ABSTRACT
Background
Amoebiasis, caused by the human pathogen Entamoeba histolytica is one of the most common parasitic diseases. The existing anti-amoebic drugs show significant side effects in humans. Therefore, for effective control of this parasite, an alternative strategy is required. Microorganisms having probiotic potential are gaining global importance due to their health-promoting effects along with the safety aspects. Therefore, the present study aimed to isolate new lactic acid bacterial strains having anti-amoebic activity as well as probiotic potential from a traditional fermented beverage consumed by an Indian tribal community.
Materials and Methods
Twenty bacterial isolates have been selected based on their tolerance ability towards acid and bile salt. Their biofunctional aspects and anti-amoebic potential are studied. The most potential one was chosen through heatmap visualization and principal component analysis based on probiotic attributes. Finally, the opted isolate was studied for its in vivo safety evaluation using mouse model.
Results and Discussion
The isolates were found as Gram positive, catalase negative and able to ferment different carbohydrates. They showed significant killing activity against Entamoeba histolytica. They were found to have a high tolerance to gastrointestinal stresses, antioxidant activity, sensitivity to various antibiotics, high auto-aggregation activity (up to 28.78%), cell-surface hydrophobicity (up to 78.75%) and antibacterial activity. Their negative activity in gelatin hydrolysis and haemolysis assay indicates that they are safe. From 16s rDNA sequencing, they were found to be Limosilactobacillus sp. Among the isolates, B-51 was considered as the best probiotic strain by heatmap visualization and principal component analysis. Importantly, the strain B-51 showed beneficial effects in mice when supplemented through oral gavage.
Conclusion
The isolated strains from rice fermented beverage showed anti-amoebic activity along with interesting probiotic traits. The in vivo study findings of the best one proved its potential for therapeutic application in humans.
INTRODUCTION
In the 21st century, attention has been increased towards the improvement of the quality of human life and as one of the instances, probiotic microorganisms are widely investigated to be used as therapeutic agents with numerous health benefits. Probiotics are defined as ‘‘live micro-organisms in the sense that, when they are administered in adequate amounts, they can confer health benefits on the host”.1 The micro-organisms with promising probiotic potential must exhibit certain necessary qualities such as tolerance to harsh gastrointestinal conditions adherence to the intestinal mucosa, anti-bacterial activity and anti-biotic sensitivity.2 Lactic Acid Bacteria (LAB) are a group of probiotic bacteria that are widely distributed in various environments such as dairy products, non-dairy fermented products, natural fruits and vegetables. The probiotic microorganisms have several health benefits such as prevention of lactose intolerance, allergies, hypertension, inflammatory bowel disease and colon cancer.
Amoebiasis is a gastrointestinal infection, caused by Entamoeba histolytica. It is the second leading cause of mortality from parasitic disease in the developing world.3 In most cases, amoebiasis is asymptomatic or manifested with mild diarrhoea or dysentery. Worldwide, 50 million invasive amoebiasis cases were reported with amoebic liver abscess and among them, about 100,000 cases were found as fatal. Non-invasive amoebiasis is treated using iodoquinol, diloxanide furoate or paromomycin whereas metronidazole and tinidazole are mainly used for the treatment of invasive amoebiasis. These drugs have been reported to show side effects such as anorexia, ataxia, headaches, nausea, skin rashes and vomiting when retained at high concentrations within the blood.4 An effective alternative therapy is therefore urgently required to combat this pathogen. In this context, the exploitation of probiotic organisms may be an alternative strategy for the treatment of amoebiasis. Haria is a popular rice-based fermented beverage consumed by several ethnic communities from central and eastern India. The beverage is prepared based on their indigenous knowledge which is followed from generation to generation. Bakhar is the starter culture used in haria and is responsible for fermentation. A change in the composition of bakhar would alter the microbial population in haria.5
The present study is aimed at isolation and characterization of LAB having anti-amoebic activity as well as good probiotic potential.
MATERIALS AND METHODS
Isolation and purification of LAB strains
Fourteen samples were randomly collected from different families of the Santal tribal community of Sriniketan Block in Birbhum District, West Bengal, India. The collected samples were centrifuged at 1000 g for 5 min. Serially diluted samples were spread over agar medium containing 2.3% peptone, 0.5% sodium chloride, 0.5% dextrose, 0.1% soluble starch, 0.03% L-Cysteine hydrochloride, 1.5% agar; pH 6.8 (Himedia, India) followed by incubation in anaerobic jar (Anaerobic Systems containing AnaeroGas Pack and Anaero Indicator, Himedia) at 37°C for 24 to 48 hr. Single colonies were randomly picked and streaked to obtain pure culture (Figure 1a). The purified cultures were preserved at -80°C in glycerol stock (20% v/v).

