ABSTRACT
Purpose
This study aimed to prepare and evaluate mucoadhesive buccal patches of Sumatriptan Succinate (SUS) made from natural polymers to improve bioavailability, patient compliance, and dose-dependent adverse effects associated with currently available dosage forms.
Materials and Methods
The solvent casting method was used with a 32 factorial design. The base polymer was water-soluble polysaccharide pullulan at 2% m/v. NaCMC, SA, PVA, and PVP-K30 was also added in various amounts. The polymer-plasticizer solution, SUS, and SS were combined and dried before being cut into 2 cm patches. The patch was water-resistant-backed and stored at room temperature in an airtight glass jar.
Results
The patches had a mass uniformity of 61.75 mg to 84.18 mg and a film thickness of 0.2 mm to 0.5 mm. Some formulations demonstrated excellent folding endurance, with some exceeding 300 folds. The drug content ranged from 6.0 to 9.2 mg, with high drug loading efficiency in formulation SB29 (95%). The patches surface pH ranged from 6.0 to 7.2. At 120 min, formulation SA19 showed significant swelling (70±3%) but achieved maximum drug release (100.2%) in a shorter time. Permeation studies discovered a link between drug release and permeation. Various analyses and accelerated stability tests revealed no significant changes in physicochemical properties.
Conclusion
This study demonstrated that the prepared mucoadhesive buccal patches of SUS might be useful in treating migraines, promising improved therapeutic outcomes, increased patient compliance, reduced side effects, and cost benefits.
INTRODUCTION
Pharmaceutical research is shifting from developing new chemical entities to developing Novel Drug Delivery Systems of existing drug molecules to maximize therapeutic action, patent protection, patient compliance, and reduce adverse effects and costs. Recently, interest has grown in developing drug delivery systems using mucoadhesive polymers to attach to body tissue to target absorptive mucosa like ocular, nasal, pulmonary, buccal, and vaginal. Out of this, drug administration through the buccal region is appealing due to ease of administration, patient compliance, and the need for relatively less complicated techniques. Buccal drug delivery involves drug delivery through the buccal mucosal lining.1
Sumatriptan Succinate (SUS) (3-[2-(dimethyl amino) ethyl ]-N-methyl-1H-indole-5-methane sulphonamide succinate), used to treat migraine, is a 5 HT-1 receptor agonist. Typically, migraine lasts up to 24 hr. Still, their duration can range from 4 to 72 hr, and patients experience migraine attacks once or twice per month.2 SUS is traditionally administered as oral tablets in doses of 25 mg, 50 mg, or 100 mg as a single dose. However, due to hepatic first-pass metabolism, its oral administration results in only 15% bioavailability. It is also reported that the side effects of SUS can be dose-dependent. To overcome this limitation, SUS is developed as 10 mg or 20 mg nasal sprays and subcutaneous injections (6 mg, taken twice within 24 hr). These alternative formulations are unfortunately expensive, poorly received by patients, and have poor retention time.2
The buccal drug delivery system holds promise for addressing unmet needs in migraine treatment. By providing direct access to the systemic circulation through the internal jugular vein, this method circumvents the first-pass metabolism, increasing the bioavailability of SUS. In addition, the buccal system features noninvasive administration, rapid onset of action, a convenient and easily accessible site for drug application, self-administration capability, low enzymatic activity, suitability for drugs or additives causing mild and reversible mucosal damage or irritation, painless administration, simple drug withdrawal, low cost, and high patient compliance.3,4
Therefore, this study aimed to investigate the viability of buccal-controlled drug delivery for SUS using natural polymers. The study evaluated and optimized different formulation variables that control the physicochemical characteristics and drug release from the mucoadhesive patch to achieve consistent and controlled release of SUS to ultimately improve the therapeutic outcomes for the treatment of migraine.
MATERIALS AND METHODS
Materials
For the study, the following materials were procured from specific suppliers: Yarrow Chem Mumbai, India, supplied the SUS, pullulan, and Sodium Carboxy Methyl Cellulose (NaCMC). SD Fine Chemicals, Bangalore, India, supplied all other chemicals, including Sodium Alginate (SA), Poly Vinyl Alcohol (PVA), Poly Vinyl Pyrollidine (PVP-K30), Poly Ethylene Glycol (PEG), Propylene Glycol (PG), and Sodium Saccharin (SS). Throughout the study, analytical-grade chemicals and reagents were used.
Methods
Formulation of Mucoadhesive Buccal Patches
The solvent casting technique was used to develop SUS mucoadhesive buccal patches. The formulation ingredients were optimized using a 32 factorial design.5 Pullulan, a water-soluble polysaccharide, was employed as the base polymer at a concentration of 2% m/v. The patches also contained various proportions of NaCMC, SA, PVA, and PVP-K30. The formulation combinations are detailed in Table 1.
