ABSTRACT
Background
Acyclovir is a widely used anti-viral drug that has low bioavailability when administered orally and is also difficult to administer parenterally due to its inherent solubility limitations. Buccal administration of Acyclovir with the aid of absorption enhancers can improve the permeability of the poorly permeable anti-viral drug.
Materials and Methods
Acyclovir-loaded buccal films were prepared by solvent casting method using piperine or zingiberene as herbal bioenhancers. The films were characterized for weight, thickness, folding endurance, surface pH, drug content, swelling index, tensile strength, mucoadhesion time and mucoadhesive strength. They were also evaluated for in vitro drug release and ex vivo permeation using porcine mucosa.
Results
The buccal films were found to be smooth and elegant with thicknesses ranging from 0.13 mm to 0.16 mm indicating the uniformity in thickness among the films. All the formulated films demonstrated excellent folding endurance of more than 200 and also the tensile strength ranged from 0.8 kg/cm2 to 3.33 kg/cm2 indicating the robustness of the buccal films. Ex vivo mucoadhesion studies revealed that the films had a mucoadhesion time of more than 100 min. In vitro drug release showed that films released the drug in a prolonged manner for more than 350 min due to the presence of chitosan in the formulation. The drug permeation from all the piperine and zingiberene-incorporated buccal films was found to be more than two-fold compared to those without the permeation enhancers.
Conclusion
Acyclovir loaded buccal films containing bioenhancers were able to successfully permeate the buccal mucosa thus achieves better bioavailability.
INTRODUCTION
Drugs delivered within or via the buccal mucosa to exert local or systemic pharmacological activities are referred to as buccal-mediated drug delivery. Drugs supplied buccally may be administered systemically or for the treatment of illnesses of the mouth or buccal cavity.1 Besides its easy accessibility, the buccal mucosa has good permeability characteristics and a rich supply of blood vessels. The buccal route is suitable for the delivery of drugs such as Acyclovir which undergo avoids first-pass metabolism in the gastrointestinal tract.2 ‘Bioavailability enhancers’ are considered drug facilitators because when combined with other molecules, they improve drug activity in a number of ways such as increasing drug bioavailability across the membrane, potentiating the drug molecule through conformational interaction, making target cells more susceptible to drugs and also acting as drug receptors.3 However, these molecules do not have any activity of their own. The ability of these molecules to permeate the biological membranes and reach systemic circulation after oral or topical administration is limited by their lipid solubility and molecular size.
Herbal bioenhancers are phyto molecules that can increase the bioavailability of a drug that is mixed with it but do not have any pharmacological activity of its own at the dosage utilized. A bigger share of medications stays sub-therapeutic dosage and is never able to reach the region of action and exercise its effects.4 Herbal bioenhancers are known to increase the bioavailability and bioefficacy of a wide range of drugs including antiviral, antifungal, and anticancer moieties.4,5 The idea of herbal bio-enhancers can be orginated from the Ayurveda system of medicine where ayurvedic preparation known as “Trikatu” in sanskrit which means three acrids was used. It includes black pepper (Piper nigrum), long pepper (Piper longum), and ginger (Zingiber officinale).6
The bio-enhancing effect of pepper species is attributed to its content of an alkaloid named piperine which is present in Piper nigrum (Black pepper) and Piper longum (Long pepper) families.7 There are two mechanisms by which piperine enhance the permeation of penetrants through the intestinal mucosa. The first nonspecific mechanism involves decreasing the secretion of HCl which aids in the rapid absorption of the drug. Piperine also increases the influence of gamma-glutamyl transpeptidase by boosting gastrointestinal tract blood flow. The second mechanism is by blocking enzymes that are involved in the biotransformation of drugs and thus lowering elimination rates.8 Gingerols have been proven to promote gastrointestinal tract motility and have analgesic, anti-pyretic, anti-microbial, and sedative activities in laboratory study animals. The main pungent principle in ginger is gingerol 6-has chemopreventive properties. The bio-enhancing action of the composition containing ginger ranges from 30 to 75%, and the bioavailability of drugs ranges from 10 to 85% when piperine and ginger are combined.8,9
One of the most potent and selective antiviral medications on the market is Acyclovir. It is frequently used to treat Herpes Simplex Virus (HSV) infections in both immune-competent and immune-compromised patients. It is currently available in intravenous and oral dosage forms.10 However, the oral bioavailability of Acyclovir varies greatly depending on dosage, ranging from 15 to 30%11 and also it is difficult to administer it intramuscularly. Moreover, Intravenous infusions or boluses need strongly alkaline (pH 10-11) solutions to avoid thrombophlebitis or perivascular inflammation.12,13
In this study, we attempt to formulate buccal films containing acyclovir and also evaluate the effect of using herbal bioenhancers on improving the permeability of acyclovir through the porcine buccal mucosa. Mucoadhesive buccal films containing acyclovir were prepared with the intention of delivering the drug through buccal mucosa thus overcoming first-pass metabolism and other limitations of oral delivery of acyclovir. The buccal films were prepared by solvent casting method and subjected to different evaluation parameters including ex vivo studies. A total of nine formulations were prepared of which three were without bio-enhancers.