Figure 1:
a. Single screened colonies of isolate B-51 and b. Microscopic observations of isolate B-51 after Gram’s staining (100 x magnification).
Phenotypic characterization
Fifty-four colonies were isolated and finally, twenty were selected based on acid (pH 4.0) and bile salt (0.3%) tolerance. Phenotypic characterization was performed using Gram staining, KOH test, catalase test, carbohydrate fermentation (using carbohydrate kit, Himedia, Mumbai, India) and their survival ability at high salinity (3% and 5% NaCl).
Anti-amoebic activity
The anti-amoebic activity of the isolates was performed by the co-culture method. The inhibitory effect of live bacterial cells on the proliferation of Entamoeba histolytica HM1:IMSS trophozoites was studied. Prior to execution, the isolates were cultured in Modified TYI-S-33 media (MTYI-S-33) at 37°C for 16 hr under microaerophilic condition.6 Entamoeba histolytica strain HM-1:IMSS, trophozoites were grown in TYI-S-33 medium containing 10% heat-inactivated adult bovine serum (Himedia, India) and antibiotic solution (Himedia, India) that contains 100 U/mL penicillin and 0.25 mg/mL streptomycin.7 E. histolytica trophozoites were seeded at 105 cells/mL in 48-well plates, whereas bacterial culture contained 1010 cells/mL. The well plates were incubated at 37°C for 24 hr.6 Post incubation, the viability of Entamoeba trophozoites was measured by trypan blue exclusion using a haemocytometer. The percentage survivability was calculated.
In vitro probiotic characterization of LAB strains
Tolerance to gastric juice, bile salt and pancreatic juice
Simulated gastric juice (3 g L-1 pepsin, pH 2.0 and 3.0) tolerance,8 bile salt (1%) tolerance9 and pancreatic juice tolerance (1 g L-1 pancreatin, pH 8.0)10 were determined in terms of viable cell count on MRSC (0.05% of L-cysteine supplemented with de Mann Rogosa Sharpe media, Himedia) agar plates. The plates were incubated at 37°C for 48 hr in anaerobic chamber.
Where, N1 represents the total count of viable colony after treatment by simulated gastric juice and N0 represents the total count of viable colony before treatment.
In vitro adhesion
In vitro adhesion involves several types of interactions such as auto-aggregation and hydrophobicity. The specific cell-to- cell interactions were determined by auto-aggregation assay. Auto-aggregation assays were performed by spectrophotometric method.11 The auto-aggregation percentage was expressed as:
Where, At represents the absorbance at time t and A0 the absorbance at t=0.
The ability to attach with hydrocarbons indicates the extent of attachment of the microorganism to the epithelial cells. This is referred to as cell surface hydrophobicity. In this present study, toluene was used as a source of hydrocarbon.12 The cell surface hydrophobicity percentage was expressed as:
Where, A0 and A1 are the absorbance before and after the extraction with toluene respectively.
Anti-bacterial activity
Anti-bacterial activity assay was performed using the cross streak method.13 The isolates were inoculated at the center of the MRS agar plates by streaking method followed by incubation at 37°C. After incubation, the indicator strains such as Escherichia coli (MTCC 1667), Staphylococcus aureus (MTCC 96), Bacillus subtilis (MTCC 121) and Pseudomonas aeruginosa (MTCC 741) were inoculated. The inhibitory activity of the isolates was determined by the extent of the inhibition zone.
Antioxidant activity
For DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging activity assay, DPPH solution (0.2 mmol L-1) was freshly prepared in ethanol.14 Then, 500 μL of DPPH solution was added with 500 μL of bacterial cell suspension and incubated at 37°C for 30 min in dark. Absorbance at 517 nm was taken. The results were compared with the scavenging activity of ascorbate solution (10 μg mL-1). The percentage of DPPH scavenging ability was calculated as:
Where, “As” represents the absorbance of bacterial cell suspension and DPPH solution, “Ab” represents the absorbance of bacterial cell suspension and ethanol and “Ac” represents the absorbance of DPPH solution and ethanol
Safety issues
Antibiotic susceptibility
Susceptibility to different antibiotics was investigated by using the Kirby-Bauer disk diffusion method.15 Eleven antibiotics of different classes such as ampicillin (10 μg), amoxyclav (30 μg), cefuroxime (30 μg), cefotaxime (30 μg), vancomycin (30 μg), gentamicin (10 μg), streptomycin (10 μg), tetracycline (30 μg), chloramphenicol (30 μg), azithromycin (15 μg) and nitrofurantoin (300 μg) were used in this study.
Gelatin hydrolysis
Gelatinase activity of the isolates was detected by gelatin liquefaction method with slight modifications.16 Bacillus subtilis (MTCC 121) was used as positive control.
Haemolysis assay
Haemolytic activity was performed in Blood agar (Himedia, Mumbai, India) supplemented with 5% (w/v) defibrinated sheep blood. Overnight grown LAB isolates were inoculated on plates by streaking and incubated at 37°C. After 48 hr, the haemolytic reaction was monitored by observing the hydrolysis of RBC.17 Staphylococcus aureus (MTCC 96) was used as positive control.
Morphological study of selected Strains by SEM
The outer structure of two most potential isolates was examined using Scanning Electron Microscopy (SEM). Briefly, the cells were harvested by centrifugation at 5000 g for 10 min and then fixed with 2.5% glutaraldehyde for 6 hr at 4°C. Subsequently, the cells were washed three times with deionized water, then dehydrated subsequently with various concentrations of ethanol (30%-90%), and finally dried to the critical point. The samples were scanned under SEM.18
Heat map and Principal component analysis
The most potential isolate was identified by heat map and principal component analysis based on probiotic potential traits. The heat map was generated using ggplot2 package of RStudio software. The relationship among the isolates was determined by Principal Component Analysis (PCA) using RStudio software.
Molecular identification of strains by 16S rDNA sequencing
The selected isolates were grown in 10 mL MRS broth supplemented with L-cysteine at 37°C. The genomic DNA was extracted by the CTAB method and checked with 0.8% agarose gel.19 The purity and concentration of the extracted genomic DNA were evaluated by using the formula (50 μg/mL x OD260 x dilution factor) and OD260/280 ratio. The DNA was amplified using primer 9F (5’-GAGTTTGATCITIGCTCAG-3’) and 1492R (5’-TACGGYTACCTTGTTACGACTT-3’). PCR cycle was: denaturation (95°C for 3 min), followed by 30 cycles of denaturation (95°C for 30 sec), annealing (57°C for 45 sec) and extension (68°C for 1 min 30 sec) followed by a final extension (68°C for 10 min). The amplified DNA was checked by 1% agarose gel electrophoresis followed by sequencing.
In vivo safety evaluation
