ABSTRACT
Background
Olanzapine causes alterations in lipid and glucose metabolism, indicated by weight gain, and altered metabolic profiles. Hesperidin has been related to numerous positive health benefits including improvements in cardiovascular and metabolic function making it a potentially suitable candidate to counteract the negative effects of olanzapine treatment. Hence the purpose of the study is to investigate the effect of hesperidin on olanzapine-induced weight gain, and associated lipid and glucose metabolic dysfunctions in female Sprague Dawley rats.
Materials and Methods
Olanzapine (2 mg/kg b.i.d. i.p.) was administered for 28 days to induce weight gain, dyslipidemia and insulin resistance in rats. Hesperidin was tested at doses of 50, 100, 200 mg/kg p.o. over 28 days. Body weight, food intake, and water intake were noted daily. Locomotor activity was recorded weekly. Novel object recognition test, Oral glucose tolerance test, and Homeostatic Model Assessment for Insulin Resistance and antioxidant biomarkers were measured followed by histopathological examination.
Results and Discussion
Treatment with hesperidin notably reduced the weight gain and hyperphagia brought on by olanzapine administration. Significant improvement in locomotor activity was observed upon hesperidin administration. Further, hesperidin resulted in a significant improvement in the discrimination ratio in the Novel object recognition test. Administration of hesperidin significantly reduced the glucose intolerance, insulin resistance and dyslipidemia induced by olanzapine treatment. Furthermore, leptin and adiponectin levels were significantly improved upon hesperidin administration. Additionally, there was a significant improvement in anti-oxidant biomarkers followed by amelioration of histological examination.
Conclusion
In conclusion, it was found that hesperidin reduced weight gain, and improved lipid, and glucose dysregulation caused by olanzapine administration. Additionally, it mitigates olanzapine-induced changes in plasma levels of leptin, and adiponectin. Hesperidin also improved cognitive behavior in olanzapine-treated rats.
INTRODUCTION
Obesity is a multifactorial disorder that majorly contributes to public health issues since it can lead to several metabolic and cardiovascular problems as well as reduced quality of life.1 The WHO estimates that 39% of adults were overweight in 2016 and 13% were obese, and by 2020, 39 million children will be overweight or obese.2 Various factors involved in the development of obesity include dietary habits, lifestyle, genetic, and environmental factors.1 Additionally, certain medications such as antipsychotics are highly linked with increased weight, and other metabolic disorders in patients.3 Olanzapine is one of the most efficacious atypical anti-psychotics used to treat schizophrenia and bipolar disorders. Administration of olanzapine demonstrates the alteration of metabolic profiles which is evident by weight gain, and changes in lipid and glucose metabolism.4,5 Significant weight gain with olanzapine has been reported in various clinical as well as preclinical studies.6–8 Further it stimulates appetite by interfering with the neurotransmitter systems that control satiety and hunger, which results in an excessive intake of calories. Additionally, the medication alters glucose and lipid metabolism, leads to insulin resistance, and disturbs metabolic homeostasis.5 Olanzapine also affects adipose tissue by promoting fat accumulation and preventing fat breakdown.9 Hormonal imbalances for instance elevated leptin levels results in increased appetite and weight gain. Olanzapine-induced sedation and decreased physical activity may also lower energy expenditure.10
Over the years, many researchers have directed their efforts in this area to understand the mechanism of olanzapine-induced weight gain, and to find an effective intervention to mitigate these unwanted side effects The seek for adjuvant therapy for the olanzapine treatment has led to the exploration of natural substances with possible anti-obesity properties. Flavonoids are highly reported for their efficacy on metabolic disorders including obesity as a potential therapeutic adjuvant.11,12
Hesperidin is a bioflavonoid, present in various citrus fruit like lemon, orange, grapefruit, tangerine etc;13 reported to possess anti-hyperlipidemic, cardioprotective, anti-diabetic, anti-hypertensive, antioxidant and anti-inflammatory properties14–17 making it a potentially suitable candidate to overcome olanzapine-induced metabolic disturbances. In preclinical research, animal models play a pivotal role to understand the underlying mechanism for olanzapine-induced obesity to identify beneficial treatment strategies.
Hence, we aim to investigate the complex interaction between olanzapine administration and weight gain as well as to explore the possible therapeutic benefits of hesperidin as a therapeutic approach in female SD rats. Understanding the mechanisms of olanzapine-induced obesity and exploring the efficacy of hesperidin to counteract the effects of olanzapine-induced obesity, could contribute towards the development of novel approaches to manage weight gain and other related metabolic disturbances associated with antipsychotic medication.
MATERIALS AND METHODS
Animals and Ethical Clearance
The IAEC of KLE College of Pharmacy, Belagavi granted ethical clearance for the animal investigations bearing resolution no. (KLECOP/CPCSEA-Reg.No.221/Po/Re/S/2000/CPCSEA, resolution no. 30). The animals were obtained from a vendor, registered at CPCSEA and were kept in a pathogen-free setting. The animals were subjected to a 12 hr cycle of light and dark, during the study.
Study Design
Female SD rats (180±10 g) were randomly divided into 5 groups, containing 6 animals in each group. These groups were (i) Normal: receive vehicle, (ii) Olz: receive olanzapine 2 mg/kg b.i.d, i.p.;18 (iii) Olz+H50: receive olanzapine 2 mg/kg b.i.d, i.p.+Hesperidin 50 mg/kg p.o;. (iv) Olz+H100: receive olanzapine 2 mg/kg b.i.d, i.p+Hesperidin 100 mg/kg p.o; (v) Olz+H200: receive olanzapine 2 mg/kg b.i.d, i.p.+Hesperidin 200 mg/kg p.o.
Body weight and BMI
The body weight (g) was monitored daily throughout the study period. Body Mass Index (BMI) of each animal was calculated using formula explained by19
Food and water intake
During the study, the food intake and water intake of the animals were monitored every day. The amount of food (g) and water (mL) remaining was subtracted from the initial amounts to determine what amount was consumed.
Locomotor activity
Locomotor activity was performed weekly during the experimental period using actophotometer. The animals were brought into the testing hall and acclimatized for 30 to 60 min before the test. Each animal was placed into the actophotometer and the number of counts for each animal was recorded.20
Novel object recognition
After the 28th treatment, NOR was performed in three sessions (i) habituation, (ii) training, and (iii) test session. During the habituation phase, each animal was permitted to explore the arena for 10 min. After 24 hr, training session was conducted; the animals were allowed to explore two identical objects that were kept in the opposing quadrants of the arena for 5 min. After 4 hr, the test session was conducted; one of the objects was replaced, and the animals were permitted to explore again for about 2 min. The time taken by the animal exploring the novel object was noted.21
Oral glucose tolerance test
After the 28th treatment, OGTT was performed as per the method explained by Salahuddin M et al., and Bagali RS et al.22,23 Overnight fasted animals were orally administered glucose dissolved in distilled water (2 g/kg of b.w.). The glucose levels of the animals were measured using a glucometer (JanaushadiSeva Kendra, India) at 0, 30, 60, and 120 min, and AUC of glucose (AUC0-120min) was calculated.
Homeostatic model assessment for insulin resistance (HOMA-IR)
The HOMA IR was calculated as per the formula explained by Chao PC et al.24 i.e.
Biochemical estimation
Following surgical anesthesia, blood from each animal was collected via cardiac puncture, and they were subsequently dissected in order to collect the adipose tissue. The adipose tissue collected was then washed, weighed, and kept in 10% v/v formalin for histological analysis by Haematoxylin and Eosin method (H & E). Total cholesterol, triglycerides, and HDL level were measured following the manufacturer’s instructions using ERBA diagnostics kits. Further, LDL, and VLDL levels were calculated as explained by Salahuddin MD et al.25 Plasma levels of leptin, adiponectin, and insulin were determined following the manufacturer’s instructions using a rat ELISA kit (KrishgenBiosystem). Oxidative biomarkers such as GSH, LPO, CAT, total thiol, and SOD were measured.26
Statistical analysis
All the data has been represented as mean±SD/SEM. To analyze the data, one-way/two-way ANOVA was used and it was followed by the required post hoc tests using GraphPad Prism Version 5. Statistically significant was determined by p-values (p<0.05, <0.01, and <0.001).
RESULTS
Effect of hesperidin on body weight, food intake, and water intake
Animals administered with Olz showed a significantly increased body weight starting from 16th day (p<0.05) to 28th day (p<0.001); whereas, Olz+H100, and Olz+H200 group resulted in significant reduction (p<0.01, and p<0.001) in body weight. The body weight of Olz+H50 did decline, however it was not statistically significant (Figure 1). Olz group resulted in a significant increase (p<0.05, p<0.01, and p<0.001) in cumulative food intake which declined significantly (p<0.05, and p<0.01) in Olz+H50, and Olz+H100 groups. Olz+H200 group showed a significant reduction (p<0.05, p<0.01, and p<0.001) in cumulative food intake (Figure 2). Further, it was observed that the water intake of the animals did not differ significantly.

