ABSTRACT
Background
The development of micro-organism resistance in humans and the environment has been linked to the inadequacy of conventional treatment methods in completely eliminating antibiotics. The purpose of this research is to look at the potential for eliminating Amoxicillin (AMO) from aqueous environments using Nickel (II) Oxide Nanoparticles (NiO nanoparticles).
Materials and Methods
In order to determine how successful NiO nanoparticles are in eliminating AMO, the impact of many important adsorption process factors, such as the initial concentrations of AMO (10-50 mg/L), pH (3-10), adsorbent dose (0.1-1 g/L), mixing rate (50-300 rpm), contact time (10-100 min), and temperature (15-45°C) was examined.
Results
The greatest AMO removal efficiency was 99.48% at pH=7, 0.8 g/L of adsorbent, and contact time of 60 min. The endothermic nature of the adsorption process was suggested by the positive value of ΔHo. The observed physical adsorption was consistent with the ΔHo evolved during adsorption, which was less than 40 KJ/mol. The negative value of ΔGo demonstrated that AMO absorbed on the NiO nanoparticles was a spontaneous process.
Conclusion
Accordingly, NiO nanoparticles could be employed as a successful adsorbent to eliminate AMO from pharmaceutical wastewater.
INTRODUCTION
The most essential substance for human activity on Earth is water.1,2 Ensuring access to clean water is essential for human well-being.3,4 Water contamination is expanding worldwide because of the quick development of industry, increment human populace, and local and rural exercises, which prompt the spread of life-threatening diseases.5 One of the most genuine ecological issues is the presence of risky and lethal contaminations in the earth,6 which is exceptionally in charge of a wide range of contamination, including area, water, and air contamination.7
The utilization of antibiotics in human and veterinary medicine has been vast.8 Incomplete metabolism of most antibiotics results in the excretion of their residues and degradation products. Consequently, these substances can infiltrate water environments via different pathways.9 The environment may be negatively impacted by the presence of antibiotic residues and metabolites, which could result in the development of antibiotic resistance in microbes and the occurrence of chronic and acute toxicity in organisms.10 Amoxicillin (AMO) is a type of antibiotic that is widely detected in wastewater, soil, and sediments. It is hydrolytically stable and not easily degraded in water. AMO is prescribed to treat various bacterial infections, e.g., pneumonia, strep throat, middle ear infections, skin infections, and urinary tract infections.11,12 It is administered orally or by injection, although the former is more common. The use of AMO presents environmental risks, including the emergence of antibiotic-resistant bacteria, as reported in studies.13
Antibiotics have limited biodegradability, high water solubility, complicated molecular architectures, and low removal effectiveness, making it challenging to remove them from water in a conventional system.14,15 Hence, several techniques such as oxidation, ozonation, reduction, photolysis, gamma-ray irradiation, and adsorption have been proposed as potential and competitive methods to eliminate antibiotics from water and wastewater.16,17
Sorption is a highly effective treatment approach for removing antibiotics due to its versatility in various scientific fields.18 It is a cost-effective and excellent alternative to primary treatment. However, when dealing with organic waste, it has a relatively slow rate of adsorption and seldom reaches equilibrium.19,20 There have been various suggestions made by researchers for the use of low-cost and nonconventional sorbents. These sorbents include waste materials from industries, agricultural biosorbents, and natural materials. These alternatives have the potential to act as inexpensive sorbents but have lower adsorption capacity.21,22
Nano-scale metal oxide adsorbents are a promising choice for adsorption applications due to their low production costs, high surface area, and ability to be regenerated by burning the adsorbed substances. Therefore, they are a material family that requires in-depth research.23,24 The objective of this study is to examine the adsorption of Amoxicillin (AMO) from aqueous solutions using NiO nanoparticles in batch mode. In addition, in analyzing the performance, effects of temperature, contact time, NiO nanoparticle mass, mixing speed and AMO concentration on adsorption efficiency are evaluated.
MATERIALS AND METHODS
Chemicals
The AMO was obtained from a Sigma Aldrich company and used without further purification. Accurately weighed AMO was dissolved in distilled water to achieve a concentration of 1000 mg/L as AMO stock solutions. The experimental solutions were created by diluting the stock solutions to attain the desired concentrations. Also, NiO nanoparticles used in this study were obtained from US Research Nanomaterials, Inc., and NaOH and HCl were purchased from Merck Company. Images were obtained by the use of the JSM26490LV apparatus (Joel, Tokyo, Japan) for Scanning Electron Microscopy (SEM) examinations. TEM images were recorded on a JEOl-2010 at an accelerating voltage of 200 kV. The pH measurement was performed through employment of pH meter 720 (Germany) comprised of a glass electrode and an internal reference electrode.
Sorption experiments
On a mechanical shaker equipped with a thermostatic water bath, the batch adsorption tests were done by shaking a certain quantity of the adsorbent with AMO solution that had the required concentration. The shaking was carried out in a 200 mL capped flask at 120 rpm. Initially, 100 mL of sorbate solution with an initial concentration of 50 mg/L was mixed with a definite amount of sorbent, and the mixture was shaken for 1 hr to achieve adsorption equilibrium. The paper filter was utilized to filtrate mixtures, and the spectrophotometer was used to report the AMO concentration in the solution. The influence of different parameters on the process of sorption was deliberated by altering the contact time (ranging from 10 to 100 min with 10 min intervals), initial concentration of the AMO solution (ranging from 10 to 50 mg/L), and adsorbent dose (0.1-1 g/L) at temperatures and mixing speed varying from 15-45°C and 100-300 rpm. The experiments were conducted at a constant pH of 7 that was maintained by adjusting with HCl and NaOH (0.1 M). The AMO sorption percentage was determined by calculating the difference between the initial and final concentration, using the equation 1:25
The amount of AMO adsorbed at equilibrium qe (mg/g) was calculated by the equation 2:26
In above equations, AMO concentration at equilibrium denoted as Ce (mg/L), while CO (mg/L) represents the initial concentration. The volume of the solution is denoted as V (L), and the mass of the adsorbent is represented as m (g).
The spontaneity, nature, and randomness of the sorption process are expressed through thermodynamic parameters (ΔG°, ΔH°, and ΔS°), which are calculated using the equations 3 and 4.20
The aforementioned equation involves the use of three variables, namely KC, R, and T. KC represents the equilibrium constant, R stands for the ideal gas constant with a value of 8.314 KJ/mol/K, and T represents the temperature in Kelvin.
RESULTS
NiO nanoparticles were analyzed using TEM to determine their grain size, morphology, and structure. The micrograph displayed in Figure 1a and b showed uniform morphology with an average diameter of 32 nm. The SEM images confirmed the TEM results, revealing the formation of small particles with diameters ranging from 30 to 42 nm. As displayed by Figure 2, an increase in the initial concentration of AMO from 10 to 50 mg/L resulted in a decrease in AMO removal efficiency from 99.48 to 86.81%. Thus, for the lowest initial concentration (10 mg/L), the highest AMO removal percentage was achieved. Raising the mixing speed to 200 rpm resulted in developing adsorption, which is attributed to enhancing the collision between AMO and adsorbent, as depicted by Figure 3. The study discovered that as the adsorbent dose increased, the percentage of removal also improved, as depicted in Figure 4.