MATERIALS AND METHODS
Materials
Acyclovir, Piperine, and Zingiberine were obtained from Yarrow Chem Products, Mumbai. Chitosan and PVP K-30 were obtained from Sigma Aldrich Solutions, Germany. All other chemicals and reagents were of analytical grade.
Preparation of acyclovir incorporated buccal films containing herbal bioenhancer
Mucoadhesive buccal films containing acyclovir were prepared by solvent casting method. Initially, chitosan was dissolved in 1% v/v acetic acid to produce a 2% w/v solution and continuously stirred using a magnetic stirrer overnight. The resultant viscous liquid was then filtered to remove the undissolved matter and the debris. To this filtrate glycerine and PVP K 30 were added. This was followed by the addition of the drug and herbal bio-enhancers (piperine and zingiberine) following which the mixture was kept overnight for swelling and deaeration of chitosan. The resultant solution was then transferred into a glass mold of 9 cm in diameter. The films were then dried in a hot air oven at 50°C and were cut into square films with an area of 1 cm2. Films were covered using butter paper and were stored in air-tight sealed plastic covers.14 The composition of the formulation is shown in Table 1.
Evaluation of the physicochemical characteristics of films
Uniformity of Weight and Thickness
The weight of each film was measured individually using a calibrated digital balance, and the average weight of three films was calculated. The thickness of three films made from each type of formulation was measured using a micrometer screw gauge.15
| Formulation code | Chitosan (g) | PVPK30 (g) | Glycerine (mL) | Acyclovir (g) | Piperine (g) | Zingiberine (g) |
|---|---|---|---|---|---|---|
| F1 | 0.75 | 0.07 | 0.4 | 0.191 | 0.084 | – |
| F2 | 0.9 | 0.07 | 0.4 | 0.191 | 0.084 | – |
| F3 | 1 | 0.07 | 0.4 | 0.191 | 0.084 | – |
| F4 | 0.75 | 0.07 | 0.4 | 0.191 | – | 0.836 |
| F5 | 0.9 | 0.07 | 0.4 | 0.191 | – | 0.836 |
| F6 | 1 | 0.07 | 0.4 | 0.191 | – | 0.836 |
| X1 | 0.75 | 0.07 | 0.4 | 0.191 | – | – |
| X2 | 0.9 | 0.07 | 0.4 | 0.191 | – | – |
| X3 | 1 | 0.07 | 0.4 | 0.191 | – | – |
Composition of casting solution of mucoadhesive buccal films of acyclovir with the incorporation of herbal bioenhancers.
Folding endurance
The folding endurance was carried out to see if the films could withstand mechanical stress and when utilized in the oral cavity if it is easy to handle and flexible. Folding endurance was measured by folding the film at the same spot again and again until it was broken or folded 300 times without breaking.16
Surface pH
In order to determine the surface pH, 2% w/v of agar was first added to a warm isotonic phosphate buffer with a pH of 6.6 in a beaker with continuous stirring. The solution was then transferred to the agar plate and the films were then left to swell for an hour. The solution was then poured into a petri dish and allowed to stand until it gels at room temperature. Finally Utilising a pen pH meter, the surface pH was determined.17
Drug content estimation
To determine the drug content buccal films were divided into small pieces and added to a 250 mL beaker containing 100 mL of phosphate buffer pH 6.6 in at 37°C. The solution was stirred continuously in a magnetic stirrer at 300 rpm for 3 hr followed by funnel filtration. The filtered solution was suitably diluted and examined for drug content at 253 nm by UV spectrophotometry.18
Percentage Swelling index
The films were initially weighed and the initial weight (W1) was noted then the buccal films were incubated on 2% w/v agar plates at 37°C. After 1-2 hr intervals and when the weights get stabilized, the films were removed, and the weight of inflated films (W2) was again determined and the swelling index was calculated according to formula.19
Where,
W1 is the initial weight of the film,
W2 is the final weight of the film,
SI is the swelling index,
Tensile Strength
The tensile strength of a buccal film is the maximum strength beyond which the film completely breaks. A universal testing machine was used to measure the tensile strength. The buccal films having a dimension of 40×15 mm, free from air bubbles were held between two clamps placed 5cm apart from each other. The films were then pulled with the help of an upper clamp at a rate of 300 mm/min until the films completely broke.20
Ex vivo mucoadhesive residence time
For measuring the mucoadhesive residence time, buccal mucosa was cut into an appropriate size and affixed to the inside of the beaker using cyanoacrylate glue. The first film was put to the buccal region for 20 sec with light pressure after being soaked with 50 μL of simulated salivary fluid. After that, a beaker was filled with 20 mL of artificial salivary fluid and put on a magnetic stirrer at 37°C and 50 rpm.