| Formulations* | Pullulan (2% m/v) mL | PVA (2% m/v) mL | SA (1% m/v) mL | PVP K-30 (2% m/v) mL | Na CMC | SUS (mg) | |
|---|---|---|---|---|---|---|---|
| SA1 | SB1 | 15.00 | 10.00 | 05.00 | 10.00 | ||
| SA2 | SB2 | 13.80 | 09.20 | 06.90 | 10.00 | ||
| SA3 | SB3 | 12.60 | 08.40 | 08.40 | 10.00 | ||
| SA4 | SB4 | 12.60 | 08.40 | 08.40 | 10.00 | ||
| SA5 | SB5 | 12.00 | 12.00 | 06.00 | 10.00 | ||
| SA6 | SB6 | 11.25 | 11.25 | 07.50 | 10.00 | ||
| SA7 | SB7 | 11.25 | 11.25 | 07.50 | 10.00 | ||
| SA8 | SB8 | 10.56 | 14.08 | 05.28 | 10.00 | ||
| SA9 | SB9 | 09.90 | 13.20 | 06.60 | 10.00 | ||
| SA10 | SB10 | 11.25 | 07.50 | 11.25 | 10.00 | ||
| SA11 | SB11 | 13.83 | 09.22 | 06.91 | 10.00 | ||
| SA12 | SB12 | 11.25 | 07.50 | 11.25 | 10.00 | ||
| SA13 | SB13 | 12.00 | 06.00 | 13.00 | 10.00 | ||
| SA14 | SB14 | 15.00 | 07.50 | 07.50 | 10.00 | ||
| SA15 | SB15 | 12.00 | 06.00 | 12.00 | 10.00 | ||
| SA16 | SB16 | 09.90 | 12.00 | 09.00 | 10.00 | ||
| SA17 | SB17 | 10.56 | 14.08 | 05.28 | 10.00 | ||
| SA18 | SB18 | 09.00 | 12.00 | 09.00 | 10.00 | ||
| SA19 | SB19 | 09.90 | 06.60 | 13.20 | 10.00 | ||
| SA20 | SA20 | 12.84 | 08.56 | 08.56 | 10.00 | ||
| SA21 | SB21 | 11.25 | 11.25 | 11.25 | 10.00 | ||
| SA22 | SB22 | 09.00 | 12.00 | 12.00 | 10.00 | ||
| SA23 | SB23 | 11.25 | 07.50 | 07.50 | 10.00 | ||
| SA24 | SB24 | 09.90 | 09.90 | 09.90 | 10.00 | ||
| SA25 | SB25 | 10.56 | 14.08 | 14.08 | 10.00 | ||
| SA26 | SB26 | 13.83 | 09.22 | 09.22 | 10.00 | ||
| SA27 | SB27 | 12.00 | 12.00 | 12.00 | 10.00 | ||
| SA28 | SB28 | 15.00 | 10.00 | 05.00 | 10.00 | ||
| SA29 | SB29 | 12.84 | 08.56 | 08.56 | 10.00 | ||
| SA30 | SB30 | 11.25 | 07.50 | 11.25 | 10.00 | ||
| SA31 | SB31 | 15.00 | 10.00 | 05.00 | 10.00 | ||
| SA32 | SB32 | 12.84 | 08.56 | 08.56 | 10.00 | ||
Formulation Combinations of Various Pullulan-based SUS Buccal Patches.
For the preparation of the patches, a clear and homogenous solution was prepared by adding 2 mL of either PEG or PG as a plasticizer to the polymeric solutions. A homogenizer (Biochem D-160, Molbiogen, Guwahati, India) was used to stir the mixture for 1 hr. Afterward, 10 mg of SUS and SS were added to the polymer-plasticizer solution and thoroughly mixed until a solution devoid of bubbles was obtained. The resultant solution was poured into Teflon-coated circular dishes 9.6 cm in diameter. The mixture was allowed to dry at room temperature for 2 hr before being dried at 60°C for 36 hr in a hot air oven (Labtronics Hot Air Oven, India). After drying, the patches were vacuum dried for 4 hr at room temperature. The dried patches were carefully detached from the Teflon-coated circular dishes and inspected for flaws or air bubbles. The flawless patches were then cut into 2 cm-diameter pieces with a stainless steel blade cutter. Pidilite® BOPP, a water-resistant backing, was applied to one side of the patch. The patch was wrapped in aluminium foil paper for storage and later analysis and put in an airtight glass jar at room temperature.6,7
Evaluations
Weight Variation, Thickness, Surface pH, and Folding endurance
Five different patches were selected at random for these evaluations. Patches were weighed in an electronic balance (Shimadzu TXB6201L, Japan) for weight variation (mass uniformity), and the thickness was measured using a standard screw gauge (Optec Standard Micrometer Screw guage-CHAAA010, India). The mean and standard deviation were then computed. The surface pH was calculated by leaving the patch on the surface of a 2% w/v agar plate (9.6 cm diameter) and allowing it to swell for 15 min. A pH electrode was placed on the patch’s swollen surface and left for one minute to equilibrate. Three times, the experiment was run, and the average was taken. The folding endurance was determined by repeatedly folding the patch at least 300 times or until the patch broke.8
Drug Content Uniformity
With intermittent shaking, 10 mL of pH 6.7 simulated saliva was used to dissolve the patch without a backing membrane. The above solution was filtered through 0.46 (μm) microfilter paper to obtain a clear solution. A UV spectrophotometer (Shimadzu 1800, Japan) at a 282 nm wavelength was used to measure the concentration of SUS in the solution after dilution.9
Swelling Studies
The patch’s diameter was measured without the backing membrane. This patch was applied to the agar plate and kept in an incubator (GLAB India Digital, India) at 37°C. The diameter of the swollen region was measured over time. The following equation was used to calculate the Swelling Index (SI) where Do is the diameter of the original patch at time zero, Dt is the diameter of the swollen patch after time ‘t’, and SI (%) is the % swelling:
Where Do is the diameter of the original patch at time zero, Dt is the diameter of the swollen patch after time t, and SI (%) is the % swelling.10
In vitro Residence Time (Ex vivo mucoadhesion time)