Figure 1:
Effect of hesperidin on body weight.
*p<0.05,**p<0.01,***p<0.001 compared to normal, ##p<0.01, ###p<0.001 compared to olanzapine.
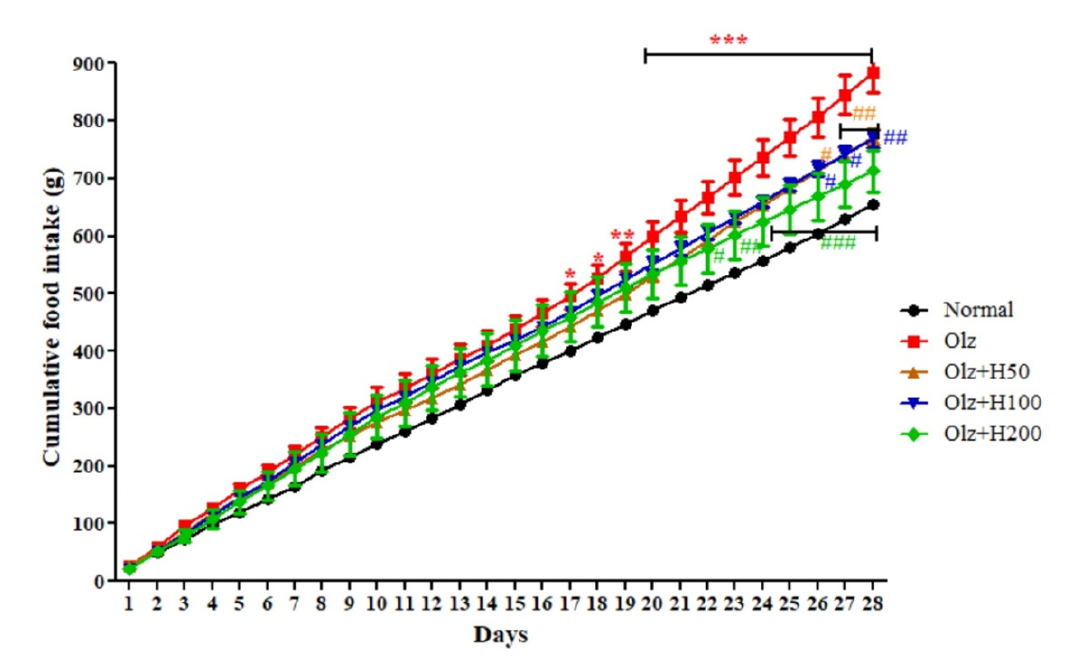
Figure 2:
Effect of hesperidin on cumulative food intake.
*p<0.05,**p<0.01,***p<0.001 compared to normal, #p<0.05, ##p<0.01, ###p<0.001 compared to olanzapine.
Effect of hesperidin on BMI and abdominal circumference
It was observed that the BMI of the Olz group had notably increased (p<0.05), which was reduced significantly in Olz+H100 (p<0.05) and Olz+H200 (p<0.01) (Figure 3). Similarly, the abdominal circumference of the Olz group was found to be significantly higher (p<0.001), which was decreased significantly in Olz+H100, and Olz+H200 group (p<0.001) and Olz+H50 group (p<0.05) (Figure 4).
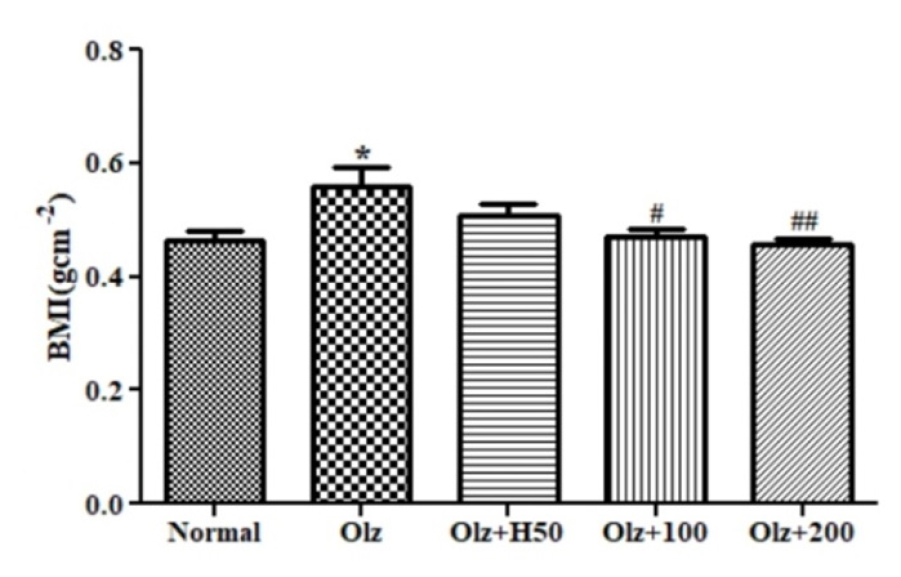
Figure 3:
Effect of hesperidin on BMI.
*p<0.05 compared to normal, #p<0.05, ##p<0.01 compared to olanzapine.
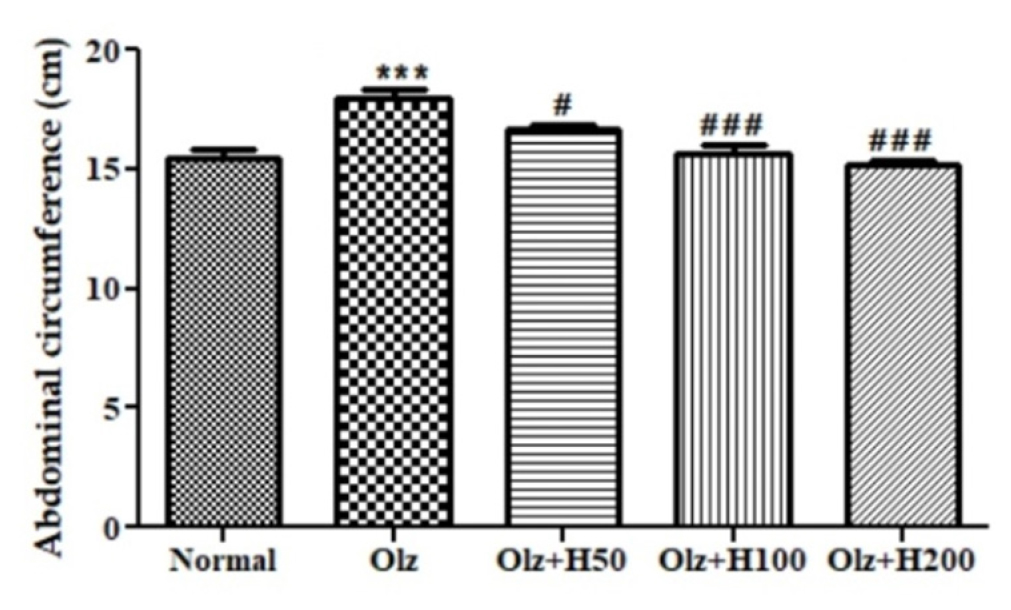
Figure 4:
Effect of hesperidin on abdominal circumference.
***p<0.001 compared to normal, and #p<0.05, ###p<0.001 compared to olanzapine.
Effect of hesperidin on locomotor activity
In comparison to the normal group, there was a significant decline in the second week (p<0.05) and in the fourth week (p<0.001). The Olz+H50, Olz+H100, and Olz+H200 groups all showed a substantial increase in locomotor activity on week four (p<0.05, p<0.01, and p<0.001, respectively) (Figure 5).
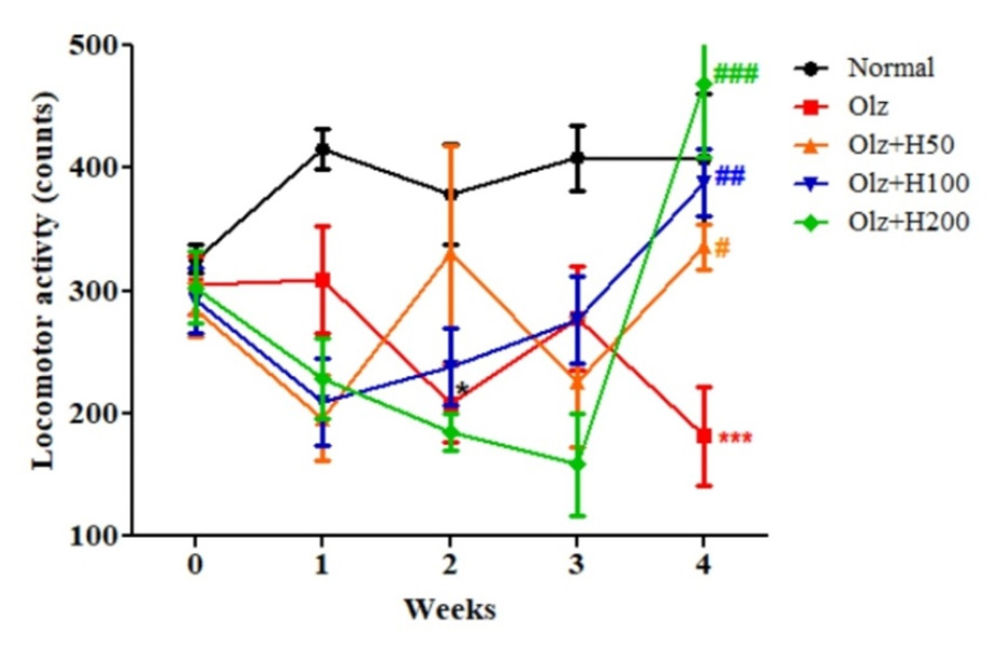
Figure 5:
Effect of hesperidin on locomotor activity.
*p<0.05,***p<0.001 compared to normal, #p<0.05, ##p<0.01, ###p<0.001 compared to olanzapine.
Effect of hesperidin on Novel object recognition test
The discrimination ratio of the Olz group was found to be significantly lower (p<0.001) than that of the normal group. In comparison to the Olz group, a significant increase (p<0.01) in the Olz+H50 group, and (p<0.001) in the Olz+H100 and Olz+H200 groups in discrimination ratio was seen (Figure 6).
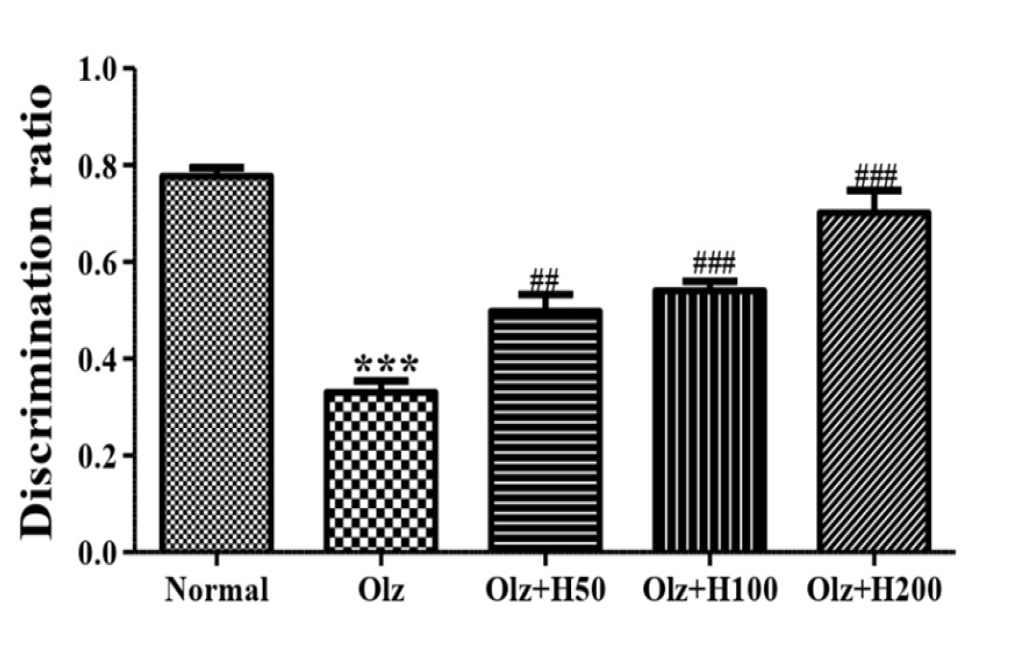
Figure 6:
Effect of hesperidin on Novel object recognition test.
***p<0.001 compared to normal, and ##p<0.01, ###p<0.001 compared to olanzapine.
Effect of hesperidin on OGTT and total AUC of glucose
The glucose level of Olz group was found to be significantly higher (p<0.001) than the normal group, but this difference was considerably reduced (p<0.001) by the co-administration of hesperidin. A significantly higher (p<0.01) AUC of glucose was observed in the Olz group than it was in the normal group. AUC of glucose was significantly declined (p<0.05) in Olz+H50 and (p<0.01) in Olz+H100 and Olz+H200 group compared to Olz group (Figure 7).