Figure 1:
TEM and SEM micrograph of NiO nanoparticles.
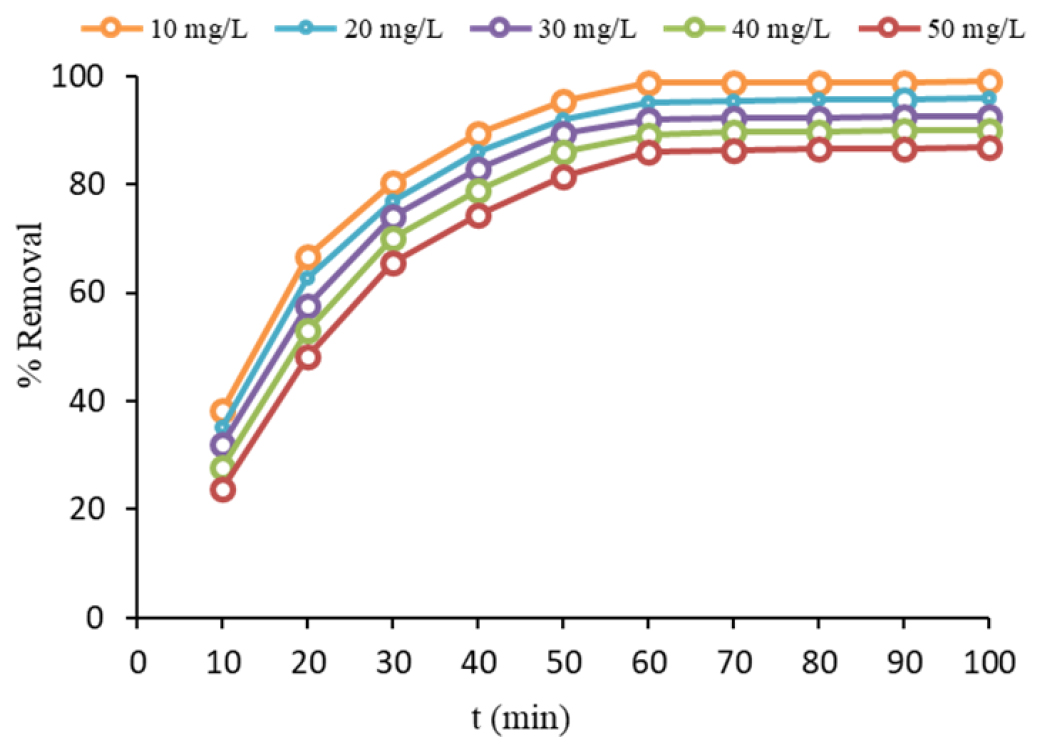
Figure 2:
Effect of AMO concentration on removal percentage (mixing speed=200 rpm, time 60 min, pH=7, T=308 K and dose=0.8 g/L).