To determine the in vitro residence time, a modified USP 23 (Wrweka ZT72, Erweka, India) disintegration testing device was used. The equipment consisted of a beaker with a capacity of 1000 mL. To this, 800 mL of simulated saliva solution (pH 6.7) was added, and the temperature was maintained at 37±1°C. A small (2 x 2 cm) piece of the patch was cut and attached to the porcine mucosa (3 cm). This mucosa was attached vertically to a glass slide and placed in phosphate buffer in the beaker using cyanoacrylate adhesive. The glass slide with mucosa began to move up and down as soon as the motor was turned on. It was noticed how long it took for the patch to separate from the porcine mucosa. Six different patches were tested simultaneously.11
In vitro Drug Release Study
The USP 23 Type II dissolution equipment was used to test the in vitro drug release (rotating paddle type, eight-station dissolution test apparatus, EDT-08Lx, Electrolab, India). Water was added to the water bath, and the temperature was maintained at 37°C.
A 1000 mL cylindrical vessel with 900 mL of pH 6.7 dissolution media was submerged in the water bath. A glass slide was attached to the prepared patch (2 cm in diameter) using cyanoacrylate adhesive before being put into the cylinder. Assisted by a movable shaft, the paddle was rotated at 50 rpm. Samples were removed (5 mL) from the cylinder at appropriate intervals (5, 10, 15, 30, 45, 60, 90, 120, 150, and 180 min), and the same quantity of fresh buffer solution was then added (dissolution medium). A UV spectrophotometer (Shimadzu 1800, Japan) set to 282 nm was used to measure the quantity of SUS released from the patch after the samples were filtered through micro filter paper (0.46 μm).The drug release mechanism was determined by finding the best fit of the release data to Higuchi and Korsmeyer-Peppas models.12,13
Mechanism of Drug Release
Several mathematical models may be pertinent when studying drug release kinetics from buccal patches. The best fit to the Higuchi model was determined to establish the kinetics of SUS release from buccal patch formulations. This model can be used to explain drug dissolution from various pharmaceutical dosage forms with modified release as a square-root time-dependent diffusion process based on Fick’s law.
The release mechanism deviates from Fick’s equation and exhibits non-Fickian behavior in various experimental situations. A more general equation can be used in these situations. A fundamental, semi-empirical model that exponentially links drug release to pass the time was developed by Korsmeyer et al. in 1983.
Where ‘n’, the release exponent, is a parameter dependent on the release mechanism and is used to characterize it. Qt/Q is the fraction of the drug released at time t. K is a constant made up of the structural and geometric features of the formulation. Peppas used this n number to describe different release methods.13
In vitro Permeation Studies
During the permeation study, the amount of SUS permeated through the porcine buccal mucosa was measured using the Franz diffusion cell. The porcine buccal mucosa was obtained from a local slaughterhouse and was utilized within 2 hr of slaughter. The mucosal epithelium was carefully removed from the surrounding tissue and placed between the donor and receptor compartments of the Franz diffusion cell. The patch was secured to the donor compartment’s mucosal surface and continually stirred on a magnetic stirrer (BioGene BO7X95Y44C, India). PBS was used in the receptor compartment, while simulated saliva was used in the donor compartment. 2 mL of samples were taken out for evaluation at intervals of 5, 10, 15, 30, 45, 60, 90, and 120 min and was replaced with fresh medium.14,15
Accelerated Stability Studies and Stability in Human Saliva
Stability testing was performed for selected patches by wrapping them in aluminum foil, placing them in glass Petri dishes, and storing them for 6 months in a stability chamber at an accelerated temperature (40±5°C and 75±5% RH). The drug content, mucoadhesion time, and changes in the appearance of all formulations were evaluated at 1, 2, 3, 4, and 6 months. The data was given as an average of 3 different measurements. Additionally, the selected patches were examined in the saliva of healthy adults. The drug content and appearance were assessed by placing the patches in 5 mL of saliva at 37±0.2°C.16
FTIR Spectral Studies
Differential Scanning Calorimetry (DSC)
X-ray Diffraction Studies
RESULTS AND DISCUSSION
Evaluation of Mucoadhesive Buccal patches
Sixty-four formulations of SUS mucoadhesive buccal patches were prepared using the solvent casting method with varied polymeric combinations of cellulose and other natural polymers as per factorial designs. Physicochemical parameters such as mass uniformity, film thickness, folding durability, drug content, drug loading efficiency, and surface pH were measured for SUS buccal patches. Table 2 shows data on physiochemical properties.
| Formulation code | Mass uniformity (mg±SD) | Film thickness (mm±SD) | Folding endurance (times) | Drug content (mg±SD) | Drug loading efficiency (%) | Surface pH |
|---|---|---|---|---|---|---|
| SA1 | 72.43±6.0 | 0.4±0.003 | >300 | 9.1±0.7 | 91 | 6.2 |
