ABSTRACT
Objectives
The purpose of this study was to assess the potential synergistic and chemo sensitizing effects of the plant-derived flavonoid EGCG (Epigallocatechin gallate) as an adjuvant therapy with doxorubicin in combating chemoresistance.
Materials and Methods
The evaluation was performed on the NCIH-460 non-small cell Lung cancer cell line, where the cells were treated with both EGCG and doxorubicin simultaneously and successionally at different combination ratios. The viability of the cells was anatomized using the SRB assay, and the quantitative assessment of synergism in the combinations was conducted using the Median effect principle and the combination index-isobologram method with the aid of CompuSyn software. To investigate the role of EGCG in doxorubicin-resistant cells and understand the molecular mechanisms underlying synergy and chemo sensitization, the interaction between EGCG and the protein vimentin was examined through in silico methods and confirmed by western blotting in resistant cells.
Results
The results indicated that simultaneous treatment with EGCG and doxorubicin exhibited synergistic effects, particularly at a combination ratio of 1:3 (EGCG:DOXO), which resulted in up to a seven-fold decrease in the IC50 value of doxorubicin. Additionally, EGCG treatment led to reduced expression of vimentin in Doxorubicin-resistant cells, shedding light on the mechanisms underlying synergy and chemo sensitization.
Conclusion
In conclusion, this study demonstrated the potential schedule-dependent synergism of combining EGCG with doxorubicin, providing a rationale for designing chemotherapeutic combinations in the treatment of Non-Small Cell Lung Cancer (NSCLC).
INTRODUCTION
High systemic toxicity and cancer resistance for existing drugs are global concerns that contribute to poor treatment outcomes and relapse after remission in cancer patients following effective therapy. Despite early detection and advanced therapy, Non-Small Cell Lung Cancer (NSCLC) accounts for 80% of lung cancer.1 The development of anti-tumor antibiotic drugs such as doxorubicin has been a big advance for effective treatment of non-small cell lung cancer. Despite NSCLC’s high responsiveness and powerful clinical advantages, with an overall response rate of 30-50%, these medicines exhibit acute or cumulative dose-related toxicity and develop drug resistance, which is unavoidable and has an impact on the overall therapeutic result.2 Thus, sensitising drug-resistant cancer cells is critical for developing a successful pharmacological therapy. With no new drugs in the pipeline that are specifically designed to decrease chemoresistance and toxicity, it is critical to look for anti-cancer agents that can replace or enhance the activity of existing anti-cancer agents. Intermediate filament proteins, like as vimentin, are thought to be important in the genesis and progression of lung cancer, including the beginning of migratory and metastatic cascades, Epithelial-to-Mesenchymal Transition (EMT), and poor prognosis. Vimentin overactivity can reduce anticancer activity within cells by forming complexes and inhibiting the activity of modulator protein 14-3-3, a protein involved for regulating signalling pathways that increase tumerogenesis, motility, and multidrug resistance.3,4 Vimentin is a canonical mesenchymal marker of cell motility and a putative motility-inducing intermediate filament that undergoes epithelial-to-mesenchymal transition.5,6 According to previous research, EMT transition plays a critical role in the development of resistance to conventional chemotherapeutic agents such as doxorubicin, and thus vimentin may be an appealing therapeutic approach for treating cancer and multidrug resistance in lung cancer because they associate with the EMT process.7
Herbs in combination with anticancer drugs have recently been discovered to be capable of increasing the efficacy of chemotherapeutic agents while minimising their toxic effects, as well as resensitizing chemoresistance developed from repeated use, because they target the specific oncogenic pathway without affecting normal cells in the body.8 Green tea (Camellia sinensis) polyphenolic component Epigallocatechin-3-Gallate (EGCG). Many scholarly publications on its effectiveness in cancer prevention have been published. It is a well-known antioxidant; its free radical scavenging and anti-inflammatory properties suppress cell proliferation.9
The purpose of this study was to assess the effects of DOX combined with EGCG in an in vitro model of lung cancer, with the goal of determining the ideal medication ratio and regimen that will generate a synergistic effect. The study also looks into the effect of combining EGCG and DOX on vimentin expression in doxorubicin-resistant cells.
MATERIALS AND METHODS
Materials
Doxorubicin, foetal bovine serum, penicillin, streptomycin, and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, USA). Sulphorhodamine B cell assay kit obtained from HiMedia RPMI media and a 1X antibiotic-antimycotic solution were obtained from Gibco, NY, USA. The other reagents were locally procured.