Figure 3:
Effect of mixing speed on removal percentage of AMO (C0=50 mg/L, time 60 min, pH=7, T=308 K and dose=0.8 g/L).

Figure 4:
Effect of NiO nanoparticle dosage on the removal of AMO (C0=50 mg/L, time 60 min, pH=7, T=308 K and mixing speed=200 rpm).
The effect of contact time and temperature on the adsorption of AMO onto NiO nanoparticle was studied (Figure 5). The results specified that the AMO adsorption enhanced with an increase in contact time and temperature. The findings pointed out that the adsorption capacity of AMO on NiO nanoparticles exhibited an increase from 46.85 mg/g at 288 K to 60.11 mg/g at 318 K. This observation suggests that higher temperatures have a favorable effect on the AMO adsorption by the adsorbent. Calculating thermodynamic parameters (Table 1) was performed based on the slope and intercept of the plot (Ln Kc vs. 1/T). The data demonstrated that the sorption increased with increasing temperature.
| T (K) | ΔGo (kJ/mol) | ΔHo (kJ/mol) | ΔSo (kJ/mol K) |
|---|---|---|---|
| 288 | -3.12 | 16.14 | 1.74 |
| 298 | -4.96 | ||
| 308 | -5.55 | ||
| 318 | -5.89 |
Thermodynamic parameters for the adsorption of AMO on NiO nanoparticle.
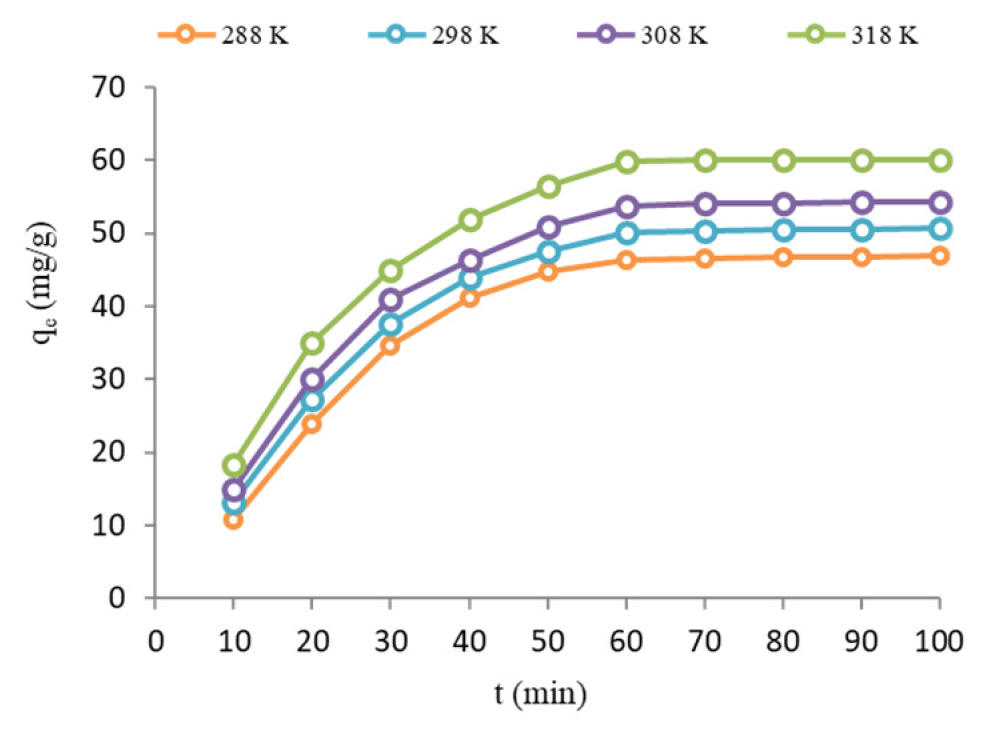
Figure 5:
Effect of AMO concentration and contact time (C0=50 mg/L, pH=7, dose=0.8 g/L and mixing speed=200 rpm).
DISCUSSION
Adsorption has shown to be one of the most effective separation techniques for water purification among the technologies used for wastewater and water treatment. Adsorption has also been shown to be a successful treatment technique for the hazardous synthetic antibiotics that are difficult to remove using conventional techniques from water and wastewater.17
Removal efficiency declined when the antibiotic concentration rose from 10 to 50 mg/L. This may be elucidated by the fact that there are less active adsorption sites available on the adsorbent surface at increased pollutant concentrations.27 Also, reduction in removal efficiency is caused by the adsorption sites on the adsorbent surface being constantly active. As the concentration of AMO increases, the adsorption capacity also increases due to the higher likelihood of contact between the adsorbate and adsorbent.28,29 Balarak et al. studied Penicillin G antibiotic adsorption using Lemna minor; their findings revealed a decrease in the efficiency of the adsorption process with an increase in antibiotic concentration, which was attributed to the fixed locations of adsorption. Nonetheless, the study also discovered that the adsorption capacity per unit mass of adsorbent increased as the contact between adsorbate molecules and the adsorbent surface increased, thereby corroborating the outcomes of this section.30 The enhancement in adsorption caused by increasing the mixing rate could be neglected due to the desorption of AMO molecules from adsorbent sites.31 Study conducted by Mostafapour et al. for the removal of erythromycin by MWCNTS confirms the results of this study.32 By the increase of the dose of NiO nanoparticle due to rising active locations, Penicillin G removal efficacy enhanced but the amount of removed Penicillin G per adsorbent unit decreased.33 Its reason is the unsaturation of the active locations on the adsorbent surface, which didn’t use the capacity of all active locations present in the adsorbent surface.34 In line with the investigation