Ex vivo mucoadhesive strength
In order to determine the ex vivo mucoadhesive strength of the buccal films a physical balance composed of two arm balances was utilized. The left arm of the physical balance was replaced by a cap made of plastic that was hung vertically by a wire. A mobile platform was kept at the bottom of the same side to hold the buccal mucosal membrane in place. The buccal mucosa was sliced into parts and extensively cleaned with phosphate buffer of pH 6.6. A piece of buccal mucosa was attached to the diffusion cell’s open end and carefully positioned within the glass beaker’s center such that it touched the buccal mucosal surface. Using cyanoacrylate adhesive, the buccal film was connected to the flat plastic cap’s bottom half and then two balance pans were balanced with 5 g of weight on the right-side pan. Following the aforementioned method, 5 g of weight was removed from the right-side pan and dropped to the pan along the film over the mucosa. After 5 min of contact time, the balance was held in place while the weight on the right side of the pan was gradually increased until the film completely separated from the mucosal surface.18
In vitro drug release studies
In vitro drug release was carried out using a USP Type II dissolution apparatus. Briefly, a buccal film was affixed to the wall of the dissolution vessel using cyanoacrylate glue halfway from the bottom. The vessel was filled with 900 mL of salivary fluid of pH 6.7 after 2 min. The dissolving medium was kept at 37°C and agitated at 50 rpm. At set intervals, 5 mL samples were withdrawn and replaced with new media. The samples were filtered using Whatman filter paper and assessed using a UV-spectrometer at 253 nm.20
Ex vivo permeation study
Ex vivo permeation study was carried out using the Franz diffusion cell having a donor and a receptor compartment with a capacity of 12 mL. A water jacket was placed over the receptor compartment to maintain a temperature of 37°C. Before starting the experiment, buccal mucosa was brought from a local slaughterhouse, and fats and adipose tissue underlying the buccal mucosa were removed using a scalpel. Then the buccal tissue was placed between the donor and receptor and the receptor compartment was filled with 12 mL of phosphate buffer pH 6.6 which served as the receptor medium. A Teflon-coated magnetic bead was placed on the receptor compartment and the entire assembly was kept on a magnetic stirrer. After giving the buccal mucosa sufficient time to stabilize in the receptor medium, 1 mL aliquots were taken out at periodic intervals and analyzed spectrophotometrically.21
RESULTS
Uniformity of Weight and Thickness
As seen in Table 2 it was observed that the weight of the films increased with an increase in the concentration of chitosan polymer. The piperine-containing buccal films had thicknesses varying from 0.13±0.005-0.16±0.001 mm and zingiberine-containing buccal films thicknesses ranged from 0.14±0.034-0.15±0.027 mm. As shown in Table 2 both piperine and zingiberine buccal films displayed uniform thickness.
Surface pH
The surface pH of piperine-incorporated films as indicated in Table 2 was found to be 6.4 to 6.5 and zingiberine incorporated formulations were found to be 6.4 to 6.6 which suggests that they are less likely to irritate the buccal mucosa and hence more comfortable for usage.
Drug content estimation
The drug content of piperine-incorporated formulations ranged from 71 to 75% and in the case of zingiberine-incorporated formulations drug content was found to be 72.3 to 86.6%. These results signify that drug content between the films remains more or less the same.
Percentage swelling index
The swelling index is an essential parameter that determines the mucoadhesive nature of the buccal films and which in turn affects the release of the drug. Films with good swelling index will be able to adhere to the buccal mucosa intimately. As seen in Figures 1 and 2, all the formulations displayed good swelling properties making it the formulation ideal for buccal application.
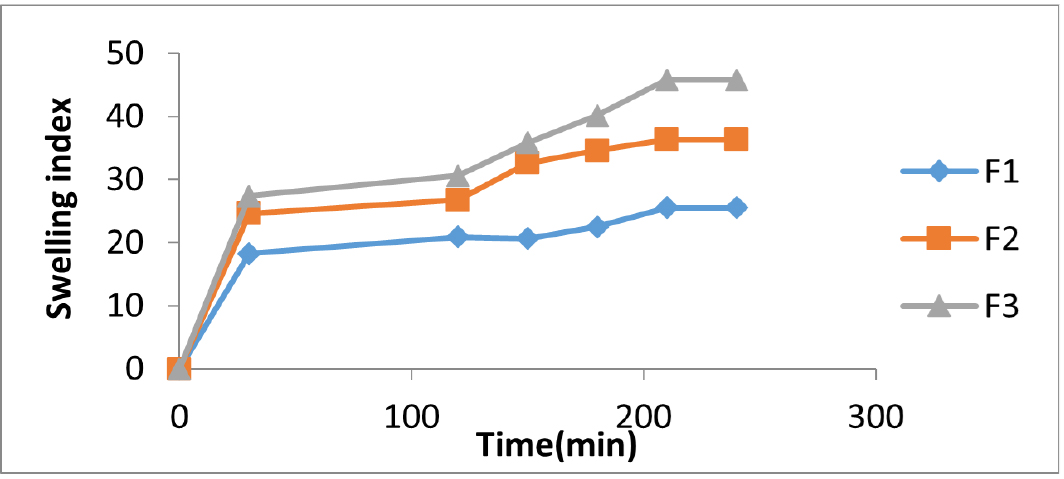
Figure 1:
Swelling index of acyclovir buccal films containing Piperine.