The most promising isolate (B-51) was studied for its in vivo safety evaluation using mouse model. Mice were housed in cages for 7 days adaptation at room temperature 25±2°. They were subjected to controlled 12 hr light/dark cycle before the experiment. During experimentation, animals were given standard animal feed and water ad libitum. All experimental procedures were approved by The University of Burdwan, Burdwan, West Bengal, India (Approval No. BU/IBSC/23/MI/16). Two groups of six Swiss Albino male mice were taken for this study. One group (treated group) was treated with bacterial suspension of 109 CFU in 100 μL of PBS daily for 4 weeks through oral gavage. The control group of mice was administered with only 100 μL of PBS. After 4 weeks of consecutive probiotic supplementation, the safety of the isolate was determined by assessing the general health status of the mice. For the assessment of health status, the parameters including body weight and organ index (liver, kidney and spleen) were calculated. Organ index was determined by organ weight/ body weight.20
Statistical analysis
The results were represented as mean±standard deviation and each experiment was carried out in a triplicate manner. The significant differences in mean values of more than two groups were assessed by ANOVA (Analysis of Variance). The significant differences in mean values between two groups were assessed by unpaired t-test. Statistical analysis was performed by GraphPad Prism software (version 8.00, GraphPad Software, USA). p-values<0.05 were considered as significant.
RESULTS
Isolation and phenotypic characterization of isolates
Fifty-four bacterial isolates were initially obtained from traditional fermented rice-based beverage, collected from Birbhum district in West Bengal, India. They were screened based on acid (pH 4.0) and bile salt (0.3%) tolerance. Twenty isolates showed good tolerance activity and so their further characterization was performed. They were found as rod shaped, Gram-positive (Figure 1b), catalase-negative and showed tolerance up to 3% of NaCl. They were able to ferment different carbohydrates and among them, sixteen were able to ferment inulin (Table 1).
| Test | B-31 | B-32 | B-34 | B-35 | B-36 | B-37 | B-43 | B-44 | B-45 | B-46 | B-47 | B-48 | B-49 | B-50 | B-51 | B-52 | B-53 | B-54 | B-55 | B-57 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactose | ± | – | – | + | ± | – | – | – | – | – | ± | ± | – | – | ± | – | ± | – | – | ± |
| Xylose | ± | – | ± | ± | ± | ± | + | ± | – | ± | ± | ± | – | – | ± | ± | ± | – | – | + |
| Maltose | + | + | + | + | + | + | + | + | + | ± | + | + | + | + | + | + | + | + | ± | + |
| Fructose | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Dextrose | + | + | + | + | + | ± | + | + | ± | ± | + | + | ± | ± | + | + | + | + | ± | + |
| Galactose | ± | ± | – | + | ± | – | ± | ± | ± | ± | + | ± | ± | ± | + | ± | + | – | – | ± |
| Raffinose | + | – | ± | + | + | ± | + | ± | ± | ± | + | + | ± | ± | + | ± | + | – | – | + |
| Trehalose | ± | – | – | + | ± | ± | ± | – | – | – | ± | ± | ± | – | ± | ± | ± | – | – | ± |
| Melibiose | + | ± | + | + | + | – | + | – | ± | ± | + | + | ± | ± | + | + | + | – | – | + |
| Sucrose | + | ± | + | + | + | ± | + | ± | – | – | + | + | ± | – | + | + | + | – | – | + |
| L-Arabinose | + | + | + | + | + | – | ± | + | + | + | + | + | + | + | + | + | + | – | + | + |
| Mannose | + | – | + | + | + | – | + | – | ± | – | + | + | ± | – | + | + | + | – | – | + |
| Inulin | + | – | + | + | + | ± | + | – | + | – | + | + | + | ± | + | + | + | + | – | + |
| Sodium gluconate | ± | – | – | – | ± | ± | – | – | – | – | ± | ± | – | – | – | – | ± | + | – | – |
| Glycerol | – | – | + | – | ± | ± | ± | – | – | – | ± | + | + | – | ± | ± | ± | – | ± | ± |
| Salicin | ± | – | ± | – | ± | – | – | – | – | – | ± | ± | – | – | ± | – | ± | – | – | – |
| Dulcitol | ± | – | – | ± | ± | – | – | – | – | – | ± | ± | – | – | – | – | ± | – | – | ± |
| Inositol | ± | – | – | ± | ± | – | – | – | – | – | ± | ± | – | – | – | – | ± | – | – | ± |
| Sorbitol | ± | – | ± | ± | ± | – | – | – | – | – | ± | ± | – | – | – | – | – | – | – | ± |
| Mannitol | + | ± | ± | ± | ± | – | ± | – | – | – | ± | ± | – | – | – | – | ± | – | ± | ± |
| Adonitol | ± | – | ± | ± | ± | – | – | – | – | – | ± | ± | – | – | ± | ± | ± | – | – | ± |
| Arabitol | ± | – | – | ± | – | – | – | – | – | – | – | – | – | – | – | ± | – | – | – | – |
| Erythritol | ± | – | – | ± | ± | – | – | – | – | – | ± | ± | – | – | – | ± | ± | – | – | ± |
| α-Methyl-D-glucoside | ± | – | – | ± | ± | – | ± | – | – | – | ± | ± | – | – | – | ± | ± | – | – | ± |
| Rhamnose | ± | – | – | ± | ± | – | – | – | – | – | ± | ± | + | – | ± | ± | ± | – | – | ± |
| Cellobiose | ± | – | – | ± | ± | – | ± | – | – | – | ± | ± | – | – | ± | ± | ± | – | – | ± |
| Melezitose | ± | – | – | ± | + | – | ± | – | – | – | ± | + | – | – | ± | ± | ± | – | – | ± |
| α-Methyl-D-Mannoside | ± | – | – | ± | ± | – | ± | – | – | – | ± | ± | – | – | ± | ± | ± | – | – | ± |
| Xylitol | ± | – | – | ± | ± | – | – | – | – | – | ± | ± | – | – | ± | ± | ± | – | – | ± |
| ONPG | – | ± | + | – | – | ± | – | ± | – | ± | – | – | – | – | – | – | – | – | – | – |
| Esculin | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | + | – | – |
| D-Arabinose | – | ± | + | ± | + | – | + | + | – | + | + | + | + | + | + | ± | + | ± | + | + |
| Citrate | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Malonate | – | ± | – | – | – | ± | – | ± | ± | ± | – | – | + | – | – | – | – | + | + | – |
| Sorbose | – | – | – | ± | ± | – | ± | – | – | – | ± | ± | – | – | ± | ± | ± | – | – | ± |
Utilization of different carbohydrates by the isolates.
Anti-amoebic activity
The present study deals with the antiproliferative activity of bacterial isolates against Entamoeba histolytica. The anti-amoebic activity assay showed that the isolates were found to have good killing activity against E. histolytica trophozoites (Figure 2a and b). Isolate B-51 was found with highest activity (87.11%) and lowest activity (72.20%) by B-48. Therefore, the bacterial strains isolated in this study can be very useful for the treatment of amoebiasis as an individual organism or in consortium.
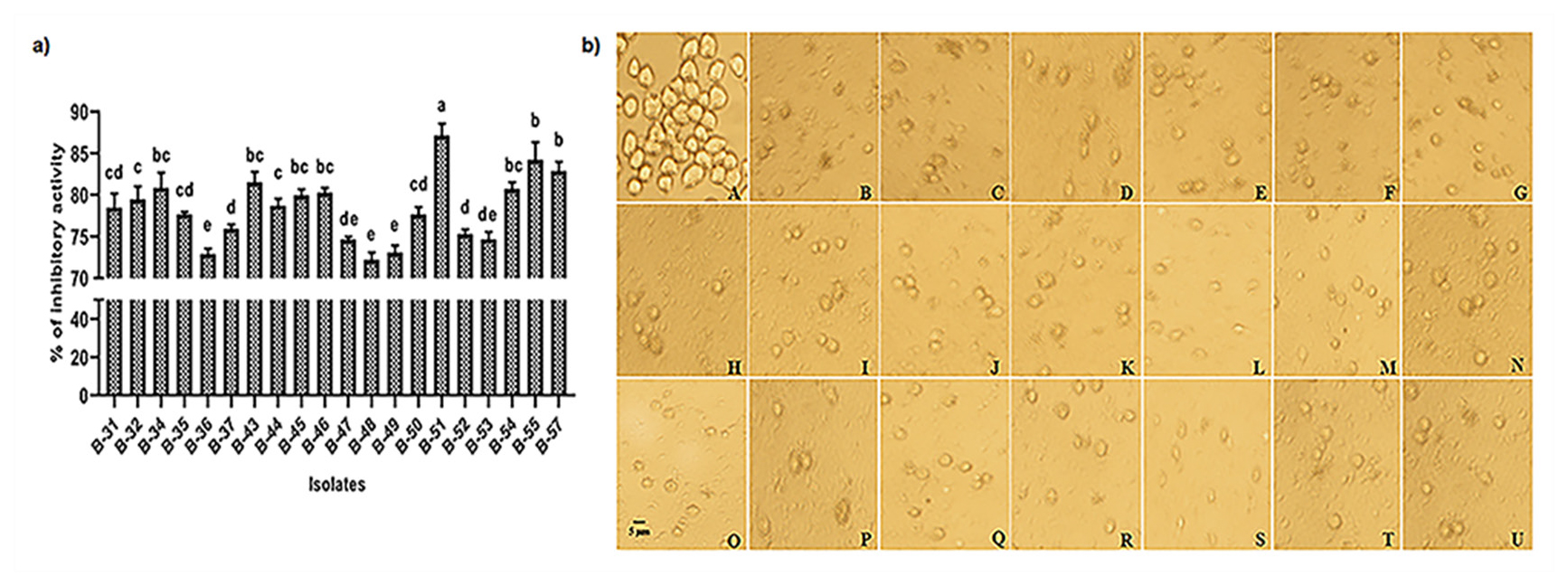
Figure 2:
a. Inhibitory activity of bacterial isolates against Entamoeba histolytica, human pathogen and b. Anti-amoebic activity: A. Control where trophozoites in amoeboid shape. B-U. Co-culture with 1010 bacterial cells/mL of isolates of B-31 to B-57: Decreased number of trophozoites due to killing activity of isolates.
In vitro probiotic characterization of LAB strains
The isolates having anti-amoebic potential were studied for their probiotic potentiality.
Tolerance to gastric juice, bile salt and pancreatic juice