Figure 7:
Effect of hesperidin on (a) OGTT and (b) AUC of OGTT.
*p<0.05, **p<0.01, ***p<0.001 compared to normal, and #p<0.05, ##p<0.01 compared to olanzapine.
Effect of hesperidin on HOMA-IR Index
It was observed that the HOMA-IR index of Olz group was increased significantly (p<0.001) than the normal group which was reduced significantly (p<0.001) with hesperidin co-administration (Figure 8).
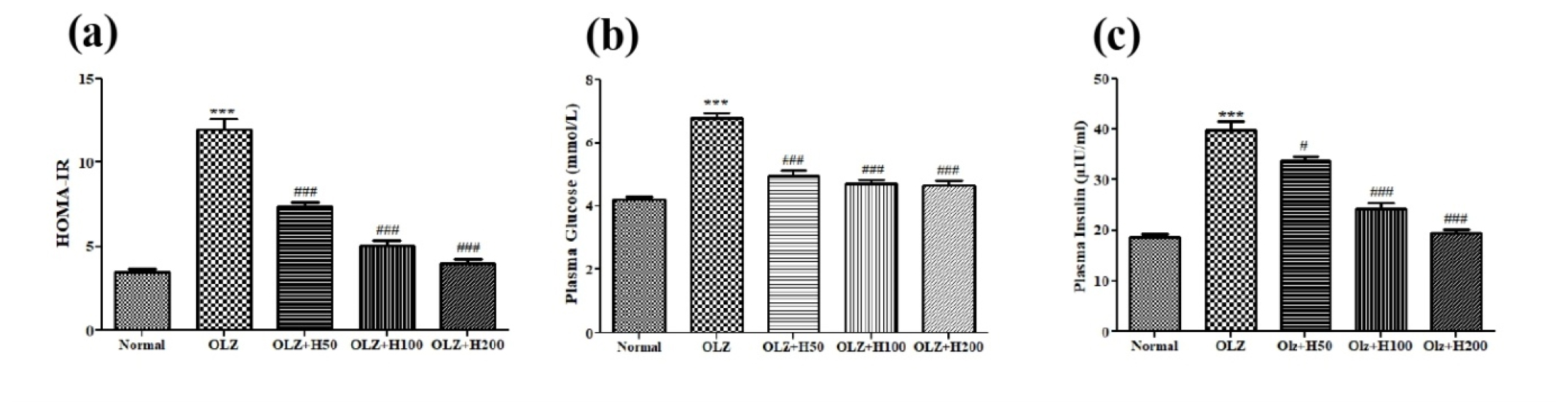
Figure 8:
Effect of hesperidin on (a) HOMA-IR (b) Plasma glucose, and (c) Plasma insulin level.
***p<0.001 compared to normal, and #p<0.05 ###p<0.001 compared to olanzapine.
Effect of hesperidin on fat pad mass
The Olz group showed a substantial rise in fat pad mass (p<0.001) than that of the normal group, which was decreased significantly in Olz+H100 (p<0.01) and Olz+H200 (p<0.001) group compared to the Olz group (Figure 9).