| SA2 | 71.23±3.2 | 0.4±0.005 | 182 | 9.1±0.5 | 91 | 6.4 |
| SA3 | 75.52±5.1 | 0.3±0.004 | 175 | 6.8±0.7 | 68 | 6.3 |
| SA4 | 7l.54±5.6 | 0.2±0.003 | 176 | 8.3±0.8 | 83 | 7.1 |
| SA5 | 74.29±7.2 | 0.4±0.005 | 194 | 7.5±0.9 | 75 | 6.9 |
| SA6 | 75.63±6.7 | 0.3±0.004 | 190 | 6.9±0.7 | 69 | 6.6 |
| SA7 | 84.l8±3.4 | 0.4±0.002 | >300 | 8.0±0.3 | 80 | 6.7 |
| SA8 | 80.63±2.0 | 0.2±0.003 | 185 | 7.2±0.4 | 72 | 7.1 |
| SA9 | 8l.53±2.9 | 0.4±0.004 | 169 | 6.0±0.6 | 60 | 7.0 |
| SA10 | 82.3l±3.l | 0.3±0.003 | 175 | 8.0±0.5 | 80 | 6.4 |
| SA11 | 8l.44±4.2 | 0.3±0.00l | 185 | 9.1±0.4 | 91 | 6.3 |
| SA12 | 83.20±5.2 | 0.4±0.005 | 196 | 6.9±0.9 | 69 | 6.2 |
| SA13 | 63.23±l.4 | 0.4±0.004 | >300 | 9.2±0.2 | 92 | 7.0 |
| SA14 | 64.58±6.6 | 0.4±0.005 | 199 | 8.4±0.5 | 84 | 6.1 |
| SA15 | 66.45±5.2 | 0.2±0.004 | 215 | 7.9±0.6 | 79 | 6.5 |
| SA16 | 62.36±7.l | 0.2±0.003 | 188 | 8.0±0.2 | 80 | 6.2 |
| SA17 | 6l.75±6.3 | 0.4±0.002 | >300 | 8.0±0.5 | 80 | 6.4 |
| SA18 | 64.23±8.l | 0.4±0.002 | 182 | 6.7±0.6 | 67 | 6.3 |
| SA19 | 75.53±5.l | 0.4±0.006 | >300 | 9.1±0.4 | 91 | 6.7 |
| SA20 | 74.23±2.3 | 0.3±0.004 | 180 | 7.3±0.6 | 73 | 6.2 |
| SA21 | 7l.56±3.4 | 0.2±0.006 | 176 | 6.8±0.7 | 68 | 6.3 |
| SA22 | 73.54±6.l | 0.4±0.004 | 169 | 8.0±0.4 | 80 | 6.7 |
| SA23 | 74.36±5.2 | 0.3±0.002 | 175 | 7.7±0.6 | 77 | 6.8 |
| SA24 | 73.45±7.0 | 0.3±0.00l | 183 | 8.7±0.5 | 87 | 6.7 |
| SA25 | 67.85±6.9 | 0.4±0.006 | 290 | 7.9±0.6 | 79 | 6.3 |
| SA26 | 66.24±3.5 | 0.2±0.006 | 256 | 7.9±0.6 | 79 | 7.0 |
| SA27 | 66.46±4.6 | 0.4±0.006 | 188 | 8.7±0.5 | 87 | 6.4 |
| SA28 | 68.34±7.0 | 0.2±0.006 | 169 | 6.0±0.6 | 60 | 6.1 |
| SA29 | 66.54±5.9 | 0.3±0.00l | 220 | 7.9±0.6 | 79 | 7.1 |
| SA30 | 68.63±3.4 | 0.3±0.002 | 230 | 7.9±0.6 | 79 | 6.9 |
| SA31 | 66.44±2.9 | 0.4±0.006 | 199 | 6.0±0.6 | 60 | 6.0 |
| SA32 | 65.63±4.8 | 0.2±0.006 | 185 | 8.7±0.5 | 87 | 7.0 |
| SB1 | 43.23±3.0 | 0.3±0.004 | 188 | 6.9±0.7 | 69 | 6.1 |
| SB2 | 42.15±1.2 | 0.4±0.002 | >300 | 8.1±0.5 | 81 | 6.0 |
| SB3 | 4l.36±4.6 | 0.5±0.00l | 199 | 6.7±0.6 | 67 | 6.4 |
| SB4 | 43.24±5.0 | 0.2±0.005 | 210 | 7.5±0.5 | 75 | 6.3 |
| SB5 | 42.54±6.9 | 0.4±0.00l | 175 | 6.9±0.4 | 69 | 6.5 |
| SB6 | 44.43±3.l | 0.3±0.003 | 179 | 8.9±0.7 | 89 | 6.2 |
| SB7 | 47.56±3.3 | 0.2±0.002 | 185 | 7.3±0.6 | 73 | 6.0 |
| SB8 | 46.36±5.7 | 0.5±0.00l | 196 | 6.5±0.6 | 65 | 7.0 |
| SB9 | 48.54±6.1 | 0.4±0.002 | >300 | 9.2±0.1 | 92 | 7.2 |
| SB10 | 47.48±4.9 | 0.4±0.001 | 210 | 9.2±0.2 | 92 | 7.1 |
| SB11 | 46.56±5.5 | 0.2±0.004 | 167 | 7.4±0.6 | 74 | 6.4 |
| SB12 | 45.21±5.1 | 0.3±0.004 | 183 | 6.8±0.7 | 68 | 6.6 |
| SB13 | 51.63±6.0 | 0.3±0.002 | 169 | 8.8±0.2 | 88 | 6.5 |
| SB14 | 50.56±2.2 | 0.4±0.006 | 230 | 9.0±0.5 | 90 | 6.3 |
| SB15 | 52.44±4.7 | 0.3±0.005 | 200 | 7.7±0.3 | 77 | 6.4 |
| SB16 | 5l.55±7.2 | 0.4±0.004 | >300 | 9.0±0.5 | 90 | 6.2 |
| SB17 | 53.33±6.9 | 0.2±0.003 | 188 | 8.5±0.1 | 85 | 6.7 |
| SB18 | 52.5l±2.6 | 0.3±0.004 | 180 | 7.4±0.3 | 74 | 6.9 |
| SB19 | 55.25±3.4 | 0.4±0.002 | 240 | 9.0±0.2 | 90 | 6.2 |
| SB20 | 54.42±4.6 | 0.3±0.004 | 199 | 8.8±0.3 | 88 | 6.0 |
| SB21 | 55.22±4.3 | 0.4±0.006 | >300 | 9.2±0.2 | 92 | 6.4 |
| SB22 | 53.50±6.0 | 0.4±0.00l | 200 | 7.5±0.5 | 75 | 6.2 |
| SB23 | 56.25±5.7 | 0.2±0.005 | 187 | 6.7±0.2 | 67 | 6.3 |
| SB24 | 53.20±7.0 | 0.3±0.005 | 179 | 8.1±0.1 | 81 | 6.0 |
| SB25 | 52.56±4.6 | 0.4±0.006 | 166 | 8.8±0.2 | 88 | 6.0 |
| SB26 | 50.45±6.6 | 0.3±0.005 | 195 | 7.3±0.6 | 73 | 6.4 |
| SB27 | 55.2l±4.8 | 0.4±0.006 | 187 | 6.7±0.2 | 67 | 6.5 |
| SB28 | 55.36±6.0 | 0.4±0.006 | 225 | 7.7±0.3 | 77 | 6.1 |
| SB29 | 54.03±5.9 | 0.3±0.005 | 210 | 7.7±0.3 | 77 | 6.2 |
| SB30 | 55.45±l.8 | 0.4±0.006 | 195 | 7.3±0.6 | 73 | 6.0 |
| SB31 | 54.65±2.7 | 0.4±0.006 | 170 | 7.4±0.3 | 74 | 6.9 |
| SB32 | 52.36±5.4 | 0.3±0.005 | 185 | 6.7±0.2 | 67 | 7.0 |
The Physico-chemical Characteristics of Pullulan Based SUS Buccal Patches.
Mass uniformity is a crucial quality since it guarantees both the accuracy of the dose and the compliance of all the constituents with the limit, further ensuring consistent adhesive properties and ultimately improving drug delivery and patient experience,21 Depending on the formulation’s ingredients, the mass of the patches in the current study varied from 40 to 85 mg. The largest intra-batch variation was notably low, indicating that all formulations showed good mass uniformity. The satisfactory mass uniformity also indicates the robustness of the process, appropriate mixing, weighing, and drying.
The thickness of an adhesive patch is an important metric since it influences patient comfort and compliance, medication release kinetics, adhesion, and retention. In the current study, patch thickness varied from 0.2±0.002 mm to 0.4±0.006 mm. The largest intra-batch variation was notably low (±0.006). Some of the designed patches demonstrated good folding endurance of >300, ensuring durability, the convenience of handling, medication integrity protection, and product quality. A patch with a high folding endurance improves functionality, dependability, and convenience for healthcare providers and patients.