Cell lines and reagents
Human non-small cell lung carcinoma cell lines NCI-H460 were obtained from the National Centre for Cell Science (NCCS), Pune, and cultured in RPMI 1640 medium supplemented with 10% foetal bovine serum. The cells are maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Sulforhodamine B (SRB) assay
SRB protein assay used to analyze cell proliferation. NCIH-460 cell suspensions of 100 μL were seeded in a 96 well microtiter plate (1×103 cells per well) and incubated in a 5 % CO2 atmosphere at +37°C. Following an overnight incubation, cells were exposed to various doses of doxorubicin and EGCG, either separately or together, in triplicate, and incubated for 24 hr. Each well’s culture media is covered with a cold fixative solution, which is then held there for an hour at 2 to 8°C. Plates are checked under a microscope to ensure that cells are fixed after several washings, and cells are then stained for at least 30 min with SRB staining solution. The unbound stains were removed by washing several times, and subsequently, the plates were air-dried. Solubilization solution was added to each well to extract the incorporated dye. The intensity of the colour was measured spectroscopically at 450 nm. SRB assay conducted according to the manufacturer’s instructions (EZ countTM Sulphorhodamine B cell assay kit) and IC50 value calculated from the triplicate reading. The values obtained by the assay were further used in CompuSyn software for drug combination studies.
Schedule dependence studies of EGCG and DOX co-administration
Chou’s diagonal constant ratio scheme10 was used for drug-combination tests. Based on the IC50 values obtained by the individual treatment of EGCG and doxorubicin in NCIH-460 cell lines, the equipotent molar ratio of EGCG: DOX was taken for the combination analysis. Cells were tested at a concentration that corresponds to 0.25, 0.5, 1, 1.5, 2 times the IC50 value of an individual drug at a different constant ratio of 1:1 to 1:5. Later, the optimum combination ratio will be calculated that will have the maximum synergistic impact on NCIH-460.11 At three separate schedules, cells were subjected to various mixture ratios (1:1, 1:2, 1:3, 1:4 and 1:5) as (1) simultaneous treatment (DOX: EGCG), (2) sequential treatment (DOX: EGCG after 72 hr), and (3) reverse sequence (EGCG: DOX after 72 hr).11 Data analysis was carried out using computational software (Calcusyn, Biosoft, Oxford, UK), that performs multiple drug dose-effect calculations employing the median effect methods described by Chou. The drug interaction between EGCG and DOXO was expressed as a Combination Index (CI) and dose reduction index (DRI). The combination index value at different Fa levels (fraction of cells affected, i.e % growth inhibition/100) was determined.12
Development of doxorubicin resistant subline
Doxorubicin resistant cell lines are established stepwise based on FDA recommendation sub-toxic initial doses of doxorubicin (10 nM) and gradually increasing their doses for every 15 days up to the final concentration of 320 nM. The cells are maintained for two months and further the three clones are developed in 96 well plates and maintained in RPMI complete medium supplement. Cell clones that survived over 4 months were selected for the drug sensitivity assay and different clones of resistant cells are regarded as DR1320, DR2320, DR3320 in the present paper.
The parental NCIH-460 cells are cultured under the similar condition as the resistant cells and maintained in culture for four months a same period of time as DR320.
The sensitivity of the parental NCIH-460 and resistant cells to doxorubicin was determined and compared by performing the SRB cytotoxicity assay.
Molecular Docking study
Three-dimension (3D) crystallographic structures of protein vimentin were retrieved from the Protein Data Bank (PDB) database (http://www.rcsb.org).
Ligand and target protein was prepared by using the LigPrep tool and Protein Preparation Wizard tool respectively that is integrated in Schrödinger suite-maestro device. The parameter set for the study includes pH neutralisation at 7.0±2.0 using Epik 2.2 and the OPLS_2005 force field was used for minimization.
The downloaded protein structures were then subjected to molecular docking using glide standard precision docking embedded in Schrodinger 2018-3 suite device Maestro 11.7.012, (Ligprep, Glide XP docking, Qik Prop) where the EGCG is used as Experimental ligand.
Western Blot
Resistant cell DR320 were starved overnight and treated with the DOXO alone (181nM for 72 hr) and in combination with EGCG at the ratio of 1:3 at different time interval (24, 48 and 72 hr). Cells are lysed with a suitable lysis buffer (01 M Tris HCl, pH 7.4, 02M NaF, 0.5M EDTA, 1% Triton X-100, 10 mM phenylmethylsulfonyl fluoride) containing protease inhibitors and cleared by centrifugation. Protein is estimated using BCA assay. Equal amount of protein was loaded into the wells of a SDS PAGE gel and subsequently the obtained protein bands from the gels are transferred to Nitrocellulose membrane. Ponceau Stain was conducted to confirm proper transfer of proteins. After extensive washing, membranes are incubated with the primary antibody solutions against target antibody vimentin at 4°C with a suitable dilution. After washing secondary antibody is added (Anti-Rabbit HRP 1:300 TBST).Western blotting performed according to the standard protocol, and protein expression is quantified using the chemiluminescent imaging system.
Statistical analysis
All the assays were performed in triplicate and subjected to statistical analysis.
Results were expressed as mean Standard deviation. The Statistical comparisons between the different sets of data was performed in GraphPad PrismTM version 3.03 applying One-way ANOVA (analysis of variance) followed by Dunnett’s multiple comparison test; p<0.05 were considered significant.