Figure 9:
Effect of hesperidin on fat pad mass.
***p<0.001 compared to normal, and ##p<0.01, ###p<0.001 compared to olanzapine.
Effect of hesperidin on lipid profile
Substantial increase (p<0.001) in TC, TG, LDL, and VLDL level on Olz group was observed which was significantly decreased (p<0.001) in Olz+H200 group. Further, significant reduction in Olz+H50 (p<0.01), and Olz+H100 group (p<0.001) was observed in TC, TG, and LDL level compared to normal group. Additionally, Olz group showed a considerable decrease (p<0.001) in the HDL level which was significantly improved (p<0.01) in Olz+H200 group (Table 1).
| Groups | TC | TG | HDL | LDL | VLDL |
|---|---|---|---|---|---|
| Normal | 108.7±3.19 | 76.5±5.57 | 22.83±0.94 | 70.53±3.06 | 15.3±1.11 |
| Olz | 142.5±3.18*** | 137±7.97*** | 15±1.23*** | 100.1±2.90*** | 27.4±1.59*** |
| Olz+H50 | 122.7±4.63## | 131.5±2.56## | 14.17±0.83 | 82.2±4.09## | 26.3±0.51 |
| Olz+H100 | 114.8±2.98### | 115±3.49### | 15.83±1.13 | 76±2.92### | 23±0.69# |
| Olz+H20O | 103.7±1.25’## | 90.83±4.02### | 20.67±1.17” | 64.83±1.01### | 18.17±0.80### |
Effect of hesperidin on lipid profile.
Effect of hesperidin on plasma leptin, and adiponectin level
Olanzapine administration showed a notable increase (p<0.001) in plasma leptin which was significantly reduced (p<0.001) in Olz+H100, and Olz+H200 compared to Olz group. Further, a significant reduction (p<0.001) in plasma adiponectin of Olz group was observed compared to normal which was improved significantly (p<0.001) in Olz+H100 and Olz+H200 group compared to Olz group (Figure 10).