In this present investigation, the isolated strains were exposed to various conditions that resemble the environment of human GI tract. The survival of LAB in gastric juice is an important prerequisite for a good probiotic. For gastric juice tolerance, the strains were exposed to simulated gastric juice of pH 2 and 3. The survival ability of the isolates was significantly different (p<0.05) at pH 2 and ranged from 92.17 to 97.41%. The highest activity was with B-51 (97.41%) after 3 hr of exposure. However, in pH 3, the highest tolerance was found for B-55 (99.02%) and the lowest was found with B-50 (96.21%) (Table 2).
| Isolates | Gastric juice (pH 2.0) | Gastric juice (pH 3.0) | ||||
|---|---|---|---|---|---|---|
| 0 min | 90 min | 180 min | 0 min | 90 min | 180 min | |
| B-31 | 97.96±0.46ab | 96.72±0.43ab | 96.24±0.39a | 98.77±0.10ab | 97.66±0.13ab | 96.75±0.45b |
| B-32 | 97.78±0.47b | 95.81±0.41b | 95.18±0.38a | 98.12±0.80ab | 97.37±0.75b | 96.84±0.84b |
| B-34 | 96.76±0.23bc | 96.01±0.21ab | 94.64±0.80b | 97.88±0.35b | 97.20±0.36b | 96.45±0.06b |
| B-35 | 96.22±0.65c | 95.75±0.57b | 95.05±0.56b | 98.34±0.15ab | 97.80±0.12ab | 97.08±0.48ab |
| B-36 | 98.33±0.18a | 96.56±0.14ab | 95.42±0.39a | 98.90±0.14a | 97.82±0.15ab | 97.41±0.14ab |
| B-37 | 96.74±0.12bc | 95.54±0.42bc | 94.36±0.60b | 98.58±0.12ab | 98.01±0.32ab | 97.14±0.33ab |
| B-43 | 98.64±0.40a | 97.45±0.19a | 95.56±0.52a | 98.28±0.97ab | 97.76±1.07ab | 97.15±1.07ab |
| B-44 | 97.40±0.42b | 96.18±0.26ab | 95.79±0.41a | 99.57±0.40a | 99.10±0.38a | 98.35±0.08a |
| B-45 | 96.49±0.11c | 94.53±0.35c | 94.02±0.49b | 98.61±0.35ab | 98.33±0.43ab | 98.04±0.47a |
| B-46 | 96.67±0.27bc | 94.64±0.11bc | 93.83±0.26b | 98.17±0.26ab | 97.39±0.35ab | 96.41±0.29b |
| B-47 | 95.46±0.07cd | 94.94±0.23bc | 92.17±0.89d | 99.39±0.24a | 98.65±0.45a | 98.70±0.06a |
| B-48 | 98.25±0.15a | 95.86±0.18b | 93.65±0.72c | 98.75±0.50ab | 98.46±0.50a | 97.05±0.58ab |
| B-49 | 92.75±0.31e | 91.99±0.34d | 90.85±0.67e | 98.59±0.79ab | 98.11±0.87ab | 97.79±0.85ab |
| B-50 | 95.49±0.11c | 94.79±0.10bc | 93.79±0.06b | 96.63±0.30b | 96.42±0.20b | 96.21±0.13b |
| B-51 | 99.41±0.10a | 97.92±0.09a | 97.41±0.12a | 99.52±0.23a | 98.78±0.10a | 98.64±0.24a |
| B-52 | 98.66±0.08a | 96.57±0.56ab | 96.12±0.36a | 99.61±0.16a | 99.29±0.11a | 97.39±0.11ab |
| B-53 | 96.80±0.29b | 95.70±0.22bc | 95.26±0.40a | 99.07±0.57a | 98.69±0.59a | 98.26±0.52a |
| B-54 | 94.71±0.11d | 94.01±0.28c | 93.36±0.29d | 97.21±0.47b | 96.86±0.46b | 96.60±0.24b |
| B-55 | 97.84±0.28b | 97.28±0.22a | 96.62±0.14a | 99.65±0.17a | 99.30±0.45a | 99.02±0.41a |
| B-57 | 97.68±0.11b | 96.99±0.10ba | 96.36±0.13a | 99.00±0.03a | 98.58±0.17a | 97.60±0.08ab |
Effect of gastric juice on the survival rate of the selected isolates.
Another important criterion is bile salt. The isolates showed 83.77 to 96.87% survival rate at 1% (w/v) bile salt solution after 3 hr of incubation (Table 3). Next to the stomach passage, probiotic microorganisms have to face the second crucial barrier, pancreatic juice in the small intestine. The isolates showed significant growth after 4 hr of incubation in pancreatic juice and their survival rate ranged between 90.16 to 96.95% (Table 3).
| Isolates | 1% Bile salt | Pancreatic juice (pH 8.0) | ||||
|---|---|---|---|---|---|---|
| 0 min | 90 min | 180 min | 0 min | 240 min | ||
| B-31 | 89.92±0.91d | 88.64±0.65e | 83.77±0.86e | 98.57±0.05b | 95.26±0.07c | |
| B-32 | 92.82±0.25c | 89.74±0.66de | 85.33±0.57de | 98.42±0.37b | 94.98±0.19c | |
| B-34 | 96.27±0.16ab | 95.22±0.03b | 94.60±0.11a | 98.27±0.15b | 95.86±0.49bc | |
| B-35 | 93.22±0.57bc | 92.75±0.35c | 91.51±0.14b | 99.05±0.37ab | 92.72±0.05e | |
| B-36 | 97.06±0.46a | 95.47±0.39ab | 94.49±0.40a | 99.01±0.09ab | 96.07±0.06b | |
| B-37 | 97.99±0.48a | 97.12±0.44a | 96.06±0.15a | 99.21±0.08ab | 94.49±0.40cd | |
| B-43 | 93.27±0.29bc | 90.83±0.15d | 88.58±0.10cd | 99.49±0.15ab | 94.01±0.04d | |
| B-44 | 94.62±0.54b | 92.01±0.11cd | 90.92±0.42bc | 99.74±0.05a | 94.25±0.22cd | |
| B-45 | 93.48±0.22bc | 92.16±0.63cd | 91.02±0.50bc | 98.26±0.42b | 92.43±0.10ef | |
| B-46 | 90.41±0.82d | 88.86±0.35e | 86.98±0.60d | 98.88±0.11b | 93.53±0.33d | |
| B-47 | 97.14±0.67a | 95.43±1.25ab | 94.59±0.98a | 99.03±0.37ab | 96.95±0.35a | |
| B-48 | 93.84±0.30bc | 92.71±0.05c | 91.65±0.11b | 98.50±0.06b | 93.67±0.19d | |
| B-49 | 97.89±0.45a | 93.89±0.59bc | 89.45±1.08c | 98.84±0.26b | 96.72±0.21ab | |
| B-50 | 94.78±0.49b | 92.10±0.09cd | 91.83±0.05b | 98.77±0.08b | 92.12±0.23ef | |
| B-51 | 97.46±0.21a | 97.19±0.06a | 96.87±0.04a | 98.51±0.21b | 96.65±0.13ab | |
| B-52 | 93.40±1.08bc | 90.81±0.20d | 88.12±0.81cd | 99.41±0.14ab | 94.85±0.03c | |
| B-53 | 90.72±0.16d | 89.39±0.95de | 87.91±0.49cd | 96.63±0.14c | 91.70±0.30f | |
| B-54 | 93.89±0.80bc | 92.09±0.11cd | 91.39±0.11b | 99.36±0.09ab | 95.86±0.26bc | |
| B-55 | 94.17±0.81bc | 92.55±0.37cd | 90.50±0.24bc | 99.68±0.07a | 90.16±0.39g | |
| B-57 | 94.90±0.03b | 91.43±0.07cd | 90.28±0.20bc | 99.70±0.04a | 94.23±0.16cd | |
Effect of bile salt and pancreatic juice on the survival rate of the selected isolates.
In vitro adhesion
Auto-aggregation and cell-surface hydrophobicity are the important properties of probiotic microorganisms which are associated with adhesion to the intestinal epithelial cells. In this present investigation, auto-aggregation ability of the isolates was measured at three different periods such as 2, 4 and 6 hr at 37°C. The highest cellular auto-aggregation value was observed for B-49 (28.78%) whereas the lowest activity was possessed by B-32 (14.66%) after 6 hr of incubation period (Table 4).
| Isolates | % of Auto-aggregation | ||
|---|---|---|---|
| 2 hr | 4 hr | 6 hr | |
| B-31 | 11.33±0.28b | 12.53±0.36bc | 16.89±0.33de |
| B-32 | 9.68±0.38b | 11.20±0.08c | 14.66±0.53e |
| B-34 | 12.25±0.16ab | 15.21±0.19ab | 19.44±0.14cd |
| B-35 | 10.01±0.78b | 12.99±0.79bc | 14.67±0.48e |
| B-36 | 8.63±0.23bc | 11.77±0.24c | 16.82±0.16de |
| B-37 | 11.04±0.34b | 14.07±0.53b | 18.11±0.48d |
| B-43 | 14.59±0.69a | 17.47±0.44a | 20.56±0.51c |
| B-44 | 13.68±0.38a | 16.30±0.61a | 20.37±0.42c |
| B-45 | 9.96±0.36b | 13.06±0.60bc | 17.23±0.41d |
| B-46 | 10.52±0.09b | 13.41±0.14bc | 17.13±0.35d |
| B-47 | 5.76±0.94cd | 10.67±0.88c | 24.99±0.47b |
| B-48 | 7.62±0.14bc | 13.80±0.22bc | 27.30±0.95a |
| B-49 | 8.14±0.74bc | 14.01±0.07b | 28.78±0.12a |
| B-50 | 8.92±0.17bc | 14.03±0.39b | 28.38±0.38a |
| B-51 | 9.74±1.08b | 12.22±1.16bc | 27.64±0.94a |
| B-52 | 7.62±0.15c | 13.33±1.51bc | 27.12±0.54a |
| B-53 | 9.27±1.45bc | 13.31±0.18bc | 26.41±0.53ab |
| B-54 | 8.97±0.42bc | 13.95±0.83b | 26.50±0.24ab |
| B-55 | 9.59±0.32bc | 13.79±0.15bc | 28.05±0.41a |
| B-57 | 4.84±1.33d | 10.44±0.67c | 25.28±0.56ab |
Evaluation of the auto-aggregation activity of twenty isolates after 2, 4 and 6 hr.
Hydrophobicity is used to measure the relative tendency of a substance whether it prefers an aqueous environment or a non-aqueous environment. Cell surface hydrophobicity was measured using toluene as a hydrocarbon. Here, isolate B-48 was observed with the highest hydrophobicity (78.75%). The lowest activity was noticed for isolate B-47 (14.16%), but B-34 had no activity (Figure 3a).