RESULTS
Schedule dependence studies for Sequential and Simultaneous Administration of EGCG and DOX
From the SRB cytotoxicity assay the IC50 value of EGCG and DOXO in NCIH-460 cell line was determined and it was found to be 600 and 300 nM respectively. First, cells were treated with EGCG and DOX at equipotent molar ratio based on their IC50 values (EGCG IC50/DOX IC50). The DOXO was found to be ~02-fold more potent than that of EGCG in NCIH-460 cells and hence 1:2 constant ratio of DOX: EGCG was administered concurrently for 72 hr. In the combination therapy of chemotherapeutic agent it is very much essential to determine the dosage and dose administration schedule to obtain the beneficial therapeutic outcome. The drug combination can be synergistic, additive, or antagonistic based on the ratio of combination and schedule of drug administration. Interactions between EGCG and doxorubicin on their concurrent administration were determined by applying isobologram analysis and denoted with the Combination Index (CI) and Dose Reduction Index (DRI). The CI analysis was based on the median-effect principle.13
From the result obtained, synergism was observed at lower inhibition levels (<75% inhibition), while additive to antagonism was observed at higher inhibition levels (≥90% inhibition) as depicted in Figure 1. As given in Table 1 among the three regimens of concurrent treatment, the greater synergistic effect was observed only with the simultaneous treatment whereas Sequential treatment of DOX, as EGCG (DOX→EGCG) and its reverse schedule EGCG→DOX shows antagonistic effect.
| Drug combination | Combination ratio (nM) | Combination Index value at | |||
|---|---|---|---|---|---|
| IC25 | IC50 | IC75 | IC90 | ||
| DOXO+ EGCG | 01:01 | 1.033±0.04 | 1.198±0.128 | 1.702±0.274 | 2.837±0.148 |
| DOXO+ EGCG | 01:02 | 0.9805±0.132 | 0.8642±0.060 | 0.92±0.035 | 1.082±0.402 |
| DOXO+ EGCG | 01:03 | 0.713±0.250 | 0.772±0.104 | 0.975±0.227 | 1.325±0.245 |
| DOXO+ EGCG | 01:04 | 1.060±0.073 | 2.064±0.245 | 4.559±0.848 | 10.65±0.246 |
| DOXO+ EGCG | 01:05 | 1.515±0.380 | 2.852±0.550 | 5.980±0.382 | 13.13±0.381 |
| DOXO÷EGCG | 01:01 | 3.008±o.554 | 2.105±0.543 | 2.49±0.097 | 3.185±0.654 |
| DOXO÷EGCG | 01:05 | 3.489±0.532 | 2.884±0.077 | 6.029±0.811 | 13.087±0.771 |
| EGCG÷DOXO | 01:01 | 2.833±0.144 | 2.486±0.211 | 2.207±0.054 | 2.048±0.181 |
| EGCG÷DOXO | 01:05 | 5.747±0.133 | 5.193±0.092 | 13.103±0.213 | 14.55±0.311 |
Heat map of the effects of EGCG and DOX at different combination ratios and regimen.
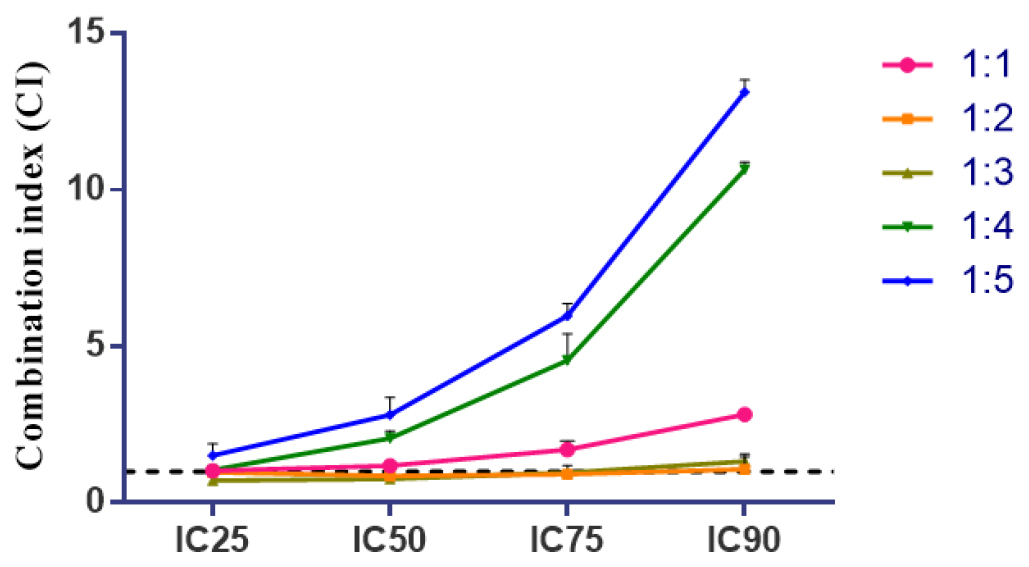
Figure 1:
The Combination Index (CI) plot of different combination ratios. Values that fall below and above the line (CI=1) would mean synergism and antagonism, respectively. Points are experimental data reported in mean±standard deviation of three independent trials.