Figure 10:
Effect of hesperidin on (a) plasma leptin, and (b) plasma adiponectin level.
***p<0.001 compared to normal, and ###p<0.001 compared to Olanzapine.
Effect of hesperidin on antioxidant biomarkers
When compared to the normal group, the LPO level of the Olz group considerably increased (p<0.001), while the LPO levels of the Olz+H50, Olz+H100, and Olz+H200 groups significantly dropped (p<0.001). The total thiol in the Olz group considerably increased (p<0.01) compared to the normal group, and it significantly decreased in the Olz+H100 (p<0.01) and Olz+H200 (p<0.001) groups compared to the Olz group. Additionally, the GSH level of the Olz group notably decreased (p<0.001) when compared to the normal group, but it significantly increased in the Olz+H100 (p<0.01) and Olz+H200 (p<0.001) groups when compared to the Olz group. There were no statistical changes in the levels of CAT and SOD (Table 2).
| Groups | LPO(Nano Moles/ mg of protein) | GSH(μMol/ mg protein) | Total thiol(μMol/ mg protein) | CAT(Unit per min/ mg of Protein) | SOD (Units/mL) |
|---|---|---|---|---|---|
| Normal | 18.31±2.20 | 24.13±1.34 | 4.19±0.99 | 9.191±2.75 | 10.03±0.91 |
| Olz | 109.5±1.91*** | 8.041±0.78*** | 18.57±3.43** | 4.208±0.50 | 10.12±0.15 |
| Olz+H50 | 37.55±3.90### | 9.639±.49 | 13.21±3.04 | 7.176±0.54 | 9.984±0.15 |
| Olz+H100 | 34.57±1.03### | 15.13±1.37” | 5.493±2.77## | 6.504±1.70 | 9.894±0.95 |
| Olz+H20O | 27.42±1.97### | 15.81±0.77### | 1.724±0.35### | 5.291±1.34 | 10.09±0.15 |
Effect of hesperidin on antioxidant biomarkers.
Effect of hesperidin on histopathological analysis of adipose tissue
Adipocytes count of Olz group was significantly elevated (p<0.01) than that of the normal group which was improved in Olz+H100 and Olz+H200 group (p<0.01) compared to the normal group. Similarly, a substantial increase (p<0.01) in adipocyte size in Olz group was observed compared to that of the normal group. Further, the adipocyte size was significantly improved in Olz+H100 (p<0.05) and Olz+H200 group (p<0.01) compared to the Olz group (Figure 11).