Drug loading efficiency is critical for mucoadhesive patches to maximize drug delivery, minimize patch size, increase stability, reduce costs, and assure constant drug release. The patches demonstrated good drug loading efficiency ranging from 78 to Surface pH in mucoadhesive patches is critical for mucosal compatibility, mucoadhesion optimization, medication stability, patient comfort, and regulatory compliance. The surface pH of all patches was tested, ranging from 6.0 to 7.5, indicating that the patches are not irritating to the mucosa.
Eight patches (SA1, SA7, SA13, SA19, SB2, SB9, SB16, and SB21) out of 64 demonstrated good physicochemical properties and folding endurance. As a result, these 8 patches were regarded as optimum formulations and were subjected to additional studies, including swelling studies, in vitro residence time, in vitro drug release, and accelerated stability experiments.
Swelling Studies
The swelling characteristic of mucoadhesive patches is crucial for mucoadhesion, sustained drug release, patient comfort, stability, and formulation optimization. The swelling characteristics of optimized SUS buccal patches are represented in Figure 1.
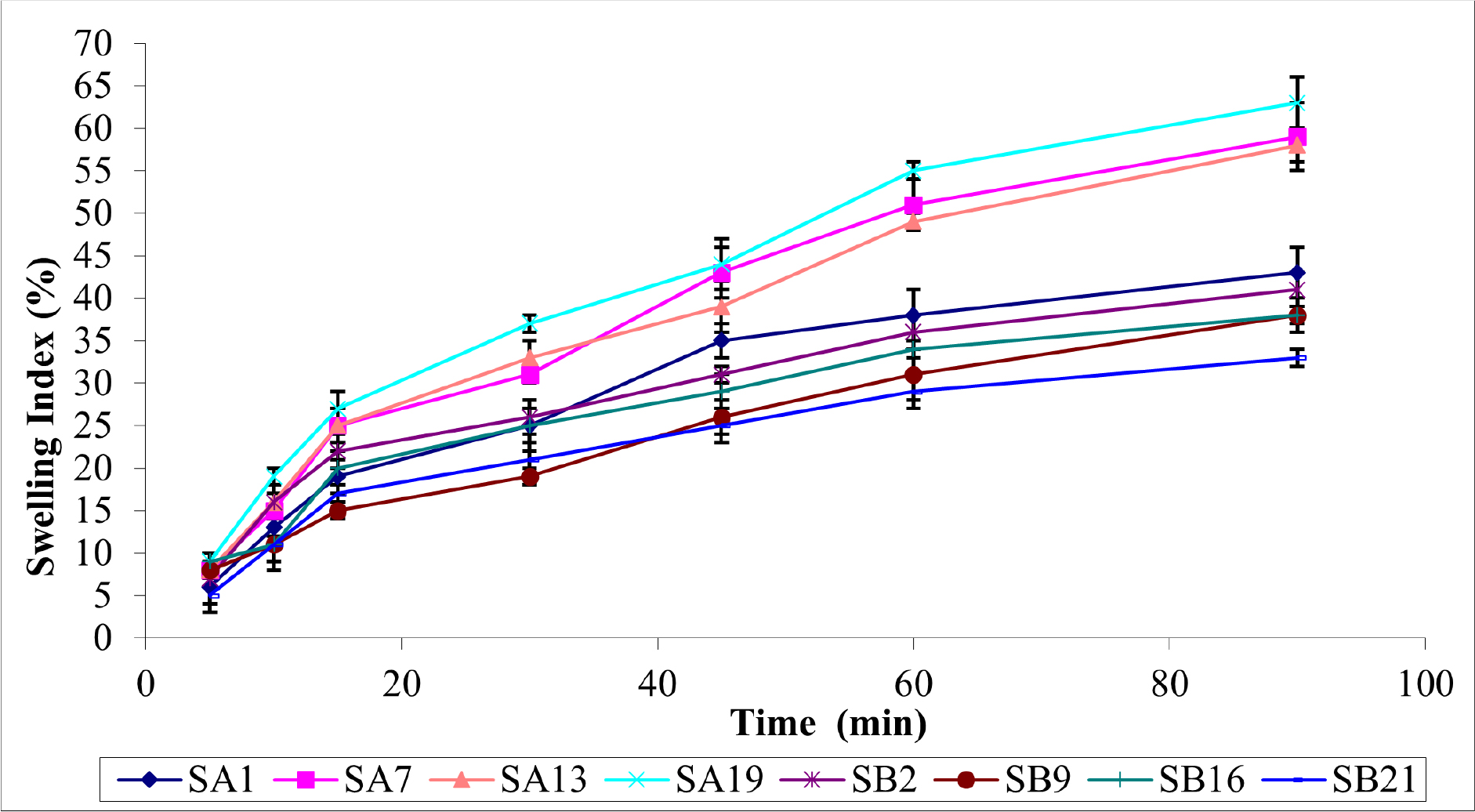
Figure 1:
Swelling Characteristics of Pullulan Based SUS Buccal Patches.
Polymer concentration, cross-linking, plasticizers, pH, ionic strength, and temperature affect mucoadhesive patch swelling. Hydrophilic polymers swell more than hydrophobic polymers. Higher polymer concentrations increase swelling. Crosslinking reduces swelling, while plasticizers increase it by increasing flexibility. Ionization and solubility affect polymer swelling. Temperature-sensitive polymers can collapse to release drugs. Understanding and optimizing these factors are essential for mucoadhesive patch swelling, transitioning from swollen to collapsed states, and enabling controlled drug release.
Taking this into account, SA19 exhibits high swelling (70±3%) at 120 min due to the presence of PVP-K30, a highly water-soluble polymer, and high PEG water uptake. Patches with PEG plasticizer have a more swelling character than patches with PG. Because of the resistance of the polymer matrix to the flow of water molecules, formulation SB21 has the lowest swelling property (35±2%) when compared to SA1 (49±1%), SA7 (67±2%), SA13 (67±1%), SB2 (45±2%), SB9 (43±3%), and SB16 (41±1%).22
In vitro Residence Time (Ex vivo mucoadhesion time)
Table 3 shows the in vitro residence time results for optimized SUS buccal patches.
| Formulations | Mucoadhesion Time (min) |
|---|---|
| SA1 | 112±2 |
| SA7 | 110±3 |
| SA13 | 125±1 |
| SA19 | 133±3 |
| SB2 | 107±3 |
| SB9 | 118±1 |
| SB16 | 128±2 |
| SB21 | 130±1 |
In vitro Residence Time for Pullulan Based SUS Buccal Patches.
In vitro residence time affects drug release, absorption, bioavailability, patient compliance, and optimal drug distribution. Several factors affect mucoadhesive buccal patch residence time in vitro. These include mucoadhesive polymer type, concentration, patch size and shape, hydration and swelling properties, saliva flow and pH, environmental conditions, and drug characteristics. Selecting a mucoadhesive polymer with high mucosal affinity and optimizing its concentration increases residence time. Optimizing patch size and shape increases contact area and patient comfort. Hydration, swelling, saliva flow, pH, and environmental conditions affect adhesion, residence time, and patch-mucosa interaction.
The current study found that the residence time of selected patches ranged from 107 to 133 min. Tmax values for SUS administration routes are as follows: 0.17 hr for subcutaneous injection, 1.50 hr for oral and intranasal administration, and 1 hr for rectal administration. Based on the Tmax of SUS, the observed residence time was deemed adequate.
In vitro Drug Release Study
In vitro drug release testing is critical for mucoadhesive buccal patches because it ensures product quality control, aids formulation development and optimization, provides information on drug release kinetics and duration, predicts in vivo performance, and aids regulatory compliance. In vitro drug release testing is critical for mucoadhesive buccal patches because it ensures product quality control, aids formulation development and optimization, provides information on drug release kinetics and duration, predicts in vivo performance, and aids regulatory compliance.
Furthermore, the ability of hydrophilic polymers to absorb water may influence the drug release rate from buccal patches, thereby increasing dissolution and, thus, the drug release rate. Figure 2 depicts the in vitro drug release from selected patches.
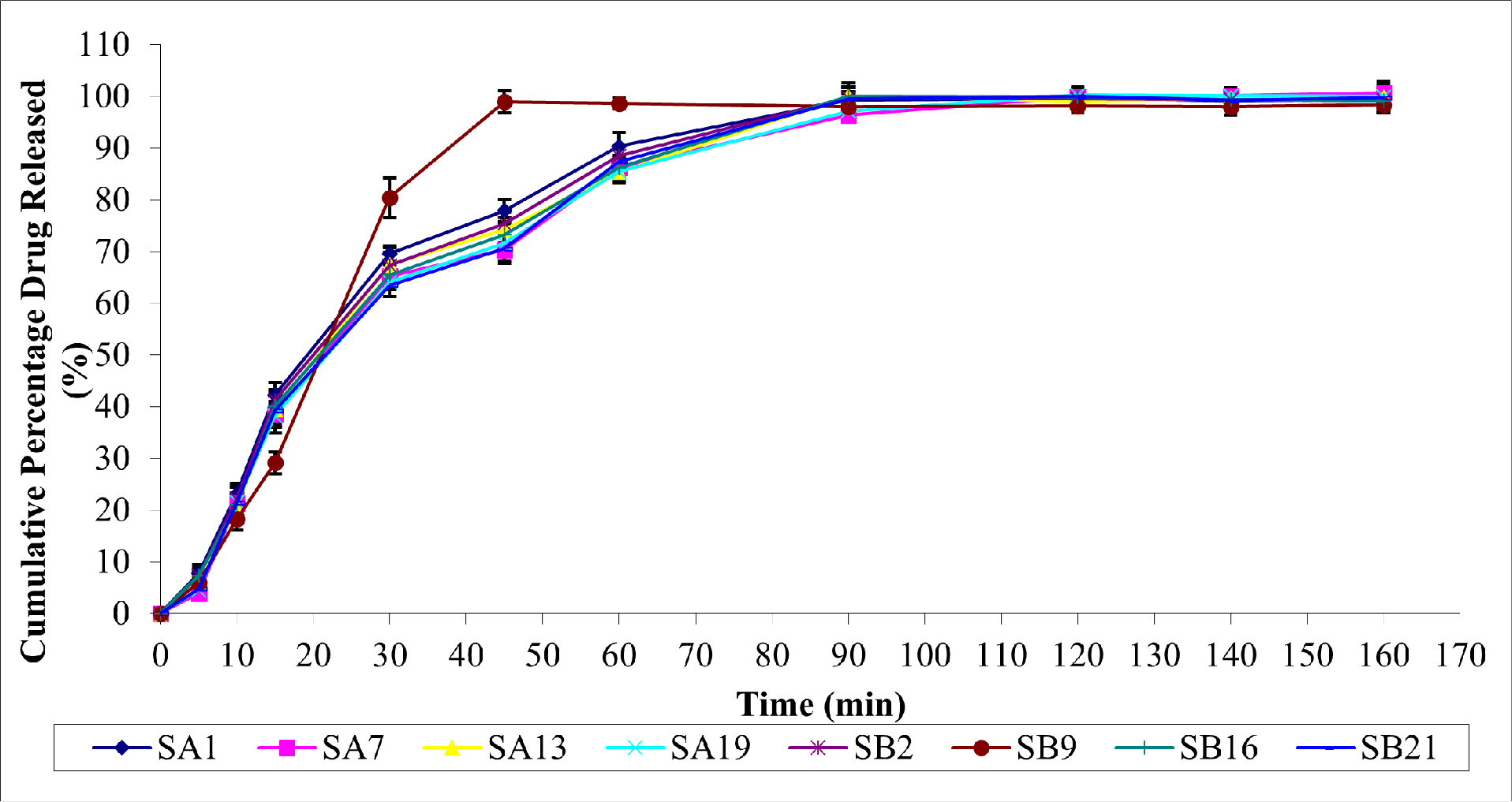
Figure 2:
In vitro Drug Release of Pullulan Based SUS Buccal Patches.
Initially, the drug, SUS, was released slowly from each patch. The rate of drug release increased significantly after 45 min. At 120 min, SA19 had the highest drug release (100.2%), sustained for up to 160 min. At 140 min, SA7 demonstrated the highest drug release (100.1%), sustained for up to 160 min. Maximum drug release percentages for SA1, SA13, SB2, SB9, SB16, and SB21 were 99.41, 99.81%, 99.18%, 98.32%, 99.1%, and 99.75%, respectively. Thus, it was demonstrated that Pullulan-based buccal patches containing the hydrophilic polymer PVP-K30 outperformed the other patches regarding drug release.
