google Scholar]

- Mehmandoust M, Khoshnavaz Y, Tuzen M, Erk N. Voltammetric sensor based on bimetallic nanocomposite for determination of favipiravir as an antiviral drug. Mikrochim Acta. 2021;188(12):434 [PubMed] | [CrossRef] | [Google Scholar]

- Akca Zeynep A, Ozok Hande Izem Ozok H, Yardim Yavuz Y, Senturk Zuhre S. Electroanalytical investigation and voltammetric quantification of antiviral drug favipiravir in the pharmaceutical formulation and urine sample using a glassy carbon electrode in anionic surfactant media. Turk J Chem. 2022;46(3):21 [PubMed] | [CrossRef] | [Google Scholar]

- ICH Harmonized Ttriplicate Guideline. Validation of analytical procedures: text and Methodology. 2005;Q2(R1) [PubMed] | [CrossRef] | [Google Scholar]