Figure 11:
Histopathological analysis of adipose tissue magnification HE at 40X (a) Normal, (b) Olanzapine, (c) Olz+H50, (d) Olz+H100, and (e) Olz+H200.
**p<0.01 compared to normal, and #p<0.05, ##p<0.01 compared to olanzapine.
DISCUSSION
Olanzapine, an antipsychotic medication of the second generation, is primarily used in the treatment of psychiatric conditions like schizophrenia and bipolar disorder.4 Although the treatment of psychological disorders has been revolutionized by these drugs, they are repeatedly linked with metabolic abnormalities such as weight gain, hyperglycemia, dyslipidemia, and insulin resistance.3,27,28 Among these conditions, the association of overweight and obesity with increased mortality, morbidity, as well as low quality of life, makes it a particular concern.4 Despite the difficulty in managing weight gain caused by antipsychotics, lifestyle changes and drug therapy are the main approaches used to manage these side effects. However, weight gain management strategies have had limited success, which illustrates the need for further research.
To contribute to existing knowledge, we assessed the potency of hesperidin on metabolic disturbances brought on by olanzapine in female Sprague Dawley rats. Several studies have demonstrated that olanzapine administration 2 mg/kg results in hyperphagia, weight gain, dyslipidemia, hyperleptinemia, and insulin resistance in female SD rats.3,29 Similar to these studies, our research revealed that rats administered with olanzapine had substantially increased food consumption during the study period than the rats in the control group, which subsequently resulted in weight gain. However, co-administration of hesperidin showed a significant reduction in food consumption as well as body weight, which implies that hesperidin may be able to mitigate olanzapine’s detrimental effects. According to previous studies, the increased lipogenesis and impaired lipolysis caused by olanzapine contributes to fat accumulation, as well as to energy homeostasis.9,30 Based on these reports, we utilized actophotometer to assess energy expenditure, and found notable reduction in the locomotor activity of the olanzapine-treated rats which was enhanced significantly by the co-administration of hesperidin. Additionally, the olanzapine group had significantly higher BMI and abdominal circumference, both of which were reduced with the co-administration of hesperidin. Compared to olanzapine group, hesperidin co-administration in this study resulted in a significantly decreased total fat mass.
Several studies have demonstrated that olanzapine treatment causes hyperglycemia and disruption of glucose metabolism which leads to insulin resistance. Long-term use of olanzapine has been linked to insulin resistance as it dramatically increased plasma levels of pro-inflammatory cytokines.27,28 In contrast, hesperidin has been shown to have an anti-hyperglycemic effect and improved insulin resistance in animal models of high-fat diets.31–33 Thus, we performed OGTT, where the olanzapine group resulted in significantly higher blood glucose levels compared to the normal group, but hesperidin treatment effectively prevented these effects. The HOMA-IR model was employed in this study to evaluate insulin resistance, and it was observed that co-administration of hesperidin with olanzapine decreased the HOMA-IR index, indicating that hesperidin treatment reduced the insulin resistance brought on by olanzapine.
Leptin and adiponectin, are the major hormones secreted by adipocytes, that have an integral role in the development of obesity and insulin resistance.34,35 Regarding the association between antipsychotics and leptin levels, multiple studies have presented contradictory results. According to some research, low levels of leptin hinder the ability of the hypothalamus to receive signals of satiety, which increases appetite and causes weight gain.10 While other studies, found elevated leptin levels, suggesting that
high levels of leptin are a consequence of weight gain.36–38 Like the latter, our study demonstrated considerably higher leptin levels in the olanzapine group compared to the normal, and these differences were mitigated by co-administration of hesperidin. Researchers suggest that leptin may not be the cause of weight gain in antipsychotic-induced models of obesity, but rather, a rise in leptin may be caused by the increased adiposity; resulting in hypothalamic leptin resistance which worsens obesity by decreasing anorexigenic and energy expenditure signals.39–41 In our investigation, it was corroborated by enlarged adipocytes which were observed in the histological analysis. The histological examination showed that whereas olanzapine resulted in the hyperplasia and hypertrophy of adipocytes; co-administration of hesperidin with olanzapine decreased the size and number of adipocytes. Moreover, leptin is also reported to exhibit a direct correlation with BMI, body weight percentage, hyperinsulinemia as well as HOMA-IR.42 In this study, we analyzed each of these parameters, and we observed that co-administration of hesperidin showed a positive effect by decreasing the body weight and BMI as well as improving the HOMA-IR index, indicating that hesperidin may have the potential efficacy to reduce the negative effects brought on by olanzapine.
Adiponectin is known as the anti-inflammatory cytokine, it promotes insulin signalling as well as lipid metabolism to maintain the lipid profile. Decreased level of adiponectin leads to chronic inflammation in adipose tissue which subsequently promotes the development of insulin resistance.43,44 Plasma adiponectin is also negatively correlated with BMI, abdominal circumference and oxidative stress.45 In consistent with these studies, our study showed a significantly decreased plasma adiponectin level in the olanzapine group, which was improved with hesperidin administration. Improvement in adiponectin level could contribute to the improvement of insulin resistance and lipid profile in this study.
Often, insulin resistance and dyslipidemia are associated and reinforce one another, raising the risk of cardiovascular and metabolic disease. It has been suggested that IR results in alterations in lipid and lipoprotein metabolism that lead to dyslipidemia.46 Hence, we measured the lipid levels including total cholesterol, total triglyceride, HDL, LDL, and VLDL, and found that olanzapine altered the lipid levels significantly. However, co-administration of hesperidin with olanzapine improved the lipid profiles, indicating its potential to mitigate olanzapine-induced dyslipidemia. These outcomes were in line with earlier research that showed hesperidin to have anti-adipogenic properties in both in vitro and in vivo experiments via upregulation of pAMPK expression and downregulation of SREBP-1C, ACC, and FAS expression.47,48
Insulin resistance, fat deposition, and oxidative stress have all been linked; overexpression of oxidative stress damages cellular structures and prevents the generation of antioxidant mechanisms, which leads to the emergence of complications associated with obesity.41 Consequently we measured markers of oxidative stress and antioxidant defence systems such as lipid peroxidation, glutathione, catalase, superoxide dismutase, and total thiol in this study. Consistent with previous studies, olanzapine disrupted the antioxidant levels in the rats, but co-administration of hesperidin significantly improved the levels of these oxidative stress markers.49–51
According to some research, olanzapine may have detrimental impacts on cognition,52–54 while others report improvement of the cognitive function.55,56 The effect of olanzapine may differ depending on the particular cognitive domain being studied, dosage, and the duration of the therapy.54,57 In this study, olanzapine administration led to impaired recognition memory and learning, which is assessed by the failure to distinguish between familiar and novel objects in the NOR test. This behavioral deficiency was significantly improved in the hesperidin treated group. This result complies with the previous studies where hesperidin has been reported to be able to improve cognitive function in rodents by inducing synapse formation and enhancing hippocampal neurogenesis processes.58,59
CONCLUSION
The present study demonstrates that hesperidin has the potential to mitigate metabolic disturbances brought on by olanzapine in female Sprague Dawley rats. Co-administration of hesperidin not only improved metabolic parameters but additionally exhibited a beneficial effect on memory performance. This shows that hesperidin may be used as an adjuvant therapy alongside olanzapine, without compromising olanzapine’s primary therapeutic effects. However, further preclinical and clinical research is required to validate these encouraging findings and translate these findings into safe and efficient therapeutic approaches for those on olanzapine therapy.
Cite this article
Taaza D, Jalalpure SS, Patil BM, Kurangi B. Hesperidin Attenuate Olanzapine-Induced Weight Gain, Dysregulation of Lipid and Glucose Metabolism in Rats. Int. J. Pharm. Investigation. 2024;14(2):409-18.
ACKNOWLEDGEMENT
The authors are thankful to the Department of Pharmacology, KLE College of Pharmacy, Belgaum for providing all the necessary research facilities for the completion of this work.
ABBREVIATIONS
| AUC | Area Under Curve |
|---|---|
| BMI | Body Mass Index |
| CAT | Catalase |
| GSH | Glutathione |
| HDL | High Density lipoprotein |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| IAEC | Institutional Animal Ethical Committee |
| LDL | Low density lipoprotein |
| LPO | Lipid peroxidation |
| NOR | Novel object recognition test |
| OGTT | Oral glucose tolerance test |
| SD | Sprague Dawley |
| SOD | Superoxide dismutase |
| TC | Total Cholesterol |
| TG | Triglyceride |
| VLDL | Very low density lipoprotein |
References
- Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol. 2021;12:706978 [PubMed] | [CrossRef] | [Google Scholar]

- WHO. [cited Jun 26 2023];Obesity and overweight. World Health Organization. 2021 Available fromhttp://www.who.int/news-room/fact-sheets/detail/obesity-and-over weight
[PubMed] | [CrossRef] | [Google Scholar]
- Cooper GD, Pickavance LC, Wilding JP, Halford JC, Goudie AJ. A parametric analysis of olanzapine-induced weight gain in female rats. Psychopharmacol (Berl). 2005;181(1):80-9. [PubMed] | [CrossRef] | [Google Scholar]

- Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva VA, et al. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017;13:2231-41. [PubMed] | [CrossRef] | [Google Scholar]

- Lord CC, Wyler SC, Wan R, Castorena CM, Ahmed N, Mathew D, et al. The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J Clin Invest. 2017;127(9):3402-6. [PubMed] | [CrossRef] | [Google Scholar]

- Schoretsanitis G, Dubath C, Grosu C, Piras M, Laaboub N, Ranjbar S, et al. Olanzapine-associated dose-dependent alterations for weight and metabolic parameters in a prospective cohort. Basic Clin Pharmacol Toxicol. 2022;130(4):531-41. [PubMed] | [CrossRef] | [Google Scholar]

- Hirsch L, Yang J, Bresee L, Jette N, Patten S, Pringsheim T, et al. Second-generation antipsychotics and metabolic side effects: A systematic review of population-based studies. Drug Saf. 2017;40(9):771-81. [PubMed] | [CrossRef] | [Google Scholar]

- Chen CC, Nakano T, Hsu LW, Chu CY, Huang KT. Early lipid metabolic effects of the anti-psychotic drug olanzapine on weight gain and the associated gene expression. Neuropsychiatr Dis Treat. 2022;18:645-57. [PubMed] | [CrossRef] | [Google Scholar]

- Albaugh VL, Judson JG, She P, Lang CH, Maresca KP, Joyal JL, et al. Olanzapine promotes fat accumulation in male rats by decreasing physical activity, repartitioning energy and increasing adipose tissue lipogenesis while impairing lipolysis. Mol Psychiatry. 2011;16(5):569-81. [PubMed] | [CrossRef] | [Google Scholar]