The Higuchi and Korsmeyer-Peppas kinetic equation described in vitro drug release. The release rates k and n for each model were calculated using Microsoft Excel 2003 and linear regression analysis. The precision of the fit was evaluated using correlation coefficients (r2). Table 4 shows the values for r2, k, and n.
| Formulations | Higuchi | Korsmeyer-Peppas | Mechanism of drug release | ||||
|---|---|---|---|---|---|---|---|
| R 2 | y | k (min-1/2) | R 2 | y | n | ||
| SA1 | 0.893 | y=11.14x-2.502 | 11.14 | 0.699 | y=0.101x-0.804 | 0.101 | Higuchi |
| SA7 | 0.920 | y=11.43x-6.606 | 11.43 | 0.669 | y=0.121x-0.960 | 0.121 | Higuchi |
| SA13 | 0.908 | y=11.33x-5.160 | 11.33 | 0.704 | y=0.110x-0.874 | 0.110 | Higuchi |
| SA19 | 0.922 | y=11.43x-6.537 | 11.43 | 0.685 | y=0.118x-0.933 | 0.118 | Higuchi |
| SB2 | 0.902 | y=11.43x-6.519 | 11.22 | 0.707 | y=0.104x-0.831 | 0.104 | Higuchi |
| SB9 | 0.792 | y=11.41x-2.683 | 11.41 | 0.661 | y=0.104x-0.831 | 0.114 | Higuchi |
| SB16 | 0.911 | y=1.28 x-4.815 | 11.28 | 0.722 | y=0.106x-0.844 | 0.106 | Higuchi |
| SB21 | 0.916 | y=11.41x-2.683 | 11.42 | 0.685 | y=0.117x-0.925 | 0.117 | Higuchi |
R2, k,and n Values of Selected Pullulan Based Buccal Patches of SUS.
Considering the r2 values for the Higuchi and Peppas kinetic models, all the selected formulations fit the Higuchi model well. According to this model, drug release from this formulation could be caused by micropore diffusion. The drug release rate from matrix-based devices increases when the drug loading exceeds the matrix’s solubility. Fickian drug release is distinguished by the released drug’s concentration-dependent linear dependence on the square root of time. The basis of diffusion is Fick’s rules, which show the macroscopic movement of molecules due to a concentration gradient. When the drug: polymer ratio increases, the drug release mechanism becomes Fickian or diffusion-based. This discovery could be attributed to the dispersion of the release medium, which solubilizes the drug and causes the buccal patches to slowly release the medication.23,24
In vitro Drug Permeation Study
In vitro drug permeation from mucoadhesive buccal patches is influenced by several factors, similar to in vitro drug release. These factors include mucoadhesive polymer selection, drug properties such as molecular weight and lipophilicity, patch formulation components such as excipients and concentrations, patch thickness, pH and buffering capacity, permeation enhancers, and environmental factors such as temperature and agitation. Permeation enhancers change the properties of the mucosal barrier to increase drug permeation. In vitro drug permeation study data are presented in Figure 3.
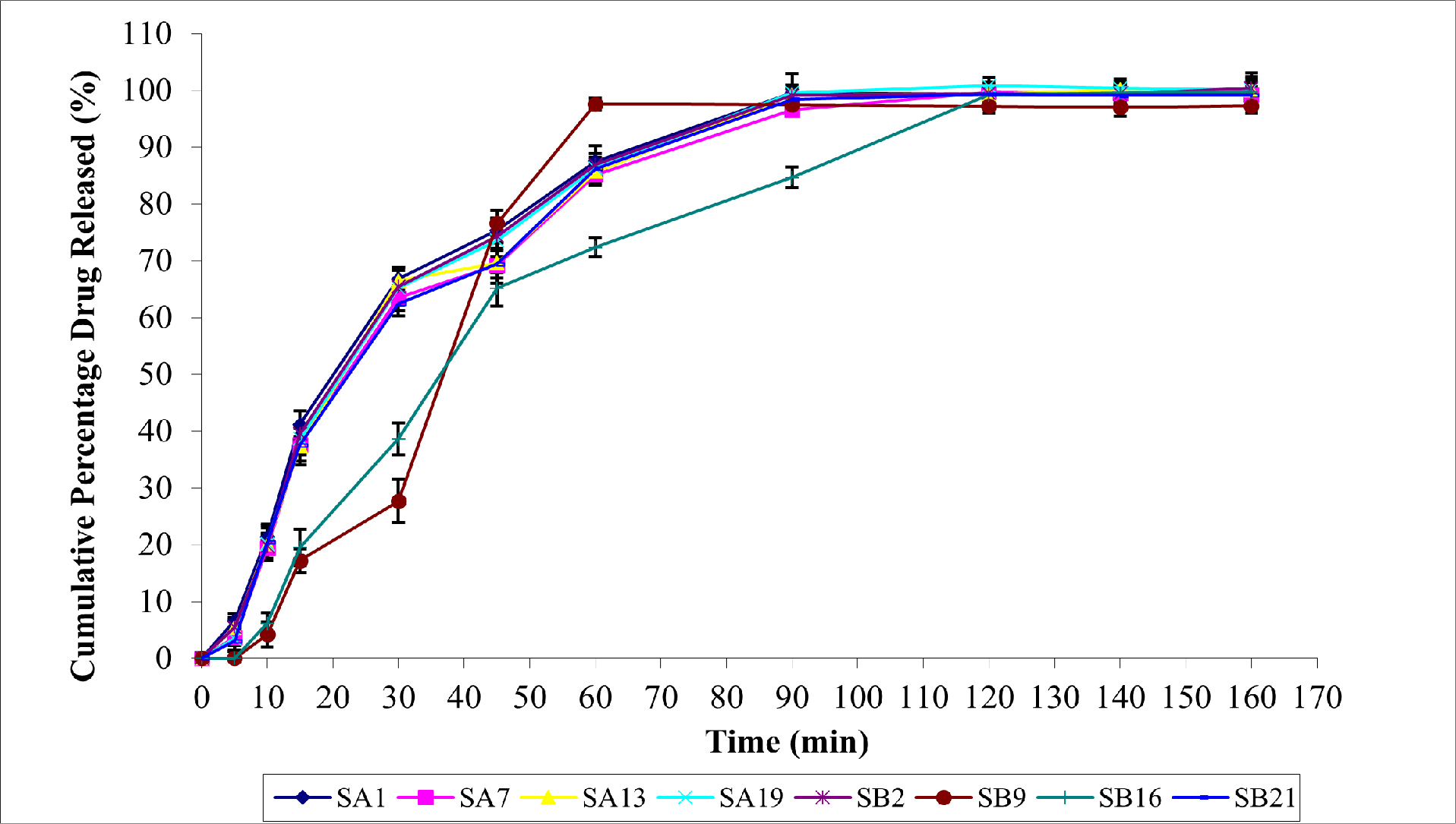
Figure 3:
The Permeation Characters of SUS Buccal Patches.
With correlation coefficients of 0.893, 0.920, 0.908, 0.922, 0.902, 0.792, 0.911, and 0.916, respectively, for SA1, SA7, SA13, SA19, SB2, SB9, SB16, and SB21, SUS permeation demonstrated a pattern resembling that of in vitro drug release. At 120 min, SA19 showed the highest permeation rate (100.8±4.2%). While other formulations (SA1, SA7, SB2, SB9, SB16, and SB21) only reached maximum drug permeation at 160 min, ranging between 97-100%, SA13 demonstrated drug permeation of 100.1±2.3% from 140 min on. This study found a significant correlation between vitro drug release and in vitro drug permeation.
The correlation between in vitro drug release and in vitro permeation was studied and plotted in Figure 4 (a-b).
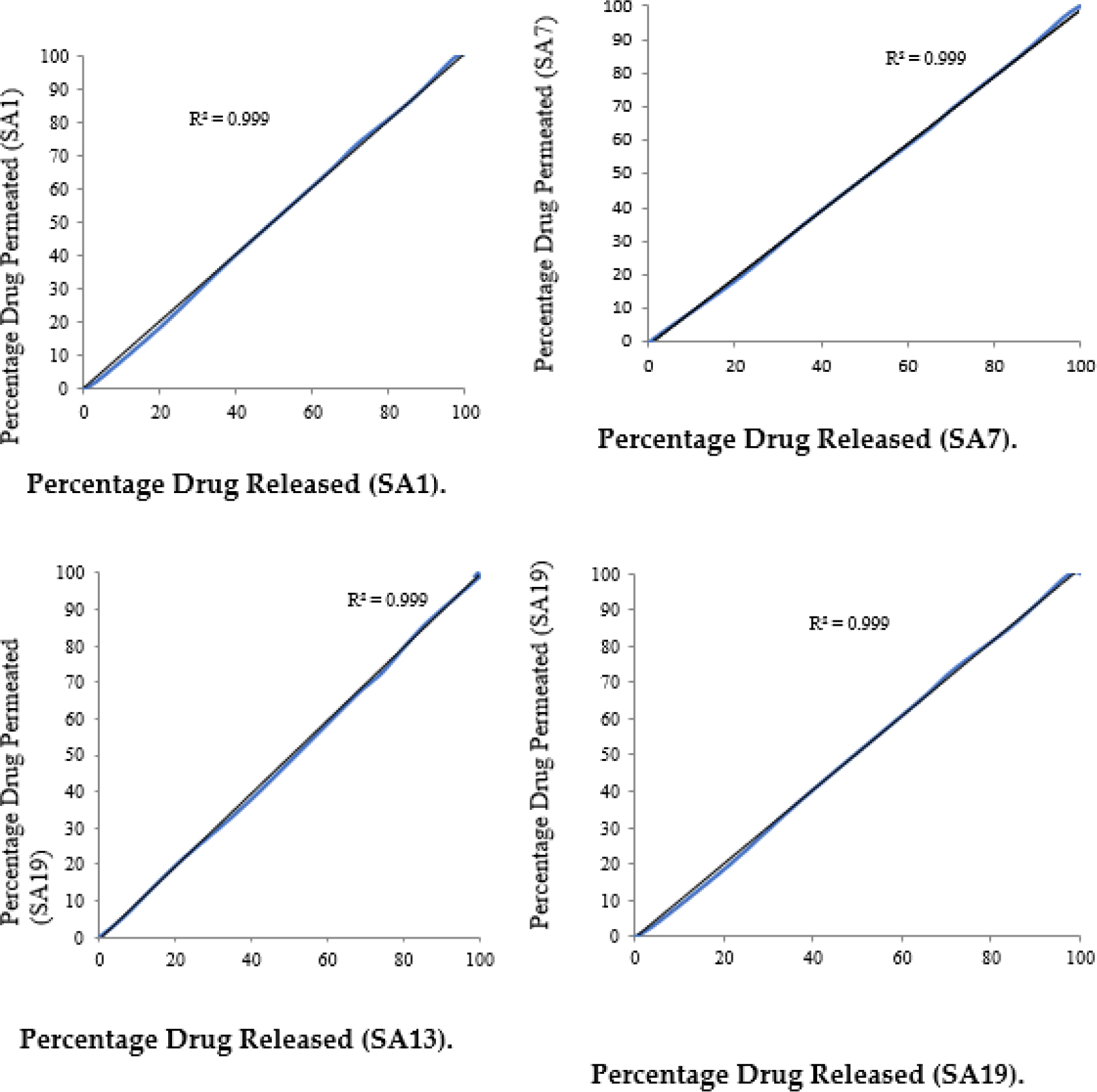
Figure 4 (a):
Correlation Between in vitro Drug Release and in vitro Drug Permeation of Optimized Patches (SA1, SA7, SA13 and SA19).
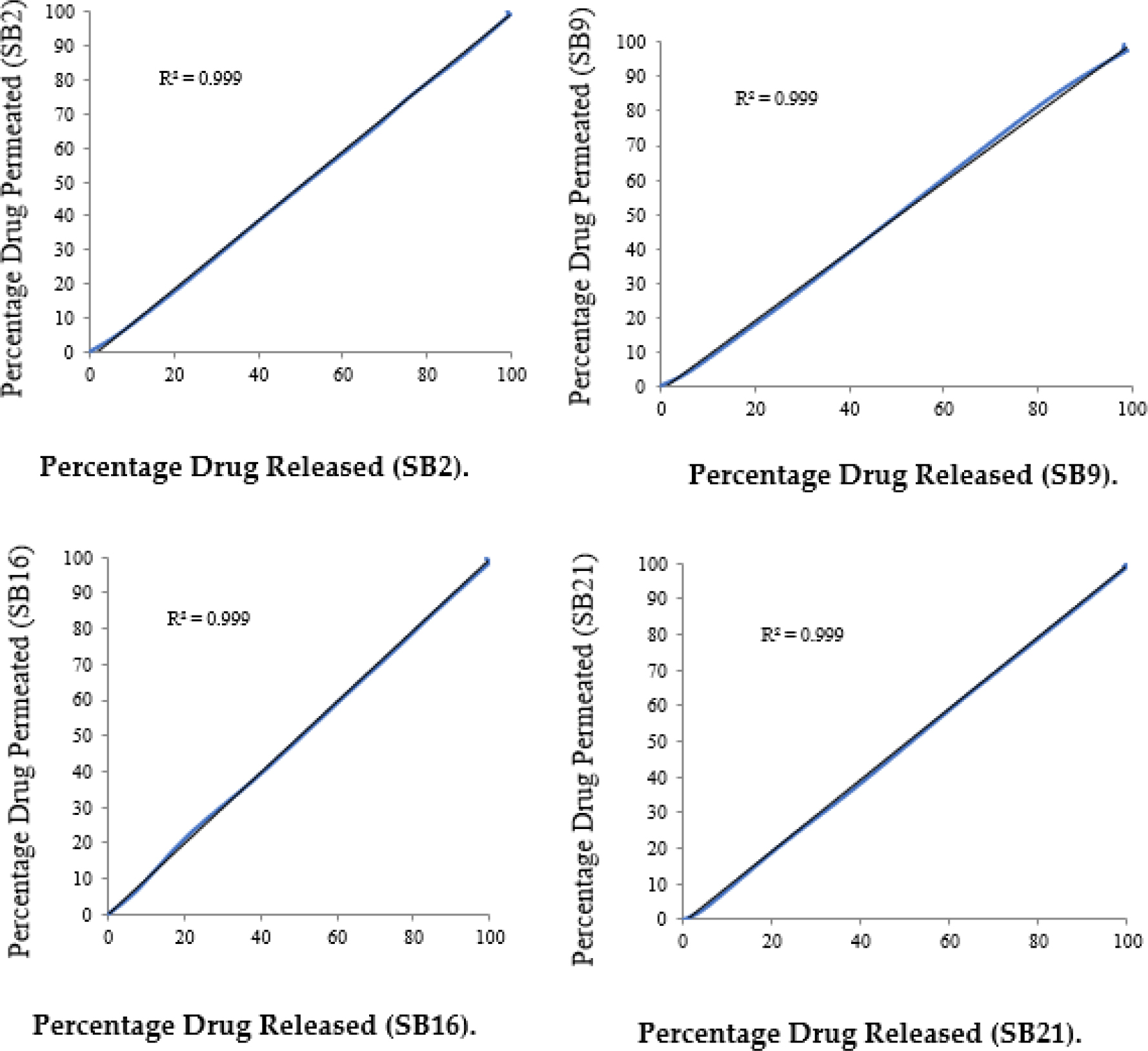
Figure 4 (b):
Correlation Between in vitro Drug Release and in vitro Drug Permeation of Optimized Patches (SB2, SB9, SB16 and SB21).
Accelerated Stability Studies
Table 5 shows the results of six-month stability studies on selected patches. Under accelerated conditions (40±0.5°C and 75% RH), selected patches showed no discernible changes in drug content, residence time, or appearance. Human saliva stability was also tested, and minor changes in appearance were observed. The drug content and residence time ranged from 9.0±0.7 mg to 8.9±0.3 mg and 130±3 to 105±1 respectively.
| Evaluation parameter | Formulation code | 1st month | 2nd month | 3rd month | 5th month | 6th month |
|---|---|---|---|---|---|---|
| Drug content(mg)* | SA1 | 9.0±0.7 | 9.0±0.8 | 8.9±0.8 | 8.9±0.9 | 8.8±0.9 |
| SA7 | 8.9±0.3 | 8.9±0.4 | 8.7±0.4 | 8.7±0.5 | 8.6±0.5 | |
| SA13 | 9.1±0.3 | 9.1±0.4 | 9.0±0.4 | 8.8±0.5 | 8.8±0.3 | |
| SA19 | 9.0±0.3 | 8.9±0.4 | 8.9±0.4 | 8.9±0.5 | 8.8±0.5 | |
| SB2 | 9.1±0.4 | 8.9±0.5 | 8.8±0.5 | 8.8±0.6 | 8.7±0.6 | |
| SB9 | 9.1±0.2 | 9.1±0.3 | 9.0±0.3 | 8.8±0.3 | 8.8±0.4 | |
| SB16 | 8.9±0.5 | 8.9±0.6 | 8.8±0.4 | 8.8±0.6 | 8.7±0.7 | |
| SB21 | 9.2±0.3 | 9.1±0.4 | 9.1±0.5 | 8.9±0.5 | 8.9±0.6 | |