Folding endurance
Folding endurance is the number of folds that are required to break the buccal film. This parameter gives an indication of the flexibility and strength of the prepared films. As shown in Table 3, The folding endurance of both piperine and zingiberine-incorporated formulations was less than 300 which indicates that films are not extremely hard but rather more flexible.
Tensile Strength
It was observed as shown in Table 3 that among the piperine formulations, F3 showed the greater tensile strength and among the zingiberine formulations, F6 showed the greater tensile strength. The tensile strength of the formulation can be attributed to the Polymers used in the formulation.
| Formulation Code | Weight (mg)* | Thickness (mm)* | Surface pH | Drug content (%)* |
|---|---|---|---|---|
| F1 | 18.66±0.025 | 0.13±0.005 | 6.5±0.025 | 71.03±0.05 |
| F2 | 21±0.013 | 0.14±0.005 | 6.6±0.020 | 73.13±0.016 |
| F3 | 26.3±0.010 | 0.16±0.0O17 | 6.4±0.020 | 75.01±0.030 |
| F4 | 19.6±0.036 | 0.14±0.034 | 6.6±0.030 | 72.37±0.012 |
| F5 | 23.3±0.024 | 0.14±0.04O | 6.4±0.052 | 82.37±0.013 |
| F6 | 32±0.017 | 0.15±0.027 | 6.4±0.023 | 86.6±0.0114 |
Data for weight, thickness, surface pH, and drug content of Acyclovir buccal films.
Ex vivo Mucoadhesion studies
Mucoadhesion is an important criterion factor for successful buccal therapy because inadequate mucoadhesion could lead to the displacement of films from the site of application. As observed in Table 3, among the piperine films, F3 formulation showed greater mucoadhesion strength and in the case of zingiberine films, F6 demonstrated the highest mucoadhesive strength and time.
In vitro drug release studies
Figures 3 and 4 show the release of acyclovir from mucoadhesive buccal films containing piperine and zingiberene respectively. It could be seen that among the piperine formulations, F3 showed the highest drug release and among the zingiberine formulations, F6 showed the maximum drug release.
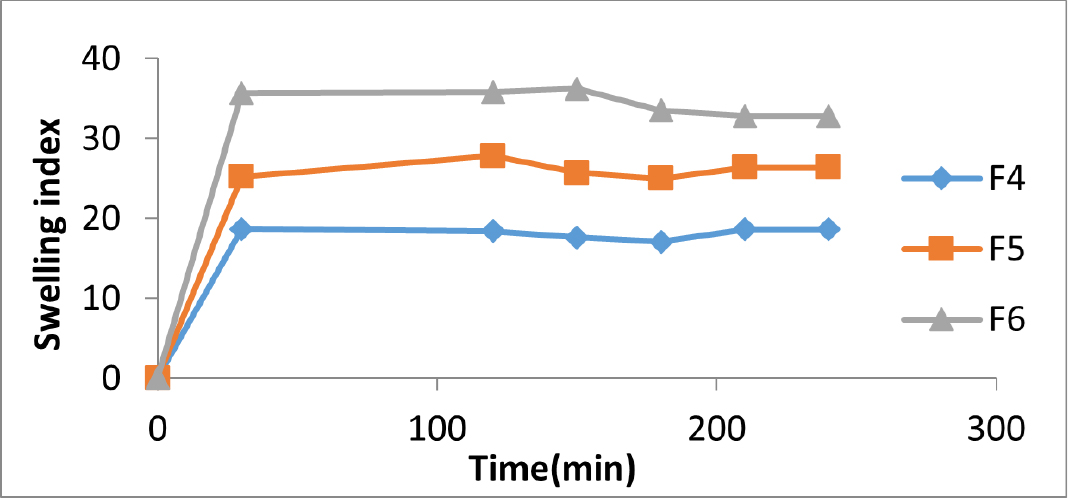
Figure 2:
Swelling index of acyclovir buccal films containing Zingiberine.