- Zugno AI, Barcelos M, Oliveira Ld, Canever L, Luca RD, Fraga DB, et al. Energy metabolism, leptin, and biochemical parameters are altered in rats subjected to the chronic administration of olanzapine. Braz J Psychiatry. 2012;34(2):168-75. [PubMed] | [CrossRef] | [Google Scholar]

- Sandoval V, Sanz-Lamora H, Arias G, Marrero PF, Haro D, Relat J, et al. Metabolic impact of flavonoids consumption in obesity: from central to peripheral. Nutrients. 2020;12(8):2393 [PubMed] | [CrossRef] | [Google Scholar]

- Gouveia HJCB, Urquiza-Martínez MV, Manhães-de-Castro R, Costa-de-Santana BJR, Villarreal JP, Mercado-Camargo R, et al. Effects of the treatment with flavonoids on metabolic syndrome components in humans: A systematic review focusing on mechanisms of action. Int J Mol Sci. 2022;23(15):8344 [PubMed] | [CrossRef] | [Google Scholar]

- Chimagave SS, Jalalpure SS, Patil AK, Kurangi BK. Development and validation of stability indicating RP-HPLC method for estimation of hesperidin in nanotransferosome and Madhiphala rasayana-an Ayurvedic marketed product. J App Pharm Sc. 2023;13(2):039-48. [CrossRef] | [Google Scholar]

- Li C, Schluesener H. Health-promoting effects of the citrus flavanone hesperidin. Crit Rev Food Sci Nutr. 2017;57(3):613-31. [PubMed] | [CrossRef] | [Google Scholar]

- Jeon HJ, Seo MJ, Choi HS, Lee OH, Lee BY. Gelidium elegans, an edible red seaweed, and hesperidin inhibit lipid accumulation and production of reactive oxygen species and reactive nitrogen species in 3T3-L1 and RAW264.7 cells. Phytother Res. 2014;28(11):1701-9. [PubMed] | [CrossRef] | [Google Scholar]

- Yoshida H, Tsuhako R, Sugita C, Kurokawa M. Glucosyl hesperidin has an anti-diabetic effect in high-fat diet-induced obese mice. Biol Pharm Bull. 2021;44(3):422-30. [PubMed] | [CrossRef] | [Google Scholar]

- Yamamoto M, Suzuki A, Hase T. Short-term effects of glucosyl hesperidin and hesperetin on blood pressure and vascular endothelial function in spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo). 2008;54(1):95-8. [PubMed] | [CrossRef] | [Google Scholar]

- Patil BM, Kulkarni NM, Unger BS. Elevation of systolic blood pressure in an animal model of olanzapine induced weight gain. Eur J Pharmacol. 2006;551(1-3):112-5. [PubMed] | [CrossRef] | [Google Scholar]

- Novelli EL, Diniz YS, Galhardi CM, Ebaid GM, Rodrigues HG, Mani F, et al. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007;41(1):111-9. [PubMed] | [CrossRef] | [Google Scholar]

- DEWS PB. The measurement of the influence of drugs on voluntary activity in mice. Br J Pharmacol Chemother. 1953;8(1):46-8. [PubMed] | [CrossRef] | [Google Scholar]

- Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. 2017;126(126):55718 [PubMed] | [CrossRef] | [Google Scholar]

- Salahuddin M, Jalalpure SS. Antidiabetic activity of aqueous fruit extract of Cucumis trigonus Roxb. in streptozotocin-induced-diabetic rats. J Ethnopharmacol. 2010;127(2):565-7. [PubMed] | [CrossRef] | [Google Scholar]

- Bagali RS, Jalalpure SS. Evaluation of antidiabetic and antioxidant effect of Schrebera swietenioides fruit ethenolic extract. Pharm Lett. 2010;2(5):278-88. [PubMed] | [CrossRef] | [Google Scholar]

- Chao PC, Li Y, Chang CH, Shieh JP, Cheng JT, Cheng KC, et al. Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomed Pharmacother. 2018;101:155-61. [PubMed] | [CrossRef] | [Google Scholar]

- Salahuddin MD, Jalalpure SS, Gadge NB. Antidiabetic activity of aqueous bark extract of Cassia glauca in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. 2010;88(2):153-60. [PubMed] | [CrossRef] | [Google Scholar]

- Shalavadi MH, Chandrashekhar VM, Avinash SP, Sowmya C, Ramkishan A. Neuroprotective activity of Stereospermum suaveolens DC against 6-OHDA induced Parkinson’s disease model. Indian J Pharmacol. 2012;44(6):737-43. [PubMed] | [CrossRef] | [Google Scholar]

- Ikegami M, Ikeda H, Ohashi T, Kai M, Osada M, Kamei A, et al. Olanzapine-induced hyperglycemia: possible involvement of histaminergic, dopaminergic and adrenergic functions in the central nervous system. Neuroendocrinology. 2013;98(3):224-32. [PubMed] | [CrossRef] | [Google Scholar]

- Wang J, Wu Q, Zhou Y, Yu L, Yu L, Deng Y, et al. The mechanisms underlying olanzapine-induced insulin resistance via the brown adipose tissue and the therapy in rats. Adipocyte. 2022;11(1):84-98. [PubMed] | [CrossRef] | [Google Scholar]

- Ullagaddi MB, Patil BM, Khanal P. Beneficial effect of Zingiber officinale on olanzapine-induced weight gain and metabolic changes. J Diabetes Metab Disord. 2021;20(1):41-8. [PubMed] | [CrossRef] | [Google Scholar]

- Vestri HS, Maianu L, Moellering DR, Garvey WT. Atypical antipsychotic drugs directly impair insulin action in adipocytes: effects on glucose transport, lipogenesis, and antilipolysis. Neuropsychopharmacology. 2007;32(4):765-72. [PubMed] | [CrossRef] | [Google Scholar]

- Peng P, Jin J, Zou G, Sui Y, Han Y, Zhao D, et al. Hesperidin prevents hyperglycemia in diabetic rats by activating the insulin receptor pathway. Exp Ther Med. 2021;21(1):53 [PubMed] | [CrossRef] | [Google Scholar]

- Rehman K, Munawar SM, Akash MSH, Buabeid MA, Chohan TA, Tariq M, et al. Hesperidin improves insulin resistance via down-regulation of inflammatory responses: biochemical analysis and in silico validation. PLOS ONE. 2020;15(1):e0227637 [PubMed] | [CrossRef] | [Google Scholar]

- Tian M, Han YB, Zhao CC, Liu L, Zhang FL. Hesperidin alleviates insulin resistance by improving HG-induced oxidative stress and mitochondrial dysfunction by restoring miR-149. Diabetol Metab Syndr. 2021;13(1):50 [PubMed] | [CrossRef] | [Google Scholar]