conducted by Zhang et al.,31 our study also demonstrated that augmenting the adsorbent dosage resulted in enhanced removal effectiveness. However, the adsorption capacity was reduced due to unsaturation of the total adsorption locations. In this study, it was determined that the ideal adsorbent dose was 0.8 gr/L. The maximum adsorption was achieved after 60 min of contact time, indicating that the equilibrium state had been reached. Further adsorption was negligible beyond this point. During the initial 30 min, the highest level of adsorption happens because of the attractive forces that develop between AMO and NiO nanoparticles. At this stage, AMO is adsorbed on the exterior surface of the nanoparticles.35 Once the outer surface is completely covered, the AMO molecules move to the inner surface of the nanoparticles through the pores.36 The adsorption process of AMO ion on the adsorbent was more efficient at elevated temperatures, signifying that the rise in temperature had a positive impact on the adsorption process. The adsorption of AMO ion on the adsorbent is an endothermic process, indicating that the degree of adsorption improved with the increase in temperature.37 The adsorption mechanism consists of both physical and chemical sorption. Dehydration of the ions becomes more facile at high temperature, thus enhancing their adsorption.38
In order to evaluate the sorption process of AMO onto NiO nanoparticle, the temperature effect at various temperatures ranging from 288 (15°C) to 318±1 K was studied under optimal conditions. The thermodynamic parameters (ΔG°, ΔH°, ΔS°) were determined to provide insights into the sorption process. The ΔG°<O indicate that the sorption process is viable and spontaneous.39 Moreover, as the temperature increases, the sorption process becomes more favorable. ΔH°>O suggests that the sorption process is endothermic in nature, meaning that it requires energy. This value also indicates that an increase in the disorder of the system leads to a more favorable sorption process.40
CONCLUSION
In conclusion, the NiO nanoparticle was employed as an efficient adsorbent for eliminating AMO from aqueous solution. The effect of various experimental parameters such as equilibration time, NiO dosage, agitation speed, AMO concentration, and temperature was studied using the batch process. In order to enlighten the studied process, the calculation of ΔGo, ΔHo, and ΔSo values. The fact that the ΔHo value was low implies that the AMO ions’ sorption onto the adsorbent was physical in nature. Additionally, the negative ΔGo values suggest that our evaluated process is both greatly favorable and spontaneous. The efficiency of NiO nanoparticles as an adsorbent for eliminating AMO antibiotics from aqueous solutions has been confirmed.
Cite this article
Naghsh N, Chandrika K, Balarak D. Thermodynamic Study of Adsorption of Amoxicillin on Synthesized NiO of Pharmaceutical Wastewater. Int. J. Pharm. Investigation. 2024;14(2):365-70.
ACKNOWLEDGEMENT
The authors are grateful from Zahedan University of Medical Sciences, because of supporting of this research (IR.ZAUMS. REC.1400.379).
ABBREVIATIONS
| AMO | Amoxicillin |
|---|
References
- Ghauch A, Tuqan A, Assi HA. Elimination of amoxicillin and ampicillin by micro scale and nanoscale iron particles. Environ Pollut. 2009;157(5):1626-35. [PubMed] | [CrossRef] | [Google Scholar]