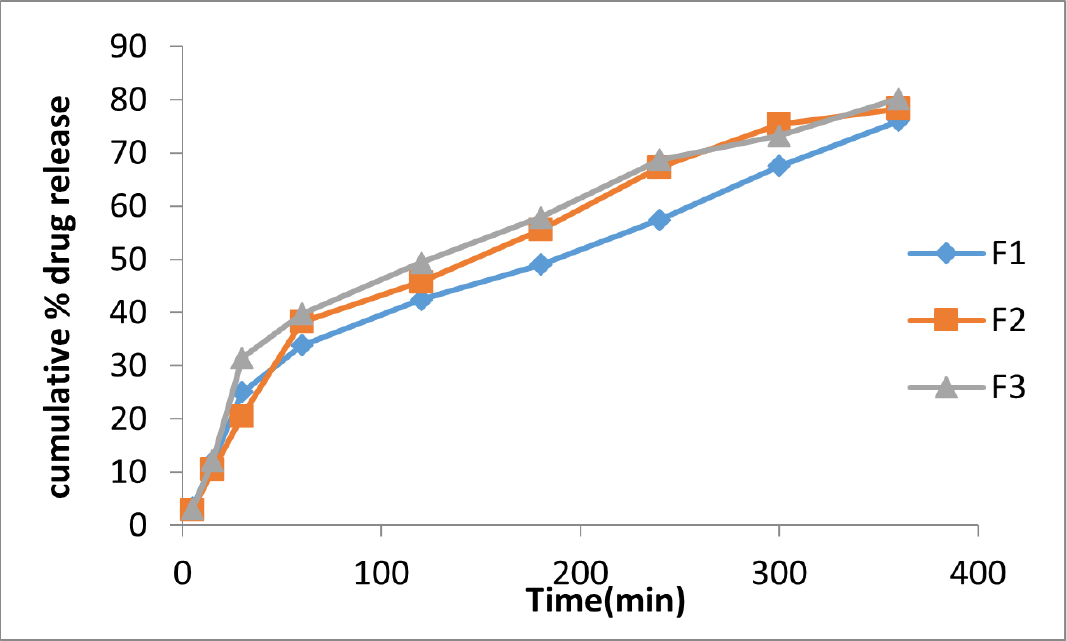
Figure 3:
Comparative in vitro drug release profiles of acyclovir buccal films containing piperine in simulated salivary fluid pH 6.7.
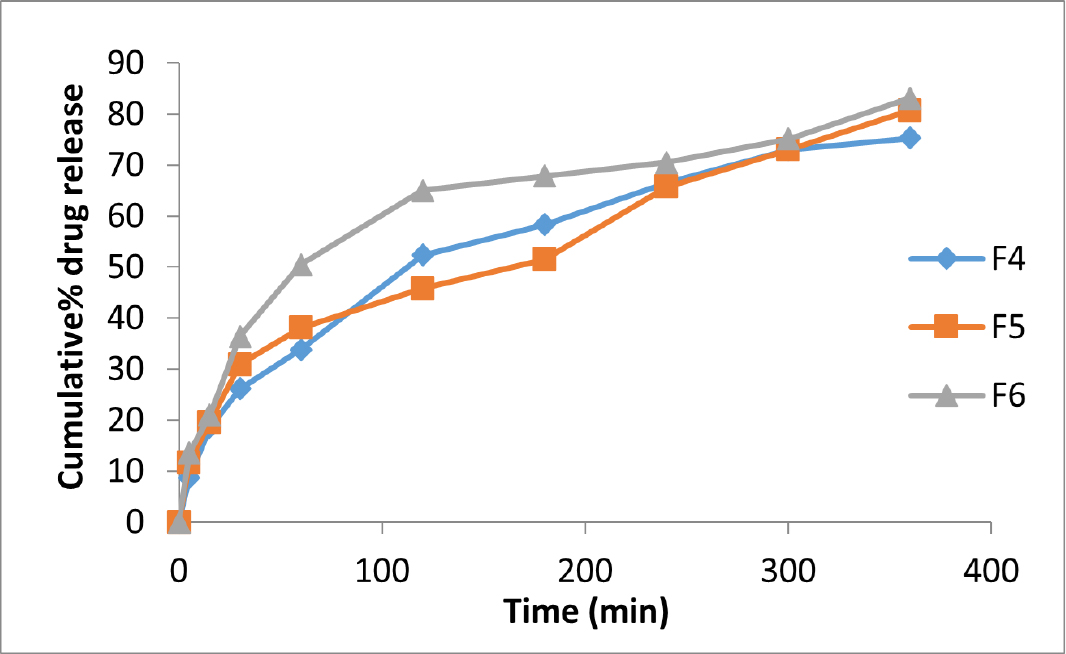
Figure 4:
Comparative in vitro drug release profiles of acyclovir buccal films containing zingiberine in simulated salivary fluid pH 6.7.