In the simultaneous treatment schedule, synergistic and additive cytotoxicity was observed in multiple combination ratios, particularly within the 1:1 to 1:4 molar ratios, with the most synergistic effect observed at 1:2 and 1:3. For the 1:2 and 1:3 combination ratios, synergistic effects were observed from IC25 to IC75. The cytotoxicity of the individual drugs was significantly enhanced over multiple drug effect level particularly when near IC50 or sub IC50 of each compound when they are combined. The combination of EGCG with DOXO reduced the concentration of the both the drug to induce 50% inhibition (Figure 2).
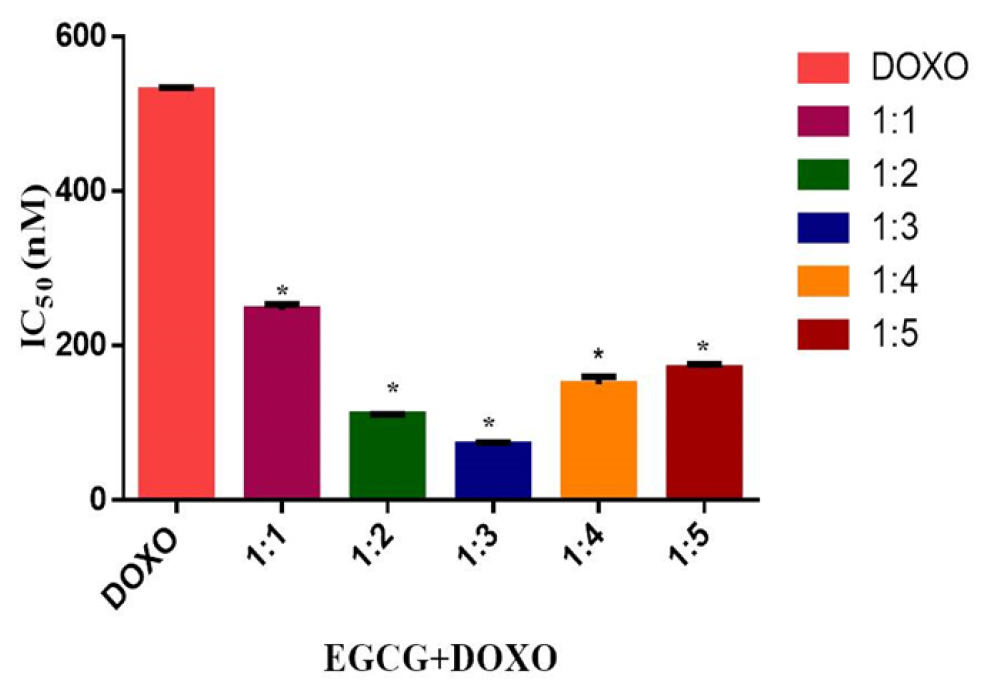
Figure 2:
IC50 were significantly lowered after using in combination at 1:3 ratios. *p<0.05 (one-way ANOVA/Dunnett’s multiple comparison test) where n=3 (triplicate).
Concentration of DOXO (nM)
Doxorubicin IC50 was remarkably reduced to 71.56±0.441 at the ratio of 1:3. Hence the toxicity of the Doxorubicin is expected to be lower by decreasing the dosage while maintaining the drug efficacy.
The DRI values of DOXO in each combination ratios at different cell growth level were generated by CompuSyn software (Table 2).
| Drug combination | Combination ratio (nM) | DRI value at | |||
|---|---|---|---|---|---|
| IC25 | IC50 | IC75 | IC90 | ||
| DOXO+ EGCG | 01:01 | 1.462±0.032 | 2.147±0.133 | 3.152±0.345 | 4.629±0.0889 |
| DOXO+ EGCG | 01:02 | 2.174±0.562 | 4.796±0.667 | 10.578± | 23.33±0.566 |
| DOXO+ EGCG | 01:03 | 3.794±0.588 | 7.406±0.334 | 14.454±0.221 | 28.209±0.234 |
| DOXO+ EGCG | 01:04 | 3.078±0.066 | 3.531±0.441 | 4.05±0.543 | 4.645±0.669 |
| DOXO+ EGCG | 01:05 | 2.527±0.443 | 3.106±0.786 | 3.818±0.700 | 4.693±0.0890 |
EGCG and DOX at different combination ratios expressed in terms of Dose Reduction Index (DRI).
The compound EGCG exhibit the strongest synergy at the ratio of 1:2 and 1:3 with the dose reduction of DOXO by more than 20 folds at high effect level i.e. DRI value of 23.33±0.566 and 28.209±0.234 with 1:2 and 1:3 ratios respectively at 90% inhibition level.