Figure 3:
a. Cell surface hydrophobicity of isolates; b. DPPH scavenging activity of ascorbate (control) and isolated strains. Values are expressed as mean±SD (n=3) and different superscript letters represent significant differences between strains measured by the Tukey’s test (p<0.05); c. Gelatin hydrolysis assay by Bacillus subtilis as positive control (1), negative control (2) and twenty bacterial isolates (3-22); d. Haemolysis activity by Staphylococcus aureus as positive control (1) and isolates (2-21) had no haemolytic activity.
Anti-bacterial activity
Lactic acid bacteria produce anti-microbial compounds that play a considerable role to maintain host health and gut microbial balance by competitive inhibition of pathogens. In the present investigation, most of the LAB isolates showed antibacterial activity against four indicator strains S. aureus, B. subtilis, P. aeruginosa and E. coli. Two isolates B-32 and B-50 did not show any antibacterial activity against P. aeruginosa and E. coli respectively (Table 5).
| Isolates | Name of the indicator strain | |||
|---|---|---|---|---|
| E. coli | S. aureas | B. subtilis | P. aeruginosa | |
| B-31 | ++ | ++ | ++ | ++ |
| B-32 | ++ | ++ | ++ | – |
| B-34 | ++ | ++ | ++ | ++ |
| B-35 | ++ | ++ | ++ | ++ |
| B-36 | ++ | ++ | ++ | ++ |
| B-37 | ++ | ++ | ++ | ++ |
| B-43 | ++ | ++ | ++ | ++ |
| B-44 | ++ | ++ | ++ | ++ |
| B-45 | ++ | ++ | ++ | ++ |
| B-46 | ++ | ++ | ++ | ++ |
| B-47 | ++ | ++ | ++ | ++ |
| B-48 | ++ | ++ | ++ | ++ |
| B-49 | ++ | ++ | ++ | ++ |
| B-50 | – | ++ | ++ | ++ |
| B-51 | ++ | ++ | ++ | ++ |
| B-52 | ++ | ++ | ++ | ++ |
| B-53 | ++ | ++ | ++ | ++ |
| B-54 | ++ | ++ | ++ | ++ |
| B-55 | ++ | ++ | ++ | ++ |
| B-57 | ++ | ++ | ++ | ++ |
Anti-microbial potential of the selected isolates.
Antioxidant activity
The antioxidants play a vital role in preventing oxidative stress-induced cellular damage and related diseases. Scavenging of DPPH free radicals is an important tool, routinely used for the antioxidant assay. In the present study, intact cells from the isolates were used for DPPH scavenging activity and compared against the scavenging activity of ascorbate (29.23%). Out of twenty, B-37 was found to have the highest (55.67%) and B-43 (10.44%) with the lowest activity (Figure 3b).
Safety issues
Safety assessment is a pivotal phase in the selection and evaluation of good probiotics. The probiotic bacteria to be consumed should be free of any acquired antibiotic resistance. A major number of the studied isolates showed sensitivity to ampicillin, amoxyclav, cefotaxime, gentamicin, tetracycline, chloramphenicol, azithromycin and nitrofurantoin (Table 6). Out of twenty isolates, isolate B-53 was found as resistant to azithromycin whereas moderate susceptibility to tetracycline was noted in two isolates and nine isolates were found to be resistant to cefuroxime. All of them showed resistance to vancomycin and streptomycin.
| Isolates | AMP (10μg) | C (30μg) | TE (30μg) | S (10μg) | GEN (10μg) | VA (30μg) | NIT (300μg) | AMC (30μg) | CXM (30μg) | AZM (15μg) | CTX (30μg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B-31 | S | S | S | R | S | R | S | S | S | S | S |
| B-32 | S | S | MS | R | S | R | S | S | R | S | S |
| B-34 | S | S | S | R | S | R | S | S | S | S | S |
| B-35 | S | S | S | R | S | R | S | S | S | S | S |
| B-36 | S | S | S | R | S | R | S | S | R | S | S |
| B-37 | S | S | S | R | S | R | S | S | S | S | S |
| B-43 | S | S | S | R | S | R | S | S | R | S | S |
| B-44 | S | S | S | R | S | R | S | S | S | S | S |
| B-45 | S | S | S | R | S | R | S | S | S | S | S |
| B-46 | S | S | S | R | S | R | S | S | R | S | S |
| B-47 | S | S | S | R | S | R | S | S | S | S | S |
| B-48 | S | S | S | R | S | R | S | S | R | S | S |
| B-49 | S | S | MS | R | S | R | S | S | R | S | S |
| B-50 | S | S | S | R | S | R | S | S | R | S | S |
| B-51 | S | S | S | R | S | R | S | S | S | S | S |
| B-52 | S | S | S | R | S | R | S | S | S | S | S |
| B-53 | S | S | S | R | S | R | S | S | S | R | S |
| B-54 | S | S | S | R | S | R | S | S | S | S | S |
| B-55 | S | S | S | R | S | R | S | S | R | S | S |
| B-57 | S | S | S | R | S | R | S | S | R | S | S |
Anti-biotic susceptibility profile of isolated LAB strains from Haria.
All the studied isolates showed negative gelatinase activity (Figure 3c). Haemolytic activity of the isolate was evaluated based on the presence of a clearance zone around the isolates on blood agar plates. No zones were found around the isolates (Figure 3d). Therefore, all these twenty isolates will be safe to use as probiotics.
Scanning electron microscopy
Cell morphology of isolates B-51 and B-55 were investigated by Scanning electron microscopy. The cells were displayed with rod-shaped and smooth-surface morphology (Figure 4).