Ex vivo permeation study
The comparative permeation profiles of the acyclovir-loaded buccal films containing bioenhancers and those without bioenhancers are shown in Figures 5 and 6. The ex vivo permeability studies clearly demonstrate the improved permeability of herbal bio-enhancers (Piperine and Zingiberine) in improving the permeability of the drug through the buccal mucosa. It was observed that among the piperine formulations, acyclovir from F3 permeated through the porcine buccal mucosa to the greatest extent. Since the percentage of piperine included in the films is kept constant, the greater mucosal drug permeation from F3 is probably due to the greater solubilizing ability of the higher-strength chitosan. It was also observed that there is not much difference between permeation enhancement by piperine and zingiberine for similar formulations.
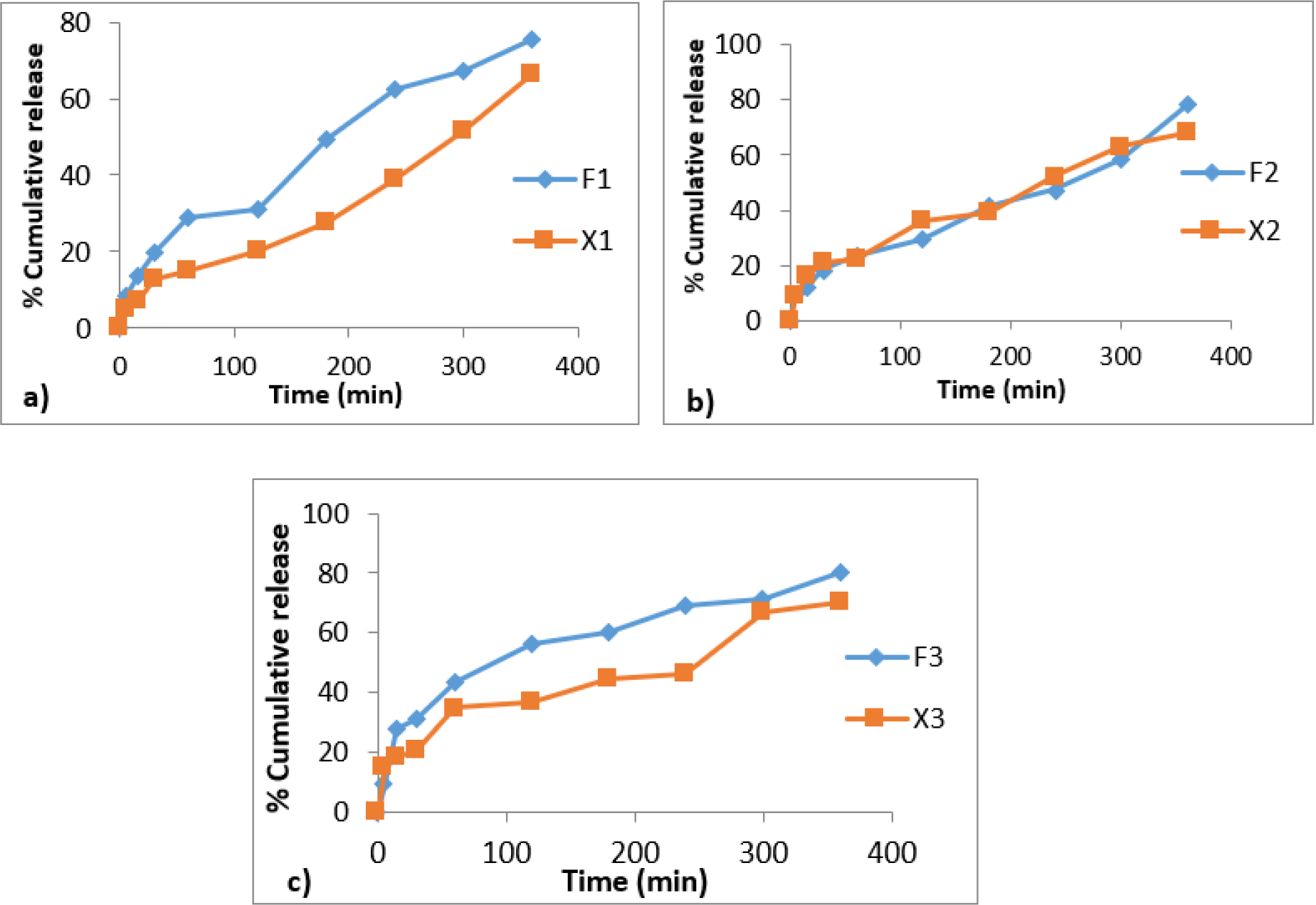
Figure 5:
Comparative ex vivo permeation profiles of acyclovir from buccal films with piperine as bioenhancer between formulations a) F1 and X1 b) F2 and X2 c) F3 and X3.