- Zou C, Shao J. Role of adipocytokines in obesity-associated insulin resistance. J Nutr Biochem. 2008;19(5):277-86. [PubMed] | [CrossRef] | [Google Scholar]

- Gunturiz Albarracín ML, Forero Torres AY. Adiponectin and leptin adipocytokines in metabolic syndrome: what is its importance?. Dubai Diabetes Endocrinol J. 2020;26(3):93-102. [CrossRef] | [Google Scholar]

- Razavi BM, Lookian F, Hosseinzadeh H. Protective effects of green tea on olanzapine-induced-metabolic syndrome in rats. Biomed Pharmacother. 2017;92:726-31. [PubMed] | [CrossRef] | [Google Scholar]

- Atmaca M, Tezcan E, Ustundag B. Plasma nitric oxide and leptin values in patients with olanzapine-induced weight gain. J Psychiatr Res. 2007;41(1-2):74-9. [PubMed] | [CrossRef] | [Google Scholar]

- Kim BJ, Sohn JW, Park CS, Hahn GH, Koo J, Noh YD, et al. Body weight and plasma levels of ghrelin and leptin during treatment with olanzapine. J Korean Med Sci. 2008;23(4):685-90. [PubMed] | [CrossRef] | [Google Scholar]

- Esen-Danaci A, Sarandöl A, Taneli F, Yurtsever F, Ozlen N. Effects of second generation antipsychotics on leptin and ghrelin. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1434-8. [PubMed] | [CrossRef] | [Google Scholar]

- Liu J, Yang X, Yu S, Zheng R. The leptin resistance. Adv Exp Med Biol. 2018;1090:145-63. [PubMed] | [CrossRef] | [Google Scholar]

- Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16(1):378-400. [PubMed] | [CrossRef] | [Google Scholar]

- Martins Mdo C, Lima Faleiro L, Fonseca A. Relationship between leptin and body mass and metabolic syndrome in an adult population. Rev Port Cardiol. 2012;31(11):711-9. Portuguese [PubMed] | [CrossRef] | [Google Scholar]

- Esteve E, Ricart W, Fernández-Real JM. Adipocytokines and insulin resistance: the possible role of lipocalin-2, retinol binding protein-4, and adiponectin. Diabetes Care. 2009;32(Suppl 2):S362-7. [PubMed] | [CrossRef] | [Google Scholar]

- Ye R, Holland WL, Gordillo R, Wang M, Wang QA, Shao M, et al. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes β-cell regeneration. eLife. 2014;3:e03851 [PubMed] | [CrossRef] | [Google Scholar]

- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752-61. [PubMed] | [CrossRef] | [Google Scholar]

- Bjornstad P, Eckel RH. Pathogenesis of lipid disorders in insulin resistance: a brief review. Curr Diabetes Rep. 2018;18:1-8. [PubMed] | [CrossRef] | [Google Scholar]

- Gómez-Zorita S, Lasa A, Abendaño N, Fernández-Quintela A, Mosqueda-Solís A, Garcia-Sobreviela MP, et al. Phenolic compounds apigenin, hesperidin and kaempferol reduce in vitro lipid accumulation in human adipocytes. J Transl Med. 2017;15(1):237 [PubMed] | [CrossRef] | [Google Scholar]

- Chen H, Nie T, Zhang P, Ma J, Shan A. Hesperidin attenuates hepatic lipid accumulation in mice fed high-fat diet and oleic acid induced HepG2 via AMPK activation. Life Sci. 2022;296:120428 [PubMed] | [CrossRef] | [Google Scholar]

- Ardakanian A, Ghasemzadeh Rahbardar M, Omidkhoda F, Razavi BM, Hosseinzadeh H. Effect of alpha-mangostin on olanzapine-induced metabolic disorders in rats. Iran J Basic Med Sci. 2022;25(2):198-207. [PubMed] | [CrossRef] | [Google Scholar]

- Eftekhari A, Azarmi Y, Parvizpur A, Eghbal MA. Involvement of oxidative stress and mitochondrial/lysosomal cross-talk in olanzapine cytotoxicity in freshly isolated rat hepatocytes. Xenobiotica. 2016;46(4):369-78. [PubMed] | [CrossRef] | [Google Scholar]

- Martins MR, Petronilho FC, Gomes KM, Dal-Pizzol F, Streck EL, Quevedo J, et al. Antipsychotic-induced oxidative stress in rat brain. Neurotox Res. 2008;13(1):63-9. [PubMed] | [CrossRef] | [Google Scholar]

- Morrens M, Wezenberg E, Verkes RJ, Hulstijn W, Ruigt GS, Sabbe BG, et al. Psychomotor and memory effects of haloperidol, olanzapine, and paroxetine in healthy subjects after short-term administration. J Clin Psychopharmacol. 2007;27(1):15-21. [PubMed] | [CrossRef] | [Google Scholar]

- Levin ED, Petro A, Beatty A. Olanzapine interactions with nicotine and mecamylamine in rats: effects on memory function. Neurotoxicol Teratol. 2005;27(3):459-64. [PubMed] | [CrossRef] | [Google Scholar]

- Babic I, Gorak A, Engel M, Sellers D, Else P, Osborne AL, et al. Liraglutide prevents metabolic side-effects and improves recognition and working memory during antipsychotic treatment in rats. J Psychopharmacol. 2018;32(5):578-90. [PubMed] | [CrossRef] | [Google Scholar]

- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25(2):233-55. [PubMed] | [CrossRef] | [Google Scholar]

- Wang CH, Li Y, Yang J, Su LY, Geng YG, Li H, et al. A randomized controlled trial of olanzapine improving memory deficits in Han Chinese patients with first-episode schizophrenia. Schizophr Res. 2013;144(1-3):129-35. [PubMed] | [CrossRef] | [Google Scholar]

- Zhou Y, Li G, Li D, Cui H, Ning Y. Dose reduction of risperidone and olanzapine can improve cognitive function and negative symptoms in stable schizophrenic patients: A single-blinded, 52-week, randomized controlled study. J Psychopharmacol. 2018;32(5):524-32. [PubMed] | [CrossRef] | [Google Scholar]

- Aranarochana A, Kaewngam S, Anosri T, Sirichoat A, Pannangrong W, Wigmore P, et al. Hesperidin reduces memory impairment associated with adult rat hippocampal neurogenesis triggered by valproic acid. Nutrients. 2021;13(12):4364 [PubMed] | [CrossRef] | [Google Scholar]

- Matias I, Diniz LP, Buosi A, Neves G, Stipursky J, Gomes FCA, et al. Flavonoid hesperidin induces synapse formation and improves memory performance through the astrocytic TGF-β1. Front Aging Neurosci. 2017;9:184 [PubMed] | [CrossRef] | [Google Scholar]