Figure 4:
Scanning electron microscopy. Morphology of isolate B-51 (a) and B-55 (b).
Heat map and Principal component analysis
To select the best probiotic strain among the twenty isolates, a heat map analysis was performed. In this study, we used a heat map to analyze the data of in vitro probiotic traits; such as cell surface hydrophobicity, auto-aggregation, antioxidant activity and tolerance to gastric juice (pH 2.0 and pH 3.0), pancreatic juice (pH 8.0) and bile salt (1%). This analysis helps us to classify isolates into different subtypes for studying their potential. The clustered data on probiotic traits of twenty potential isolates were divided into three major clusters A, B and C (Figure 5a). From overall study, isolate B-51 showed the best results in all the probiotic phenotypic features as detected by colour intensity.
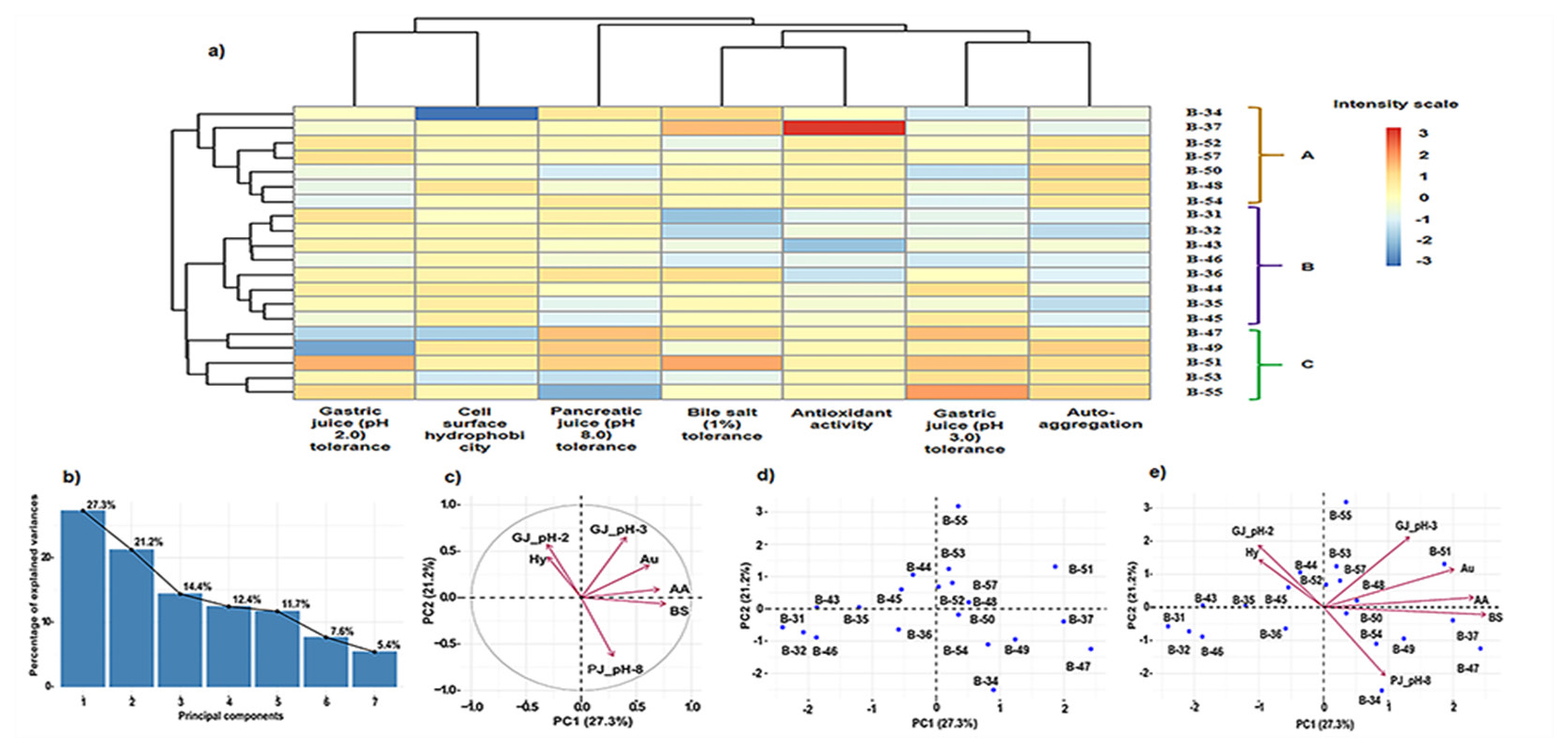
Figure 5:
a. Heat map clustering. The clustered data on probiotic traits of twenty potential isolates were divided into three major clusters A, B and C. In the cluster A, seven isolates such as B-34, B-37, B-52, B-57, B-50, B-48 and B-54 were existed, B-57 showed an average result in all phenotypic studies. In the cluster B, there are eight isolates B-31, B-32, B-43, B-46, B-36, B-44, B-35 and B-45. In the cluster C, isolates B-47, B-49, B-51, B-53 and B-55 were present and they displayed the highest results among three clusters. Here isolate B-51 showed good results in all the probiotic phenotypic studies as detected by the colour intensity. Figure. 5b-e Principal component analysis. Scree plot of seven principal components with percentage of variances for twenty isolates (5b), Projection of seven variables (5c), Projection of twenty LAB isolates (5d), PCA biplot projection based on probiotic attributes for selection of potential isolates where the percentage of variance of first two components were explained (5e).
In this present study, PCA was performed for the selection of the most potential probiotic candidate. This analysis is used to reduce the set of dependent variables (i.e., probiotic characteristics) to a smaller set of independent variables (i.e., studied isolates) based on patterns of correlation among these variables (Table 7). The Scree plot generated seven principal components (Figure 5b). The variables such as gastric juice (pH 3.0) tolerance, auto-aggregation and antioxidant activity were positively correlated to PCI and PC2, suggesting that these variables are contributing for the selection of the most relevant isolates. Another two variables such as bile salt and pancreatic juice were contributed in PCI but their position in the negative side of PC2 indicates negative correlation with PC2. Rest two variables, Gastric juice (pH 2.0) tolerance and cell-surface hydrophobicity contributed in PC2 but they were placed on the negative side of PCI (Figure 5c). The isolates present in quadrant I, II and IV show good correlation with respective variables (Figure 5d and 5e). Based on PCA analysis, again, the isolate B-51 (Accession no. OM112208) is considered as the most promising probiotic.
| Variables | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 | PC 7 |
|---|---|---|---|---|---|---|---|
| Gastric juice (pH 2.0) tolerance | -0.2235604 | 0.47187124 | -0.5902887 | 0.172925406 | -0.19383500 | 0.55725933 | 0.03007227 |
| Gastric juice (pH 3.0) tolerance | 0.2937711 | 0.53514796 | -0.2919628 | -0.361400680 | 0.24762978 | -0.42400506 | -0.41274893 |
| Bile salt (1%) tolerance | 0.5551109 | -0.05496895 | -0.3402422 | -0.004493276 | -0.38277451 | -0.25535097 | 0.60110312 |
| Pancreatic juice (pH 8.0) tolerance | 0.2102562 | -0.51778613 | -0.2732221 | -0.608297946 | -0.05193331 | 0.41790847 | -0.25625886 |
| Auto-aggregation | 0.4465000 | 0.28946369 | 0.3466297 | -0.095798020 | 0.49360755 | 0.48481093 | 0.32989256 |
| Cell-surface hydrophobicity | -0.2206526 | 0.36127352 | 0.4264839 | -0.563877403 | -0.54800409 | 0.03415798 | 0.13954335 |
| Antioxidant activity | 0.5131411 | 0.07431242 | 0.2717742 | 0.377233066 | -0.45600130 | 0.18336232 | -0.52291393 |
| Standard deviation | 1.3821 | 1.2189 | 1.0030 | 0.9326 | 0.9043 | 0.7313 | 0.61291 |
| Proportion of Variance | 0.2729 | 0.2122 | 0.1437 | 0.1242 | 0.1168 | 0.0764 | 0.05367 |
| Cumulative Proportion | 0.2729 | 0.4851 | 0.6289 | 0.7531 | 0.8699 | 0.9463 | 1.00000 |
Correlation of the variables to the principal components of the PCA analysis based on component loadings.
Molecular identification
Eleven promising isolates were selected for molecular identification by 16S rRNA gene sequencing (Figure 6). Alignments were performed using BEAST (Basic Local Alignment Search Tool) and the sequences were identified as Limosilactobacillus fermentum. The sequences were deposited in GenBank, NCBI (National Center for Biotechnology Information) and accession numbers were obtained (Table 8).
| Isolate | Species | NCBI Genbank accession ID |
|---|---|---|
| B-35 | Limosilactobacillus fermentum | OM108316 |
| B-36 | Limosilactobacillus fermentum | OM108435 |
| B-37 | Limosilactobacillus fermentum | OM108446 |
| B-44 | Limosilactobacillus fermentum | OM108466 |
| B-47 | Limosilactobacillus fermentum | OM108486 |
| B-48 | Limosilactobacillus fermentum | OM108654 |
| B-49 | Limosilactobacillus fermentum | OM112313 |
| B-51 | Limosilactobacillus fermentum | OM112208 |
| B-52 | Limosilactobacillus fermentum | OM110923 |
| B-54 | Limosilactobacillus fermentum | OM112310 |
| B-57 | Limosilactobacillus fermentum | OM112215 |
Identification of selected LAB isolates by 16S rRNA sequencing and their GenBank accession No.