- Malakootian M, Balarak D, Mahdavi Y, Sadeghi SH, Amirmahani N. Removal of antibiotics from wastewater by Azolla filiculoides: kinetic and equilibrium studies. IJAPBS. 2015;4(7):105-13. [PubMed] | [CrossRef] | [Google Scholar]

- Chen WR, Huang CH. Adsorption and transformation of tetracycline antibiotics with aluminum oxide. Chemosphere. 2010;79(8):779-85. [PubMed] | [CrossRef] | [Google Scholar]

- Liu H, Yang Y, Kang J, Fan M, Qu J. Removal of tetracycline from water by Fe-Mn binary oxide. J Environ Sci (China). 2012;24(2):242-7. [PubMed] | [CrossRef] | [Google Scholar]

- Zhao Y, Geng J, Wang X, Gu X, Gao S. Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J Colloid Interface Sci. 2011;361(1):247-51. [PubMed] | [CrossRef] | [Google Scholar]

- Prado N, Ochoa J, Amrane A. Biodegradation and biosorption of tetracycline and Tylosin antibiotics in activated sludge system. Process Biochem. 2009;44(11):1302-6. [CrossRef] | [Google Scholar]

- Kang J, Liu H, Zheng YM, Qu J, Chen JP. Systematic study of synergistic and antagonistic effects on adsorption of tetracycline and copper onto a chitosan. J Colloid Interface Sci. 2010;344(1):117-25. [PubMed] | [CrossRef] | [Google Scholar]

- Shao L, Ren Z, Zhang G, Chen L. Facile synthesis, characterization of a MnFe2O4/activated carbon magnetic composite and its effectiveness in tetracycline removal. Mater Chem Phys. 2012;135(1):16-24. [CrossRef] | [Google Scholar]

- Brigante M, Schulz PC. Remotion of the antibiotic tetracycline by titania and titania-silica composed materials. J Hazard Mater. 2011;192(3):1597-608. [PubMed] | [CrossRef] | [Google Scholar]