Doxorubicin sensitivity in resistant clones and NCIH-460 cell lines by SRB assay
Doxorubicin sensitivity in parental NCIH-460 and on the different clones of resistant cells (DR320) was determined by SRB assay to quantify cell viability. Both the cell lines are treated with the Doxorubicin ranging from 25 to 400 nM for 48 hr. Relatively established resistant clone showed higher IC50 values of 3.191±0.3, 3.246±0.18 and 2.0917±0.42 μM for DR1, DR2 and DR3 respectively compared to that of the NCIH-460 parental cells with IC50 of 0.36 μM (Figure 3). The treatment of the cells with different concentration of EGCG exerted equal cytotoxicity in NCIH-460 and resistant cells by SRB assay (data not shown) with the IC50 value of 600±0.211 and 638±0.34 nM.
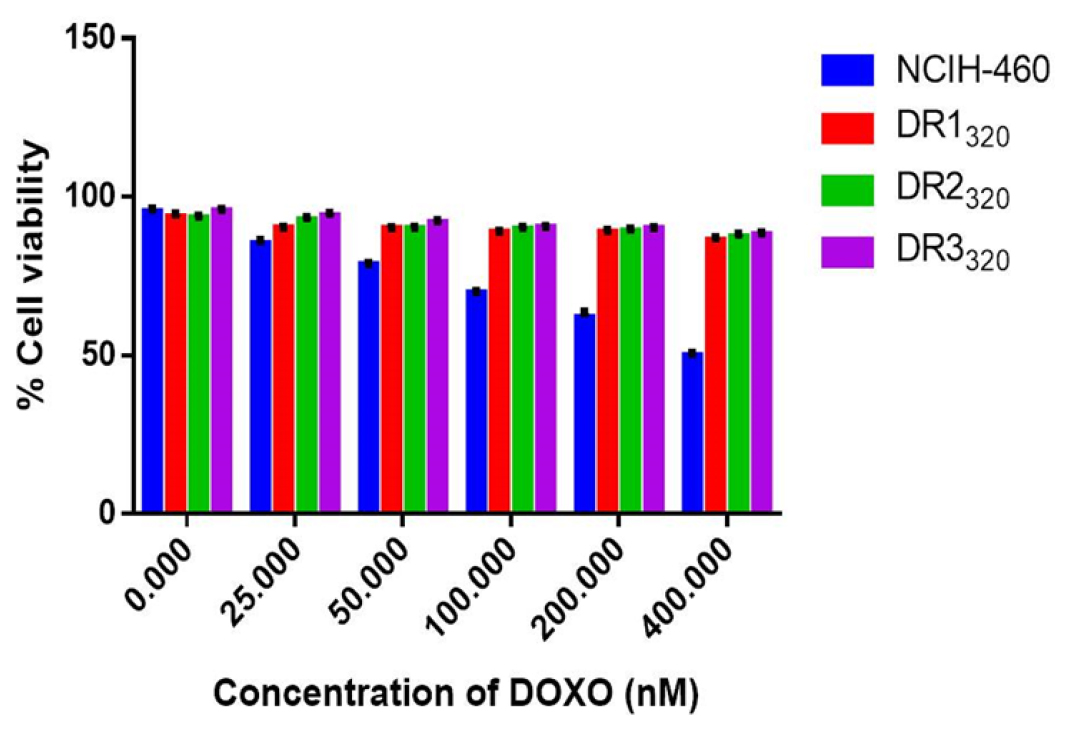
Figure 3:
Effect of different concentration of Doxorubicin on cell viability of parenteral and resistant cells.
Molecular docking of EGCG using Vimentin as receptor protein involved in doxorubicin resistance
The molecular docking study of vimentin was conducted and capacity of EGCG to occupy the active site of the protein speculates its potential to decrease resistance to Doxorubicin was determined. Molecular docking results demonstrates the promising interaction of EGCG with binding cleft of protein vimentin and explored good affinity defined in terms of docking score of -9.883, with minimal binding energy.
The active residues involved in protein binding was shown in Table 3 and forms three Hydrogen bond with amino acid GLU359 (two bonds), PRO378 as in Figure 4 (A, B). These interaction and affinity of EGCG with vimentin suggests the one of the possible mechanism of action of its synergistic effect with that of Doxorubicin and its resistance.
| Protein | Docking score | Active Residue | Number of Hydrogen bond | Residues making hydrogen bond with EGCG |
|---|---|---|---|---|
| Intermediate filament protein Vimentin (PDB ID:3KLT). | -9.883 | MSE379, PRO378,SER377,LEU374, VAL347,SER345, CYS343, GLU359, LYS357,GLU356, VAL353, LEU387,CYS380,LEU383, PHE233,ALA235,VAL252, PHE251,VAL329,SER331,PHE428, PHE302, ILE408, GLN410. | 03 | GLU359, PRO378. |
Molecular docking data of EGCG with vimentin.