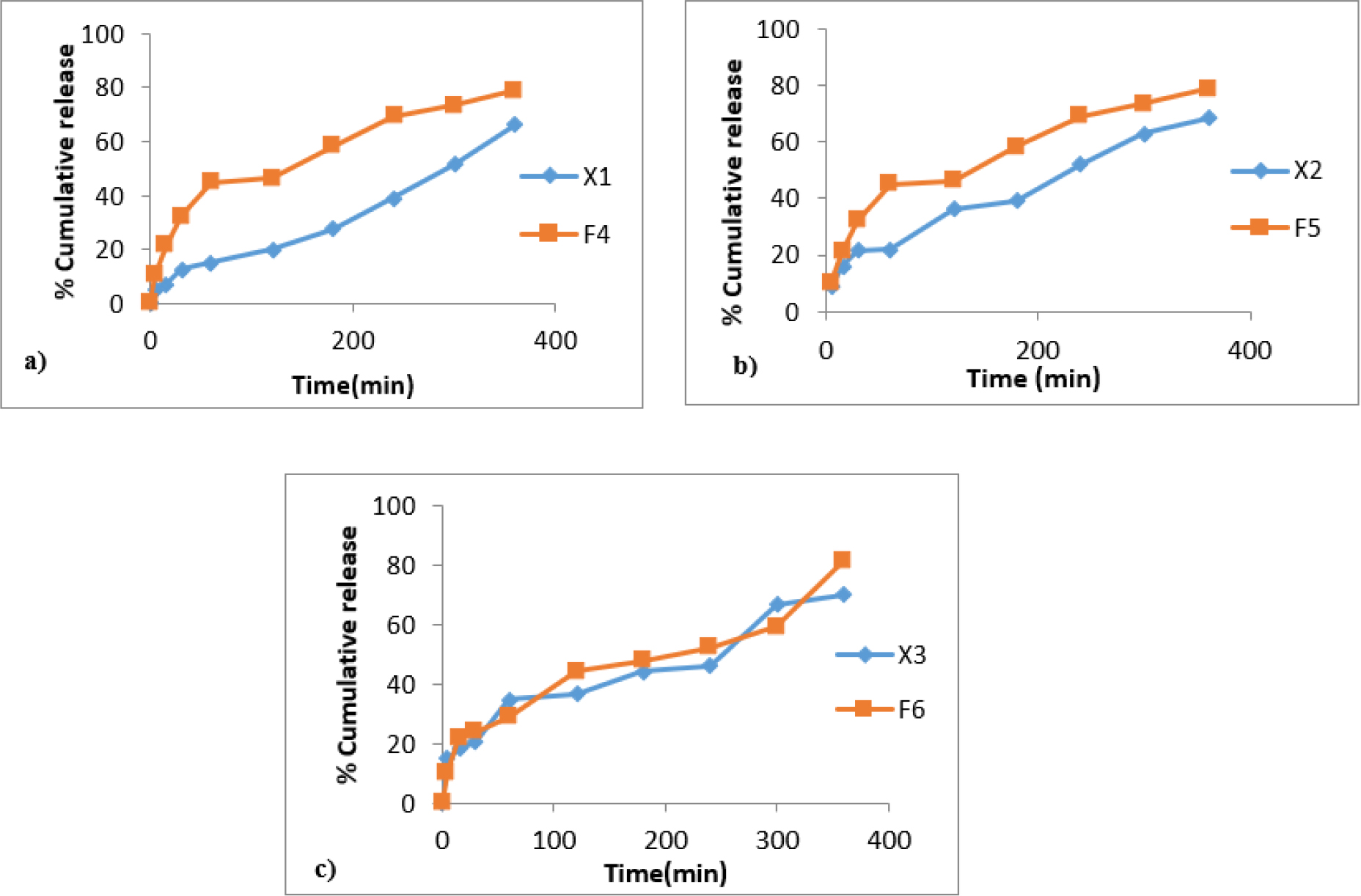
Figure 6:
Comparative ex vivo permeation profiles of acyclovir from buccal films with zingiberine as bioenhancers between formulations a) F4 and X1 b) F5 and X2 c) F6 and X3.