- Moein H, Balarak D, Meshkinain A, Chandrika K, Yazdani N. Effects of operational parameters on the removal of tetracycline from aqueous solutions by electrocoagulation. Int J Pharm Investig. 2021;11(1):23-6. [CrossRef] | [Google Scholar]

- Balarak D, Mostafapour FK, Joghtaei A. Thermodynamic analysis for adsorption of amoxicillin onto magnetic carbon nanotubes. Br J Pharm Res. 2017;16(6):1-11. [CrossRef] | [Google Scholar]

- Balarak D, Khatibi AD, Chandrika K. Antibiotics removal from aqueous solution and pharmaceutical wastewater by adsorption process: a review. Int J Pharm Investig. 2020;10(2):106-11. [CrossRef] | [Google Scholar]

- Zhao Y, Gu X, Gao S, Geng J, Wang X. Adsorption of tetracycline (TC) onto montmorillonite: cations and humic acid effects. Geoderma. 2012;183-184:12-8. [CrossRef] | [Google Scholar]

- Li Z, Schulz L, Ackley C, Fenske N. Adsorption of tetracycline on kaolinite with pH-dependent surface charges. J Colloid Interface Sci. 2010;351(1):254-60. [PubMed] | [CrossRef] | [Google Scholar]

- Chang PH, Li Z, Jean J, Jiang W, Wang CJ, Lin KH, et al. Adsorption of tetracycline on 2: 1 layered non-swelling clay mineral illite. Appl Clay Sci. 2012;67-68:158-63. [CrossRef] | [Google Scholar]

- Chang PH, Li Z, Jiang WT, Jean JS. Adsorption and intercalation of tetracycline by swelling clay minerals. Appl Clay Sci. 2009;46(1):27-36. [CrossRef] | [Google Scholar]

- de Godos I, Muñoz R, Guieysse B. Tetracycline removal during wastewater treatment in high-rate algal ponds. J Hazard Mater. 2012;229-230:446-9. [PubMed] | [CrossRef] | [Google Scholar]

- Liu P, Liu WJ, Jiang H, Chen JJ, Li WW, Yu HQ, et al. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour Technol. 2012;121:235-40. [PubMed] | [CrossRef] | [Google Scholar]

- Balarak D, Mahdavi Y, Mostafapour FK. Application of alumina-coated carbon nanotubes in removal of tetracycline from aqueous solution. Br J Pharm Res. 2016;12(1):1-11. [CrossRef] | [Google Scholar]

- Ji LG, Chen W, Duan L, Zhu D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: a comparative study using activated carbon and graphite as adsorbents. Environ Sci Technol. 2009;43(7):2322-7. [PubMed] | [CrossRef] | [Google Scholar]

- Gao Y, Li Y, Zhang L, Huang H, Hu J, Shah SM, et al. Adsorption and removal of tetracycline antibiotics from aqueous solution by grapheme oxide. J Colloid Interface Sci. 2012;368(1):540-6. [PubMed] | [CrossRef] | [Google Scholar]

- Rahardjo AK, Susanto MJ, Kurniawan A, Indraswati N, Ismadji S. Modified Ponorogo bentonite for the removal of ampicillin from wastewater. J Hazard Mater. 2011;190(1-3):1001-8. [PubMed] | [CrossRef] | [Google Scholar]

- Chang PH, Li Z, Yu TL, Munkhbayer S, Kuo TH, Hung YC, et al. Sorptive removal of tetracycline from water by palygorskite. J Hazard Mater. 2009;165(1-3):148-55. [PubMed] | [CrossRef] | [Google Scholar]

- Balarak D, Chandrika K. Batch studies on biosorption of ciprofloxacin on freshwater macro alga Lemna minor. Int J Pharm Investig. 2019;9(3):117-21. [CrossRef] | [Google Scholar]

- Mostafapour FK, Bazi M, Siddiqui SH, Bagheri H, Balarak D. Highly efficient adsorption and removal of amoxicillin from aqueous solution by magnetic graphene oxide nanocomposite. Int J Pharm Investig. 2021;11(4):384-8. [CrossRef] | [Google Scholar]