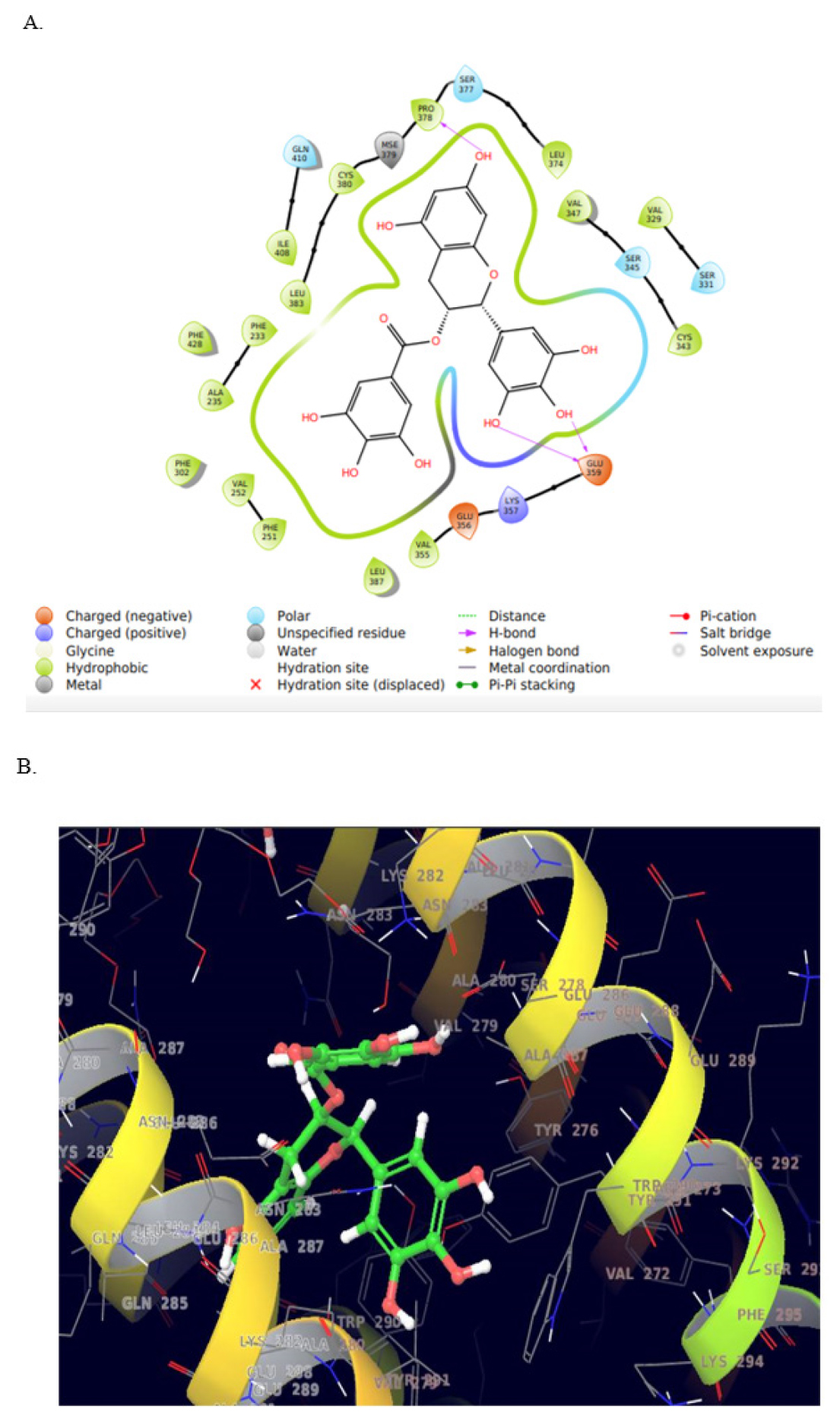
Figure 4:
Molecular docking image of EGCG with intermediate filament protein vimentin (A) Effect of interaction of different amino acid residues of vimentin with EGCG. The light pink color indicates the carbon Hydrogen bond of interaction between drug and amino acid residues of the protein (B) the docking conformations of EGCG with vimentin.
Mechanistic studies of chemosensitization and synergistic activity by inhibiting vimentin
The western blot data in the Figure 5 demonstrates that DR320 cell clones potentially acquired the resistant to doxorubicin via epithelial to mesenchymal transition as there is over expression of EMT protein vimentin in all the clones of resistant cells when compared to that of parental NCIH-460 cells.