Figure 6:
Agarose gel electrophoresis of purified PCR products of 16S rRNA gene from isolates: (M) DNA molecular marker-100 bp, (1) B-35, (2) B-36, (3) B-37, (4) B-44, (5) B-47, (6) B-48, (7) B-49, (8) B-51, (9) B-52, (10) B-55 and (11) B-57.
In vivo safety evaluation
In this study isolate B-51 was found as the most promising candidate based on the probiotic potential assays. It was further studied for its in vivo safety aspects. The percentage of body weight of B-51 treated mice was significantly higher (p<0.05) than control mice on the 28th day of the experiment. Almost 3.66% of average body weight increased by the treated group of mice in comparison to control group (Figure 7a). Additionally, no significant difference was observed in kidney index (Figure 5b), liver index (Figure 7c) and spleen index (Figure 7d) between these two groups.
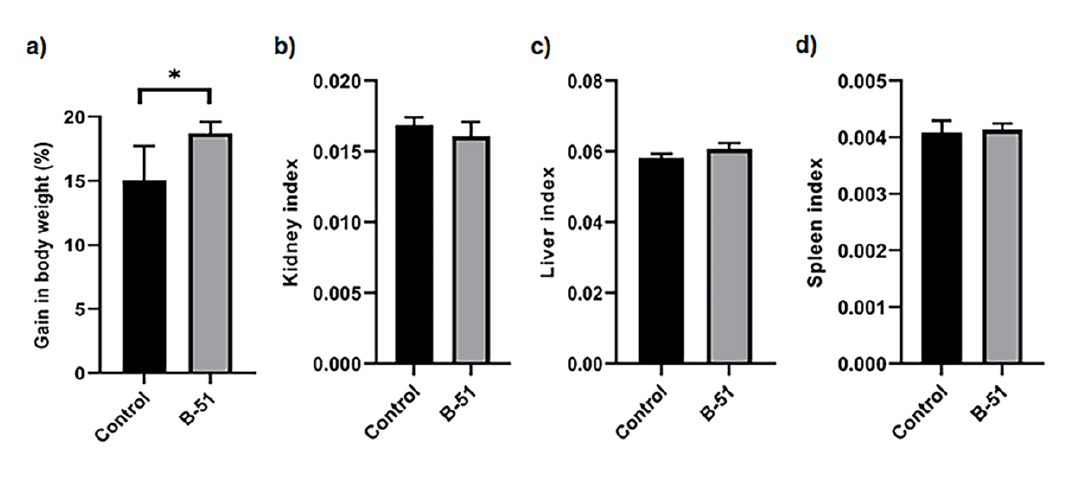
Figure 7:
Effect of probiotic B-51 supplementation on body weight gain (a), kidney index (b), liver index (c) and spleen index (d) in two groups of experimental mice. Data are represented as means±standard deviations.*p<0.05.
DISCUSSION
The ancestral knowledge of haria production has been utilzed by various ethnic communities in India. The present research work involves isolation, identification and characterization of lactic acid bacteria from a fermented rice beer collected in the district of Birbhum, West Bengal. Twenty isolates were obtained and were found as Gram positive, rod shaped and catalase negative and capable to utilize different carbohydrates. Among them, sixteen isolates could utilize the prebiotic inulin. All the isolates showed significant tolerance to salt without reducing their growth.
The normal gut microflora has a crucial role in controlling gastrointestinal diseases and maintaining the human health either by boosting the immune system or by preventing pathogen from colonization in the body. Amoebiasis, caused by human pathogen Entamoeba histolytica may alter the microbial population in gut, which could have a negative effect on a person’s health. Consequently, unlimited use of the antiprotozoal drug could raise drug resistance in E. histolytica.21 The studied isolates especially B-51, B-55 and B-57 showed good killing activity against E. histolytica trophozoites. Thus, these isolates can be used as alternative therapeutic agents for the prevention and treatment of amoebiasis.22
Probiotic micro-organisms must survive the effect of pepsin, bile salt and pancreatin throughout the time of GI passage; thereby survival in GIT is a predominant factor for selecting a good probiotic. In simulated gastric juice of pH 2 and 3, the isolates were found to have good survivability (>90%) even after 3 hr of exposure. The survival rate is significantly higher compared to most of the reported strains.10 In 1% bile salt, isolate B-51 was found with the highest survivability (96.87%) and the lowest survivability was noted for B-31 (83.77%). Thus their tolerance is quite high and comparable to the best reports when analyzed against other published data where the survival rate of probiotic LAB under 0.3% bile salt ranged between 74.32 to 82.91%.23 Pancreatic enzymes are involved in digestion of carbohydrates, proteins and fats in foods. Ability to tolerate the pancreatic enzymes is another important selection criteria of a good probiotic strain. In the present investigation, all the isolates exhibited high tolerance to pancreatic juice with 1 mg/mL concentration of pancreatin for 4 hr. The report says that Lactobacillus plantarum GCC_19M1 showed 68.42% survival in 0.5% pancreatin.24 Thus these isolated strains would definitely add resources to the existing probiotic strains.
Adhesion is an important trait for an ideal probiotic micro-organism for colonization and proliferation in the gastrointestinal tract before exertion of beneficial effect on the host. It involves several types of interactions such as auto-aggregation and hydrophobicity. The isolates showed significant auto-aggregation activity as compared to published reports; 13.8 to 36.2% after 16 hr of incubation.25 The microbial cell surface with hydrophobic nature interacts with the non-aqueous environment and this property enables it to adhere to the intestinal surface. Isolates were observed with the significant hydrophobicity that ranged from 14.16 to 78.75%. In the previous report, the probiotic isolates showed 8.4 to 66.3% of hydrophobicity to toluene.26 Therefore, the bacterial isolates in this study showed comparatively similar or better adhesion traits.
Another crucial aspect of LAB is to produce antimicrobial compounds that play a considerable role in maintaining host health and gut microbial balance by competitive inhibition of pathogens. They are extensively used as bio-conservatives against several pathogens e.g. S. aureus, L. monocytogenes and E. coli.23 The LAB isolates were studied against four indicator strains S. aureus, B. subtilis, P. aeruginosa and E. coli. The isolates showed good antibacterial activity as detected by cross-streak method.
The antioxidants play a vital role in preventing oxidative stress-induced cellular damage and related diseases. In recent days, various findings have shed new light on the appreciation of the antioxidative potentiality of probiotics. The scavenging activity of the studied isolates ranged from 10.44 to 55.67%. In other research study, twelve probiotic isolates were reported with 6.69 to 37.74% DPPH scavenging activity.27
For probiotic bacteria, it is important to check their safety but the long history of safe ingestion of some probiotics could be considered as the best evidence of their safety. Nowadays, the evaluation of antimicrobial susceptibility is of great concern for isolates with probiotic potentiality. They should not serve antibiotic resistance genes which may further increase the chances of horizontal transfer to intestinal pathogens. To check the antibiotic susceptibility of the isolates eleven common antibiotics were taken. The isolates were found to be sensitive to a major group of antibiotics except vancomycin and streptomycin. Nine isolates were found as resistant to cefuroxime and two of them showed moderately sensitive to tetracycline. Lactobacillus strains are reported to have intrinsic or natural resistance which is clinically important for the treatment of several diseases.15 So, it can be assumed that the resistance of isolates against these antibiotics are probably not acquired but intrinsic and thus they will be safe to consume as probiotics. The isolates were examined for their gelatinase and haemolytic activity where all of them were found to be negative for both gelatinase and haemolytic activity. Therefore, all these twenty isolates are considered to be potential probiotics from their safety standpoints.17
A heat map represents a good overview of the values with different colour intensities that denote the largest and smallest values in the data matrix besides clustering of similar values in the rows or columns.28 The link between the isolates was assessed based on their probiotic traits in order to distinguish the most promising candidate for future studies. Apparently, heat-map clustered twenty isolates into three main clusters. Isolate B-51 was found as the most promising based on the overall results of heat map.
PCA was performed to draw a final conclusion for the selection of most promising probiotic strain.29 From the scree plot, the first four Principal Components (PCs) explaining 75.31% of total variation, while PC1 and PC2 accounting for 27.29% and 21.22% respectively. From the analysis, highest the factor score obtained for the isolate B-51 and it was considered as the most potential candidate.
16S rRNA gene sequencing is frequently used for the identification of microorganisms.9 In this present study eleven promising isolates were identified as Limosilactobacillus fermentum by 16S rRNA gene sequencing. Limosilactobacillus fermentum, was previously known as Lactobacillus fermentum and later it has been changed with taxonomic evaluation of Lactobacillaceae.30 Probiotic L. fermentum strains are reported with numerous health benefits in prevention and treatment of several diseases including alcoholic liver disease, colorectal cancer, hyperlipidemia, gastrointestinal and upper respiratory tract infections.31,32
For therapeutic applications of potential probiotics, in vivo safety assessment is an important prerequisite. In this present study, isolate B-51 was found as the most promising and there by further studied for its in vivo safety assessment. The effects of isolate B-51 on the general health of the mice was detected in terms of measurement of body weight gain and organ indices. After 4-weeks feeding, no significant differences in organ indices, behavioral abnormality, and body weight loss were observed. These indicating that consumption of B-51 did not have adverse effect on the general health of mice. Our results support that supplementation of isolate B-51 may have beneficial effect on the mice. The in vivo safety assessment result is thus supported by other previous findings.20
CONCLUSION
This study was undertaken to isolate promising probiotic strains from a traditional rice-based fermented beverage against human pathogen Entamoeba histolytica. Twenty isolates showed significant anti-amoebic activity and probiotic potential. The isolates were found to have high survival ability under stressful simulated gastrointestinal conditions. Moreover, they showed good auto-aggregation capability, cell-surface hydrophobicity, antibacterial activity and antioxidant capability. They qualified the safety evaluation. Eleven Limosilactobacillus fermentum strains were successfully identified. From our analysis, B-51 is the best among twenty isolates to have high probiotic attributes as revealed by heat map and PCA analysis. It could exert the beneficial effects in mice also. Thus isolate B-51 can be appraised as a potentially safe probiotic strain for human. Therefore, this result demonstrates the potentiality for the development of functional foods and drinks that may have positive impact on human health by incorporating probiotic microorganism.
Cite this article
Das M, Biswas P, Dam S. Isolation and Potential Probiotic Characterization of Lactic Acid Bacteria from an Ethnic Fermented Beverage having Antibacterial Activity against Human Pathogen Entamoeba histolytica. Int. J. Pharm. Investigation. 2024;14(2):554-67.
ACKNOWLEDGEMENT
We thank the University Grant Commission, Delhi, India for Research fellowship of MD [Award Letter No. F.15-6(NOV.201 7)/2018(NET)].
ABBREVIATIONS
| LAB | Lactic acid bacteria |
|---|---|
| MRS | de Mann Rogosa Sharpe |
| PBS | Phosphate-buffered saline |
| CFU | Colony forming units |
| OD | Optical density |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| SEM | Scanning electron microscopy |
| ANOVA | Analysis of variance |
| PCA | Principal component analysis |
| PCR | Polymerase chain reaction |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| BLAST | Basic Local Alignment Search Tool |
| NCBI | National Center for Biotechnology Information |
References
- FAO/WHO. Report on drafting guidelines for the evaluation of probiotics in food. Guidelines for the evaluation of probiotics in food. London, Ontario, Canada: joint FAO/WHO working group meeting. 2002 In [Google Scholar]