- Bazi M, Balarak D, Khatibi AD, Siddiqui SH, Mostafapour FK. Investigation of isotherm, kinetics and thermodynamics of ciprofloxacin adsorption by molecularly imprinted polymer from aqueous solutions. Int J Pharm Investig. 2021;11(3):269-73. [CrossRef] | [Google Scholar]

- Ahmed MJ, Theydan SK. Microwave assisted preparation of microporous activated carbon from siris seed pods for adsorption of metronidazole antibiotic. Chem Eng J. 2013;214:310-8. [CrossRef] | [Google Scholar]

- Moussavi G, Alahabadi A, Yaghmaeian K, Eskandari M. Preparation, characterization and adsorption potential of the NH4Cl-induced activated carbon for the removal of amoxicillin antibiotic from water. Chem Eng J. 2013;217:119-28. [CrossRef] | [Google Scholar]

- Ocampo-Pérez R, Leyva-Ramos R, Rivera-Utrilla J, Flores-Cano JV, Sánchez-Polo M. Modeling adsorption rate of tetracyclines on activated carbons from aqueous phase. Chem Eng Res Des. 2015;104:579-88. [CrossRef] | [Google Scholar]

- Balarak D, Mostafapour FK. Isotherm and kinetic study on the adsorption of penicillin G from aqueous solution by using modified canola. J Rafsanjan Univ Med Sci. 2016;15:101-14. [CrossRef] | [Google Scholar]

- Zhang L, Liu X, Yang L, Pan F, Lv JZhang L, Song X, Liu X, Yang L, Pan F, Lv J, et al. Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem Eng J.. 2011;178:26-33. [CrossRef] | [Google Scholar]

- Mostafapour FK, Dashtizade M, Balarak D. Adsorption thermodynamics, kinetics and mechanism for the adsorption of erythromycin onto multi-walled carbon nanotubes. Br J Pharm Res. 2018;24(6):1-11. [CrossRef] | [Google Scholar]

- Jafari M, Aghamiri SF, Khaghanic G. Batch adsorption of cephalosporins antibiotics from aqueous solution by means of multi-walled carbon nanotubes. World Applied Sci Journal. 2011;14(11):1642-50. [CrossRef] | [Google Scholar]

- Chen Y, Wang F, Duan L, Yang H, Gao J. Tetracycline adsorption onto rice husk ash, an agricultural waste: Iits kinetic and thermodynamic studies. J Mol Liq. 2016;222:487-94. [CrossRef] | [Google Scholar]

- Balarak D, Baniasadi M, Bazzi M. Adsorption equilibrium and thermodynamic studies of ciprofloxacin from aqueous solutions by magnetic bentonite nanocomposites. Int J Pharm Investig. 2020;10(3):339-43. [CrossRef] | [Google Scholar]

- Zisti F, Alizadeh R, Azhdari Tehrani AZ, Morsali A, Eichhorn SH, Rawson JM, et al. Synthesis, characterization and single crystal X-ray analysis of Zn(II) phenanthridine complexes. J Mol Struct. 2019;1181:579-86. [CrossRef] | [Google Scholar]

- Zisti F, Eichhorn SH, Alizadeh R, Tehrani AA, Morsali A, Rawson JM, et al. Single crystals and nanoparticles of Zn(II) supramolecular compounds via sonochemical method: synthesis, characterization and structural studies. Inorg Chim Acta. 2019;496:118995 [CrossRef] | [Google Scholar]

- Zisti F, Tehrani AA, Alizadeh R, Abbasi H, Morsali A, Eichhorn SH, et al. Synthesis and structural characterization of three nano-structured Ag(I) coordination polymers; Syntheses, characterization and X-ray crystal structural analysis. J Solid State Chem. 2019;271:29-39. [CrossRef] | [Google Scholar]

- Balarak D, Ganji F, Chandrika K, Haseeb S. Montmorillonite nanoparticles effectiveness in removal of amoxicillin from water solutions. Int J Pharm Investig. 2020;10(2):122-6. [CrossRef] | [Google Scholar]

- Mostafapour FK, Haseeb S, Balarak D, Moein H, Sajadi AA, Jalalzaei Z, et al. Thermodynamic study of amoxicillin and naphthalene adsorption on activated carbon derived from Salvadora persica. Int J Pharm Investig. 2021;11(1):41-5. [CrossRef] | [Google Scholar]