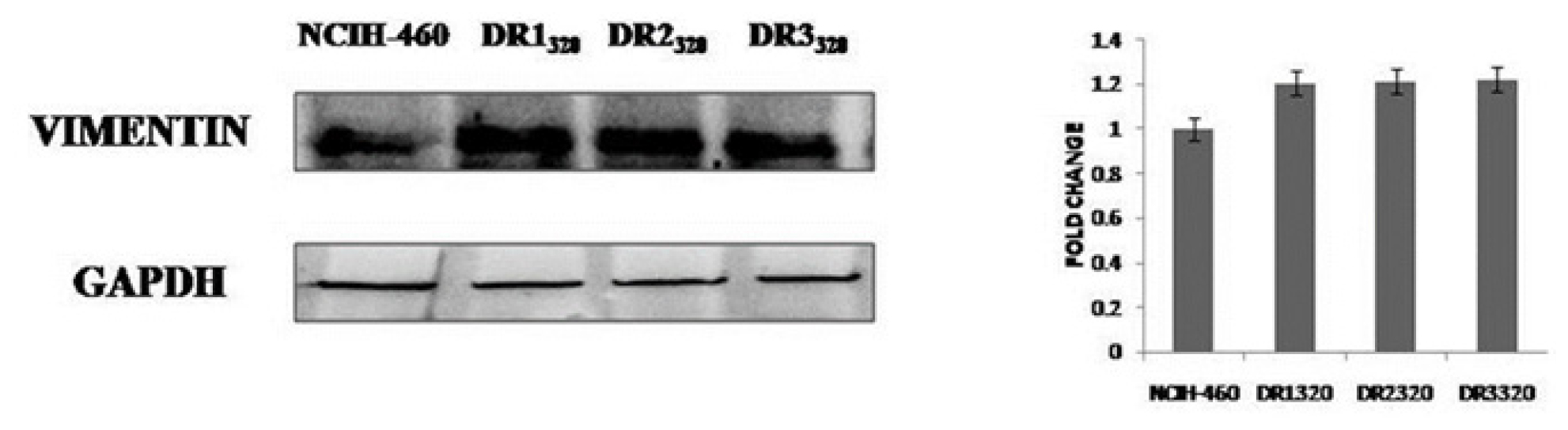
Figure 5:
Expression of EMT protein Vimentin in parentral NCIH-460 cells and in different clones of Doxorubicin resistant cells. A bar graph indicates the fold changes analysis of bands using ImageJ software.
The DR350 clone was chosen for additional research and was treated with DOXO (300 nM for 72 hr), 600 nM EGCG alone, and 600 nM EGCG combined with EGCG at a ratio of 1:2 and 1:3. The information in Figure 6 showed that, as compared to control cells, cells treated with Doxorubicin alone had higher protein levels. As a result, our findings suggest that Doxorubicin resistance may be brought on by an increase in the level of vimentin, a protein thought to be involved in the epithelial to mesenchymal transition that results in drug resistance. Compared to cells treated with EGCG and doxorubicin alone, EGCG and Doxo treatment at ratios of 1:2 and 1:3 reduced the production of the protein vimentin.

Figure 6:
Inhibition of NCIH-460 cell’s vimentin expression when treated with EGCG alone and in combination with Doxorubicin at the ratios of 1:2 and 1:3. A bar graph indicates the fold changes analysis of bands using ImageJ software.
DISCUSSION
The therapeutic outcome of any novel drug combinations depends on the compound’s dosage regimen, drug concentration ratios and mode of action. In the combination therapy of chemotherapeutic agent it is very much essential to determine the dosage and dose administration schedule to obtain the beneficial therapeutic outcome. The drug combination can be synergistic, additive, or antagonistic based on the ratio of combination and schedule of drug administration. Certain ratios and schedule of administration are synergistic for some class of compounds, and others are antagonistic. The quantitative synergy study results indicate that EGCG shows the maximal synergy with pharmacologically achievable concentrations of DOX over multiple combination ratios (1:1-1:3) when given simultaneously and not sequentially. The synergistic effect was greatest at the drug ratio of 1:3 especially at the cytotoxic level of 25-75% that could be the most favourable combination ratio. Doxorubicin IC50 was remarkably reduced to 71.56±0.441 at the ratio of 1:3. Hence the toxicity of the Doxorubicin is expected to be lower by decreasing the dosage while maintaining the drug efficacy.
The synergistic impact of EGCG and DOXO against NCIH-460 cell lines suggests that EGCG makes cells more susceptible to the cytotoxic effects of Doxorubicin and reduces the likelihood that cancer cells may acquire a resistance to the drug. Therefore, understanding the mechanism underlying these impacts is crucial.
The study of EMT-related protein expression was investigated since cells resistant to doxorubicin under the microscope showed discernible morphological alterations. Vimentin is one of the major signalling molecule and adaptor protein which involved in initiation, progression of tumors, epithelial to mesenchymal transition and thus chemoresistance and also in the metastatic spread of cancer.14
As molecular docking studies shows the great interaction of EGCG with vimentin further the western blot was conducted to determine the protein expression pattern of vimentin in resistant cells and EGCG treated resistant cell lines. The western blotting, results show the possibility of Doxorubicin resistance is due to the increase in the level of vimentin a protein that is implicated in the epithelial to mesenchymal transition responsible for the drug resistance. Treatment of EGCG and Doxo combination at ratio of 1:2 and 1:3 inhibited the protein vimentin expression as compared with cells treated with EGCG and doxorubicin alone. Higher level of inhibition was observed with DOXO: EGCG 1:3 ratio indicating the ideal combination ratio for the treatment of Doxorubicin resistant cells. Thus the mechanistic study result suggests the down regulation of protein vimentin by EGCG and DOXO combination that might be implicated in the synergism by repressing doxorubicin resistance in non-small cell lung cancer.