| Residence time (min)* | SA1 | 110±2 | 110±3 | 109±3 | 109±4 | 108±4 |
| SA7 | 106±3 | 106±4 | 105±4 | 105±5 | 104±5 | |
| SA13 | 124±2 | 124±3 | 123±3 | 122±3 | 122±4 | |
| SA19 | 132±3 | 132±4 | 130±4 | 130±5 | 128±5 | |
| SB2 | 106±3 | 106±3 | 106±4 | 105±4 | 104±4 | |
| SB9 | 117±1 | 117±2 | 115±2 | 114±3 | 114±4 | |
| SB16 | 124±2 | 123±2 | 122±2 | 122±3 | 121±3 | |
| SB21 | 125±1 | 124±1 | 124±2 | 123±2 | 123±3 | |
| Appearance | SA1 | No change | No change | No change | No change | Change in texture |
| SA7 | No change | No change | No change | No change | No change | |
| SA13 | No change | No change | No change | No change | No change | |
| SA19 | No change | No change | No change | No change | No change | |
| SB2 | No change | No change | No change | No change | Change in texture | |
| SB9 | No change | No change | No change | No change | Change in texture | |
| SB16 | No change | No change | No change | No change | Change in texture | |
| SB21 | No change | No change | No change | No change | Change in texture |
Accelerated Stability Study of Pullulan-Based SUS Buccal Patches.
FTIR Spectral Studies
FTIR spectra of the selected formulation (SA19) are given in Figure 5.
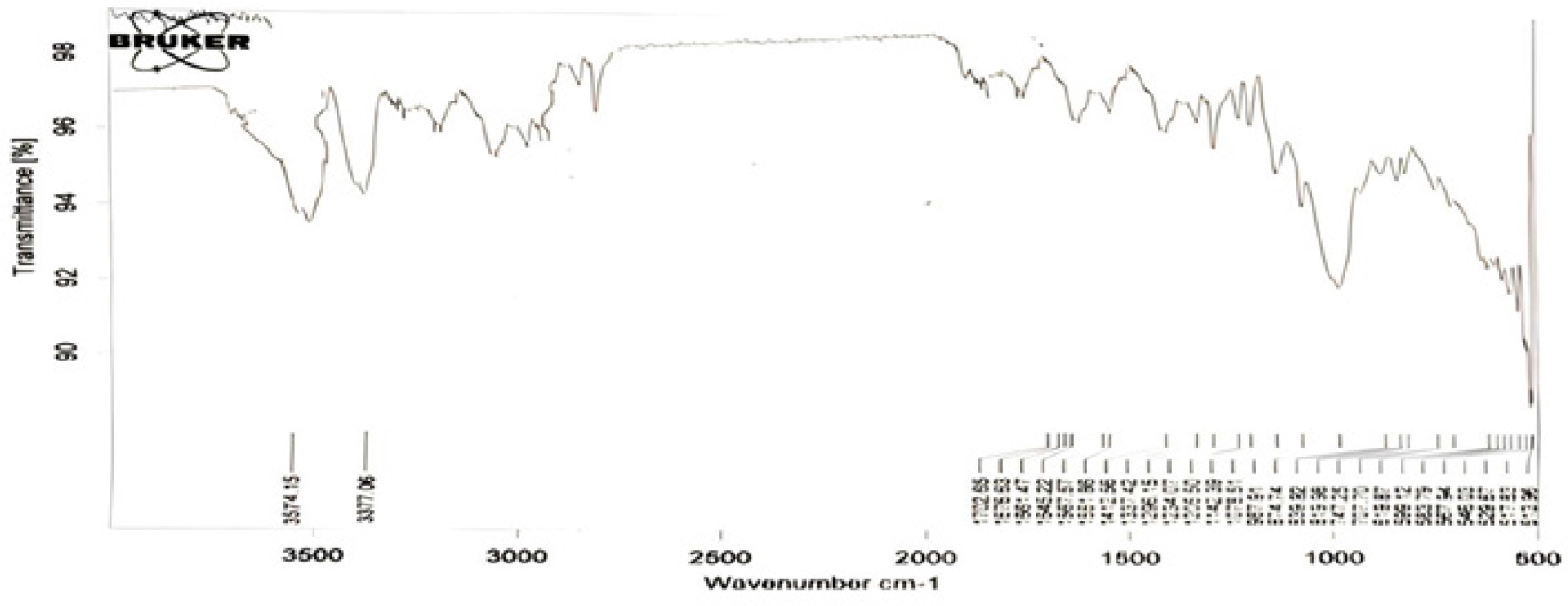
Figure 5:
FTIR Spectral Studies of Selected Formulation SA19.
The spectra show the main peaks around the necessary wave numbers, such as aliphatic primary amine at 3374 cm-1, OH stretching vibrations at 3574 cm-1, hydrocarbons (alkane,alkene, and CH bond) at 1142 cm-1, 1296 cm-1, 1205 cm-1, and sulfonyl group at 1337 cm-1. This could demonstrate no interaction between the drug and the polymers. As a result, the purity and integrity of the drug were preserved in the formulation.
Differential Scanning Calorimetry
Figure 6 shows the DSC thermal analysis of optimized formulation (SA19).
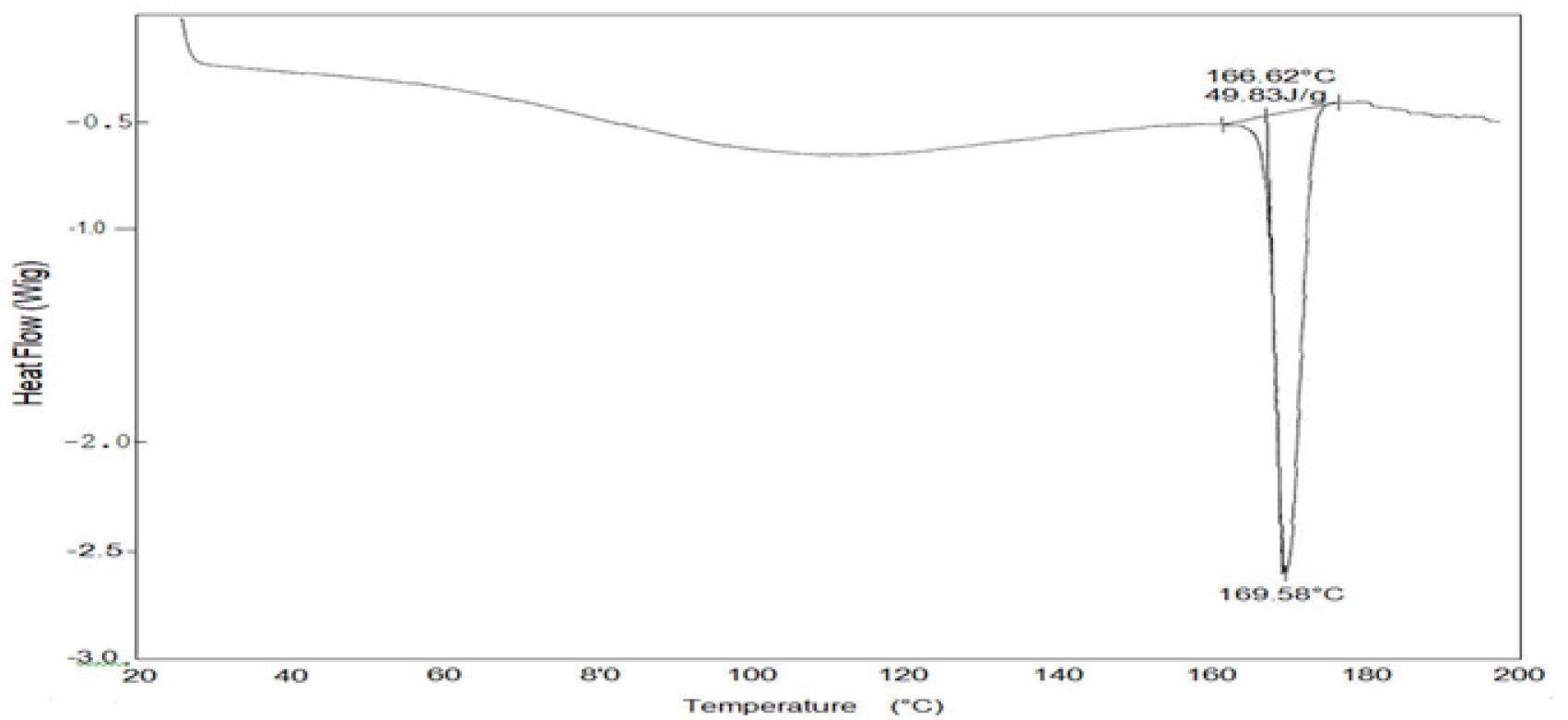
Figure 6:
DSC Thermal Analysis of Selected Formulation SA19.
The selected mucoadhesive buccal formulation SA19 was subjected to DSC analysis. The results showed that the sample remained stable up to a temperature of 169°C. This finding implies no interactions between the drug and the polymers used in the formulation were observed. The DSC analysis revealed important information about the formulation’s thermal behavior, confirming its stability within the tested temperature range.
XRD studies
The XRD pattern of the selected formulation (SA19) is shown in Figure 7.
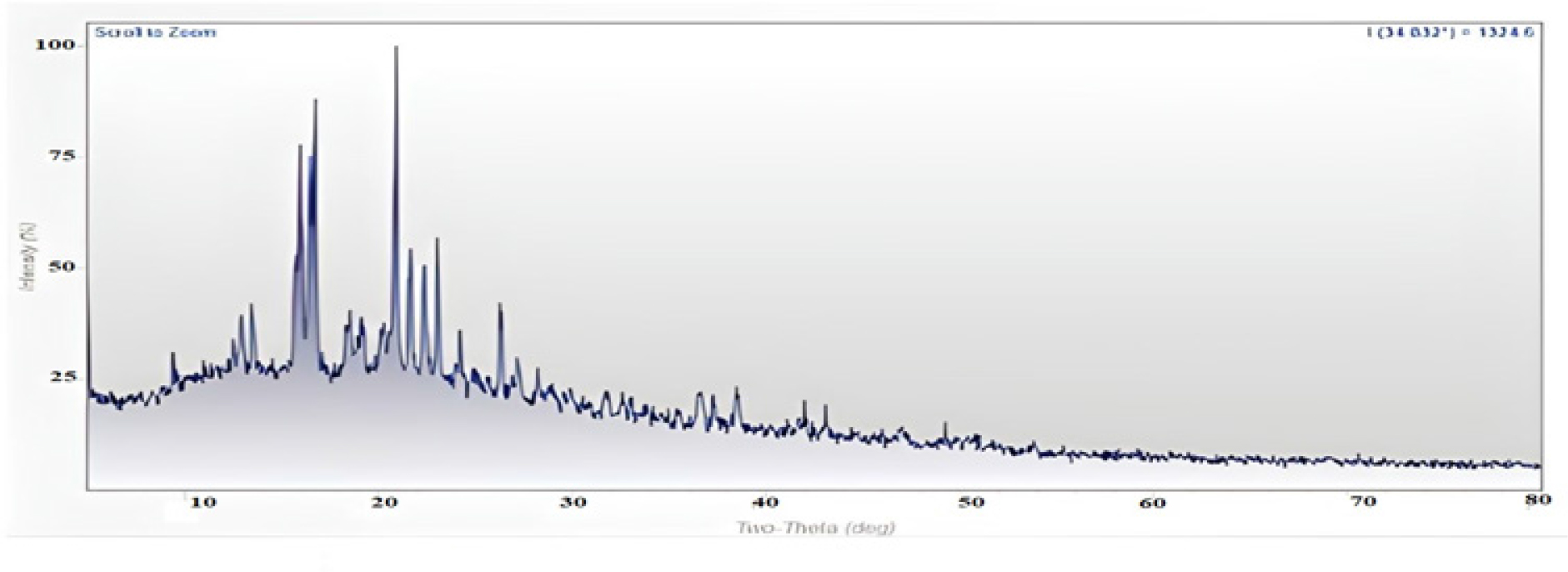
Figure 7:
XRD Pattern of Selected Formulation SA19.
Using an X-ray diffractometer, the XRD patterns were detected. The diffraction degree was measured at a scanning rate of 40/min. The drug and polymers individually exhibit intense crystalline peaks in the 10- to 40-degree range; however, in the optimized formulation, the peak intensity varies significantly, indicating a change in crystalline nature due to intermolecular interactions. However, when this observation is interpreted in conjunction with DSC and FTIR spectra, it can be concluded that the formulation was stable under test conditions.
CONCLUSION
This study investigated the viability of using natural mucoadhesive polymers for buccal-controlled drug delivery in treating migraines. Through exhaustive evaluation and optimization of formulation variables, the study was able to achieve controlled and consistent drug release. The formulated patches displayed desirable physicochemical properties, including uniformity, adequate thickness, and durability. In addition, the drug loading efficiency, SI, in vitro residence time, and in vitro drug release profiles were suitable for buccal mucoadhesive delivery of SUS. The Higuchi model accurately described the kinetics of drug release, and a correlation was observed between drug release and permeation. Stability tests confirmed the integrity and drug content of the patches. This study demonstrated the potential of mucoadhesive buccal patches for controlled drug delivery in migraines, promising improved therapeutic outcomes, increased patient compliance, reduced side effects, and cost benefits.
Cite this article:
Murali S, Prasanth VV The Effect of the Mixture of Natural and Cellulose-Based Polymers on the Physicochemical Properties of Sumatriptan Succinate Mucoadhesive Buccal Patches. Int. J. Pharm. Investigation. 2024;14(1):191-203.
ACKNOWLEDGEMENT
The authors thank SciWrite Global (www.sciwriteglobal.com), a medical communications company, for the editorial support.
ABBREVIATIONS
| SUS | Sumatriptan Succinate |
|---|---|
| NaCMC | Sodium carboxy methyl cellulose |
| SA | Sodium Alginate |
| PVA | Poly Vinyl Alcohol |
| PVP K-30 | Poly Vinyl Pyrrolidine K-30 |
| PG | Propylene Glycol |
| PEG | Poly Ethylene Glycol |
| SS | Sodium Saccharin |
| RH | Relative Humidity |
| SI | Swelling Index |
| DSC | Differential Scanning Calorimetry |
| XRD | X-ray Diffraction |
| FTIR | Fourier Transform Infrared |
References
- Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery-a promising option for orally less efficient drugs. J Control Release. 2006;114(1):15-40. [PubMed] | [CrossRef] | [Google Scholar]