- Mojgani N, Hussaini F, Vaseji N. Characterization of indigenous lactobacillus strains for probiotic properties. Jundishapur J Microbiol. 2015;8(2):e17523 [PubMed] | [CrossRef] | [Google Scholar]

- Savaş-Erdeve S, Gökay S, Dallar Y. Efficacy and safety of Saccharomyces boulardii in amebiasis-associated diarrhea in children. Turk J Pediatr. 2009;51(3):220-4. [PubMed] | [Google Scholar]

- Andersson KE. Pharmacokinetics of nitroimidazoles. Spectrum of adverse reactions. Scand J Infect Dis Suppl. 1981;26:60-7. [PubMed] | [Google Scholar]

- Ray M, Ghosh K, Singh S, Chandra Mondal K. Folk to functional: an explorative overview of rice-based fermented foods and beverages in India. J Ethn Foods. 2016;3(1):5-18. [CrossRef] | [Google Scholar]

- Sarjapuram N, Mekala N, Singh M, Tatu U. The potential of Lactobacillus casei and Entercoccus faecium combination as a preventive probiotic against Entamoeba. Probiotics Antimicrob Proteins. 2017;9(2):142-9. [PubMed] | [CrossRef] | [Google Scholar]

- Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431-2. [PubMed] | [CrossRef] | [Google Scholar]

- Charteris WP, Kelly PM, Morelli L, Collins JK. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol. 1998;84(5):759-68. [PubMed] | [CrossRef] | [Google Scholar]

- Muñoz-Quezada S, Chenoll E, Vieites JM, Genovés S, Maldonado J, Bermúdez-Brito M, et al. Isolation, identification and characterisation of three novel probiotic strains (Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036) from the faeces of exclusively breast-fed infants. Br J Nutr. 2013;109(Suppl 2):S51-62. [PubMed] | [CrossRef] | [Google Scholar]

- Maragkoudakis PA, Zoumpopoulou G, Miaris C, Kalantzopoulos G, Pot B, Tsakalidou E, et al. Probiotic potential of Lactobacillus strains isolated from dairy products. Int Dairy J. 2006;16(3):189-99. [CrossRef] | [Google Scholar]

- Devi SM, Archer AC, Halami PM. Screening, characterization and in vitro evaluation of probiotic properties among lactic acid bacteria through comparative analysis. Probiotics Antimicrob Proteins. 2015;7(3):181-92. [PubMed] | [CrossRef] | [Google Scholar]

- Sahoo TK, Jena PK, Nagar N, Patel AK, Seshadri S. In vitro Evaluation of probiotic Properties of lactic acid Bacteria from the Gut of Labeo rohita and catla catla. Probiotics Antimicrob Proteins. 2015;7(2):126-36. [PubMed] | [CrossRef] | [Google Scholar]

- Lertcanawanichakul M, Sawangnop S. A comparison of two methods used for measuring the antagonistic activity of Bacillus species. Walailak J Sci Technol. 2008;5:161-71. [CrossRef] | [Google Scholar]

- Das D, Goyal A. Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT Food Sci Technol. 2015;61(1):263-8. [CrossRef] | [Google Scholar]

- Charteris WP, Kelly PM, Morelli L, Collins JK. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot. 1998;61(12):1636-43. [PubMed] | [CrossRef] | [Google Scholar]

- El-Gendy AO, Brede DA, Essam TM, Amin MA, Ahmed SH, Holo H, et al. Purification and characterization of bacteriocins-like inhibitory substances from food isolated Enterococcus faecalis OS13 with activity against nosocomial enterococci. Sci Rep. 2021;11(1):3795 [PubMed] | [CrossRef] | [Google Scholar]

- Rao KP, Chennappa G, Suraj U, Nagaraja H, Raj AP, Sreenivasa MY, et al. Probiotic potential of lactobacillus strains isolated from sorghum-based traditional fermented food. Probiotics Antimicrob Proteins. 2015;7(2):146-56. [PubMed] | [CrossRef] | [Google Scholar]

- Guo Y, Tian X, Huang R, Tao X, Shah NP, Wei H, et al. A physiological comparative study of acid tolerance of Lactobacillus plantarum ZDY 2013 and L. plantarum ATCC 8014 at membrane and cytoplasm levels. Ann Microbiol. 2017;67(10):669-77. [CrossRef] | [Google Scholar]

- Liu W, Bao Q, Jirimutu QM, Qing M, Siriguleng Chen X. Isolation and identification of lactic acid bacteria from Tarag in Eastern Inner Mongolia of China by 16S rRNA sequences and DGGE analysis. Microbiol Res. 2012;167(2):110-5. [PubMed] | [CrossRef] | [Google Scholar]

- Li M, Wang Y, Cui H, Li Y, Sun Y, Qiu HJ, et al. Characterization of lactic acid bacteria isolated from the gastrointestinal tract of a wild boar as potential probiotics. Front Vet Sci. 2020;7:49 [PubMed] | [CrossRef] | [Google Scholar]

- Bansal D, Malla N, Mahajan RC. Drug resistance in amoebiasis. Indian J Med Res. 2006;123(2):115-8. [PubMed] | [Google Scholar]

- Sulaiman N. Lactobacillus salivarius bacteriocin and supernatant activity against Entamoeba histolytica in vitro and in vivo 2015. [PubMed] | [Google Scholar]

- Samedi L, Charles ALJAoAS. Isolation and characterization of potential probiotic Lactobacilli from leaves of food plants for possible additives in pellet feeding. Annals of Agricultural Sciences. 2019;64(1):55-62. [CrossRef] | [Google Scholar]

- Nath S, Sikidar J, Roy M, Deb B. In vitro screening of probiotic properties of Lactobacillus plantarum isolated from fermented milk product. Food Qual Saf. 2020;4(4):213-23. [CrossRef] | [Google Scholar]

- Collado MC, Surono I, Meriluoto J, Salminen S. Indigenous dadih lactic acid bacteria: cell-surface properties and interactions with pathogens. J Food Sci. 2007;72(3):M89-93. [PubMed] | [CrossRef] | [Google Scholar]

- Byakika S, Mukisa IM, Byaruhanga YB, Muyanja C. Probiotic potential of lactic acid starter cultures isolated from a traditional fermented sorghum-millet beverage. Int J Microbiol. 2020;2020:7825943 [PubMed] | [CrossRef] | [Google Scholar]

- Chen P, Zhang Q, Dang H, Liu X, Tian F, Zhao J, et al. Screening for potential new probiotic based on probiotic properties and α-glucosidase inhibitory activity. Food Control. 2014;35(1):65-72. [CrossRef] | [Google Scholar]

- Mallappa RH, Singh DK, Rokana N, Pradhan D, Batish VK, Grover S, et al. Screening and selection of probiotic Lactobacillus strains of Indian gut origin based on assessment of desired probiotic attributes combined with principal component and heatmap analysis. LWT. 2019;105:272-81. [CrossRef] | [Google Scholar]

- Angmo K, Kumari A, Savitri BTC, Bhalla TC. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT Food Sci Technol. 2016;66:428-35. [CrossRef] | [Google Scholar]

- Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782-858. [PubMed] | [CrossRef] | [Google Scholar]

- Naghmouchi K, Belguesmia Y, Bendali F, Spano G, Seal BS, Drider D, et al. Lactobacillus fermentum: a bacterial species with potential for food preservation and biomedical applications. Crit Rev Food Sci Nutr. 2020;60(20):3387-99. [PubMed] | [CrossRef] | [Google Scholar]

- Ranga C, Naved T, Thakkar A. Regulatory Pathway for the Approval of Novel Candidate Vaccine. Int J Pharm Investigation. 2024;14(2):1-9. [PubMed] | [CrossRef] | [Google Scholar]