CONCLUSION
In conclusion, the therapeutic outcome of any novel drug combinations depends on the compound’s dosage regimen, drug concentration ratios and mode of action. Certain ratios and schedule of administration are synergistic for some class of compounds, and others are antagonistic. The quantitative synergy study results indicate that EGCG shows the maximal synergy with pharmacologically achievable concentrations of DOX over a multiple combination ratio (1:1-1:3) when given simultaneously and not sequentially. The synergistic effect was greatest at the drug ratio of 1:3 especially at the cytotoxic level of 25-75% that could be the most favourable combination ratio. The results also suggest the down regulation of protein vimentin by EGCG that might be implicated in the synergism by repressing doxorubicin resistance in non-small cell Lung cancer.
Cite this article
Somayaji A, Shastry CS. Synergestic Cytotoxicity of Doxorubicin in Combination with Epigallocatechin 3-gallate in Non Small Cell Lung Cancer by Inhibiting Protein Vimentin. Int. J. Pharm. Investigation. 2024;14(2):343-9.
ACKNOWLEDGEMENT
The authors are grateful to NGSM Institute of Pharmaceutical Sciences and NITTE (Deemed to be University) for the facilities provided for carrying out this work.
ABBREVIATIONS
| ANOVA | Analysis of variance |
|---|---|
| CI | Combination Index |
| DMSO | Dimethyl sulfoxide |
| DRI | Dose Reduction Index |
| DOX | Doxorubicin |
| EGCG | Epigallocatechin-3-gallate |
| EMT | Epithelial-to-Mesenchymal transition |
| IC50 | Half maximal inhibitory concentration |
| NCCS | National Centre for Cell Science |
| NSCLC | Non-small cell lung cancer |
| PDB | Protein Data Bank |
| SDS PAGE | Sodium Dodecyl-sulfate Polyacrylamide Gel Electrophoresis |
| SRB | Sulforhodamine B |
References
- Zarogoulidis K, Zarogoulidis P, Darwiche K, Boutsikou E, Machairiotis N, Tsakiridis K, et al. treatment of nonsmall cell lung cancer (NSCLC). J Thorac Dis. 2013;5(4)(Suppl 4):S389-96. [PubMed] | [CrossRef] | [Google Scholar]

- Mi J, Zhang X, Rabbani ZN, Liu Y, Reddy SK, Su Z, et al. RNA aptamer-targeted inhibition of NF-kappa B suppresses non-small cell lung cancer resistance to doxorubicin. Mol Ther. 2008;16(1):66-73. [PubMed] | [CrossRef] | [Google Scholar]

- Tadokoro A, Kanaji N, Liu D, Yokomise H, Haba R, Ishii T, et al. Vimentin regulates invasiveness and is a poor prognostic marker in non-small cell lung cancer. Anticancer Res. 2016;36(4):1545-51. [PubMed] | [Google Scholar]

- Kidd ME, Shumaker DK, Ridge KM. The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol. 2014;50(1):1-6. [PubMed] | [CrossRef] | [Google Scholar]

- Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol. 2013;25(5):600-12. [PubMed] | [CrossRef] | [Google Scholar]

- Mendez MG, Kojima SI, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24(6):1838-51. [PubMed] | [CrossRef] | [Google Scholar]

- Li J, Liu H, Yu J, Yu H. Chemoresistance to doxorubicin induces epithelial-mesenchymal transition via upregulation of transforming growth factor β signaling in HCT116 colon cancer cells. Mol Med Rep. 2015;12(1):192-8. [PubMed] | [CrossRef] | [Google Scholar]

- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A, et al. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66(2):1234-40. [PubMed] | [CrossRef] | [Google Scholar]

- Cavet ME, Harrington KL, Vollmer TR, Ward KW, Zhang JZ. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol Vis. 2011;17:533-42. [PubMed] | [Google Scholar]

- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621-81. [PubMed] | [CrossRef] | [Google Scholar]

- Tun JO, Salvador-Reyes LA, Velarde MC, Saito N, Suwanborirux K, Concepcion GP, et al. Synergistic cytotoxicity of renieramycin M and doxorubicin in MCF-7 breast cancer cells. Mar Drugs. 2019;17(9):536 [PubMed] | [CrossRef] | [Google Scholar]

- Chou TC, Otter GM, Sirotnak FM. Schedule-dependent synergism of Taxol or Taxotere with edatrexate against human breast cancer cells in vitro. Cancer Chemother Pharmacol. 1996;37(3):222-8. [PubMed] | [CrossRef] | [Google Scholar]

- Chou T-C. The combination index (CI < 1) as the definition of synergism and of synergy claims. Synergy. 2018;7:49-50. [CrossRef] | [Google Scholar]

- Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68(18):3033-46. [PubMed] | [CrossRef] | [Google Scholar]