- Morilllo LE. Migraine headache. Clin Evid. 2003;10:1547-65. [PubMed] | [CrossRef] | [Google Scholar]

- Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci. 1998;1(1):15-30. [PubMed] | [Google Scholar]

- Varshosaz J, Dehghan Z. Development and characterization of buccoadhesive nifedipine tablets. Eur J Pharm Biopharm. 2002;54(2):135-41. [PubMed] | [CrossRef] | [Google Scholar]

- Prasanth VV, Puratchikody A, Sam TM, Ashok KB. Development and characterization of Eudragit based mucoadhesive buccal patches of salbutamol sulphate. Saudi Pharm J. 2011;19(4):207-14. [CrossRef] | [Google Scholar]

- Shidhaye SS, Saindane NS, Sutar S, Kadam V. Mucoadhesive bilayered patches for administration of sumatriptan succinate. AAPS PharmSciTech. 2008;9(3):909-16. [PubMed] | [CrossRef] | [Google Scholar]

- Guo JH, Cooklock KM. The effects of backing materials and multilayered systems on the characteristics of bioadhesive buccal patches. J Pharm Pharmacol. 1996;48(3):255-7. [PubMed] | [CrossRef] | [Google Scholar]

- Skulason S, Asgeirsdottir MS, Magnusson JP, Kristmundsdottir T. Evaluation of polymeric films for buccal drug delivery. Pharmazie. 2009;64(3):197-201. [PubMed] | [Google Scholar]

- Navneet v PC. Effect of novel mucoadhesive buccal patches of carvedilol on isoprenaline induced tachycardia. J Adv Pharm Technol Res. 2018;5:96-103. [CrossRef] | [Google Scholar]

- Kok KP, Choy FW. Polymeric films as vehicle for buccal delivery: swelling, mechanical and mucoadhesive properties. J Pharm Pharm Sci. 1999;2(2):53-61. [PubMed] | [Google Scholar]

- Vaishali B, Kuldeep V, Dinesh KM, Pankaj D. Formulation design and evaluation of mucoadhesive buccal patch of ketorolac for the treatment of periodontitis. IJISRT. 2021;6:256-65. ISSN No:-2456-2165 [PubMed] | [Google Scholar]

- Rajan R, Sheba RND, Kajal G, Sanjay KD, Jasmina K, Arunabha N, et al. Design and evaluation of chlorpheniramine maleate from different Eudragit based matrix patches: effect of platicizer and chemical enhancers. Ars Pharm. 2010;50:177-94. [PubMed] | [Google Scholar]

- Singh S, Soni R, Rawat MK, Jain A, Deshpande SB, Singh SK, et al. Array. Chem Pharm Bull (Tokyo). 2010;58(3):307-11. [PubMed] | [CrossRef] | [Google Scholar]

- Wanasathop A, Patel PB, Choi HA, Li SK. Permeability of buccal mucosa. Pharmaceutics. 2021;13(11):1-22. [PubMed] | [CrossRef] | [Google Scholar]

- Prasanth VV, Puratchikody A, Mathew ST, Ashok KB. Effect of permeation enhancers in the mucoadhesive buccal patches of salbutamol sulphate for unidirectional buccal drug delivery. Res Pharm Sci. 2014;9(4):259-68. [PubMed] | [Google Scholar]

- Majithiya RJ, Ghosh PK, Umrethia ML, Murthy RS. Thermoreversible mucoadhesive gel for nasal delivery of sumatriptan. AAPS PharmSciTech. 2006;7(3):67 [PubMed] | [CrossRef] | [Google Scholar]

- James RL. “Metaprolol Tartarate”, in analytical profiles of drug substances. 2005;12:325 [PubMed] | [CrossRef] | [Google Scholar]

- John GH, Wyka BE. “Clotrimazole”, in analytical profiles of drug substances. 2005;11:225 [PubMed] | [CrossRef] | [Google Scholar]

- Jug M, Bećirević-Laćan M. Influence of hydroxypropyl-beta-cyclodextrin complexation on piroxicam release from buccoadhesive tablets. Eur J Pharm Sci. 2004;21(2-3):251-60. [PubMed] | [CrossRef] | [Google Scholar]

- Yukinao K, Hitoshi K, Yasuyuki B, Hiroshi Y, Tetsuya O, Yoshino K, et al. Controlled release of lidocaine hydrochloride from buccal muco-adhesive films with solid dispersion. Int J Pharm. 1997;158(2):147-55. [CrossRef] | [Google Scholar]

- Zaid AN, Al-Ramahi RJ, Ghoush AA, Qaddumi A, Zaaror YA, Al-Ramahi Rowa J, Abeer AG, Aiman Q, Yara AZ, et al. Weight and content uniformity of lorazepam half-tablets: A study of correlation of a low drug content product. Saudi Pharm J. 2013;21(1):71-5. [PubMed] | [CrossRef] | [Google Scholar]

- Lucy SCW, Paul WSH, Lee FW. Matrix swelling: A simple model describing the extent of swelling of HPMC matrices. Int J Pharm. 1995;116(2):159-68. [PubMed] | [CrossRef] | [Google Scholar]

- Higuchi T. Rate of release of drug medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50(10):874-5. [PubMed] | [CrossRef] | [Google Scholar]

- Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanism of potassium chloride release from compressed, hydrophilic, polymeric matrices: effect of entrapped air. J Pharm Sci. 1983;72(10):1189-91. [PubMed] | [CrossRef] | [Google Scholar]