DISCUSSION
The main objective of the current investigation was to prepare mucoadhesive buccal films of acyclovir and assess them for various parameters, especially the effect of herbal bioenhancers on improving the permeability of the drug. The buccal films were prepared by solvent casting method which is a cost-effective method of formulating the films with minimal use of ingredients. Evaluation of the weight variation and thickness of the buccal films revealed that the weight of both piperine and zingiberene-incorporated formulations was found to be varying depending on the concentrations of the chitosan polymer. Further, the near to neutral pH of the film ensures that there is no irritation to the buccal mucosa upon application. The minimal variations in the drug content indicate that the drug was more or less uniformly distributed in different regions of the film. The formulated buccal films displayed good swelling index values that can be attributed to the presence of PVP, which is a hydrophilic polymer that displays good film hydration properties and increases the extent of swelling.14 The only parameter that was found lacking was the folding endurance which was less than ideal, this could probably be due to the fact that a small part of the drug was found to be precipitated which indicates that the drug could not remain in the solubilized condition affecting the film’s durability.22 Further analysis of tensile strength revealed that the tensile strength was proportional to the concentration of the chitosan used Moreover, the use of PVP in the formulation gave additional mechanical strength and rigidity to the films.23 The Acyclovir-loaded buccal films displayed excellent mucoadhesive properties due to the mucoadhesive nature of chitosan used in the formulation. İt was observed that the mucoadhesive strength and time of formulations increased with an increase in chitosan concentration. These findings indicate that choosing a polymer with mucoadhesive nature and concentration of that polymer is an important consideration for preparing mucoadhesive buccal films.23 From the drug release profile of the Buccal films, a prolonged release was observed, this is due to a higher swelling profile and slower release of the film from chitosan-based films which further validates the findings of swelling studies.24
| Formulation code | Folding endurance | Tensile strength (kg/cm2)* | Ex vivo mucoadhesive strength (g)* | Ex vivo mucoadhesive time (min)* |
|---|---|---|---|---|
| F1 | 218 | O.8±0.013 | 4.9±0.025 | 138±0.02 |
| F2 | 228 | O.83±0.032 | 6.2±0.017 | 168±0.031 |
| F3 | 275 | 2.11±0.015 | 8.7±0.02O | 174±0.026 |
| F4 | 230 | 3.I3±0.03O | 4.6±0.01O | 144±0.011 |
| F5 | 243 | 3.21±0.025 | 6.9±0.034 | 15O±0.027 |
| F6 | 257 | 3.33±0.034 | 8.3±0.026 | 165±0.015 |
Data of folding endurance, tensile strength, ex vivo mucoadhesive strength and ex vivo mucoadhesive time.
The mucoadhesive buccal films with bioenhancers (F1-F6) were evaluated for all the parameters and those without bioenhancers were considered for ex vivo permeation studies for comparing the enhanced permeability of the bioenhancers. The permeation studies showed that the drug permeation from all the piperine films was significantly greater than from the films prepared without the permeation enhancers but with similar polymer compositions. This indicates that the presence of piperine increases the membrane permeation capabilities of the drug. Piperine is considered the first bioenhancer to be ever used and is reported to improve the permeability of many drugs.25 It has been suggested that piperine by virtue of its high lipid solubility modulates the fluidity and permeability characteristics of the mucosal membranes, resulting in improved permeability.26 Similar results were obtained in the case of formulations containing zingiberine as bioenhancer.
CONCLUSION
Ex vivo studies demonstrated that the addition of bioenhancers piperine and zingiberine improved the permeation profiles of acyclovir. Most of the studies report the use of piperine for enhancing oral absorption of drugs. This study demonstrates that it is possible to promote buccal mucosal permeation of drugs by herbal bioenhancers, piperine and zingiberine. However further investigations such as in vivo buccal absorption studies in animals and stability studies are needed to validate the findings of the study.
Cite this article
Chandran A, Koland M, Sindhoor SM. Enhancing Permeability of Acyclovir from Mucoadhesive Buccal films Utilizing Herbal Bioenhancers: An in vitro Investigation. Int. J. Pharm. Investigation. 2024;14(1):127-34.
ACKNOWLEDGEMENT
The authors are thankful to NGSM Institute of Pharmaceutical Sciences, NITTE (Deemed to be University) for providing the necessary facilities to carry out the project work.
ABBREVIATIONS
| HCl | Hydrochloric Acid |
|---|---|
| HSV | Herpes Simplex Virus |
| PVP | Polyvinyl Pyrolidine |
| SI | Swelling Index |
| USP | United States Pharmacopoeia |
| UV | Ultraviolet |
References
- Hao J, Heng PWS. Buccal delivery systems. Drug Dev Ind Pharm. 2003;29(8):821-32. [PubMed] | [CrossRef] | [Google Scholar]

- Chinna Reddy P, Chaitanya KSC, Madhusudan Rao Y. A review on bioadhesive buccal drug delivery systems: current status of formulation and evaluation methods. Daru. 2011;19(6):385-403. [PubMed] | [Google Scholar]

- Kesarwani K, Gupta R, Mukerjee A. Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed. 2013;3(4):253-66. [PubMed] | [CrossRef] | [Google Scholar]

- Singh R, Devi S, Patel JH, Patel UD, Bhavsar SK, Thaker AM, et al. Indian herbal bioenhancers: a review. Pharmacogn Rev. 2009;3:80-2. [PubMed] | [CrossRef] | [Google Scholar]

- Dudhatra GB, Mody SK, Awale MM, Patel HB, Modi CM, Kumar A, et al. A comprehensive review on pharmacotherapeutics of herbal bioenhancers. Scientific World Journal. 2012;2012:637953 [PubMed] | [CrossRef] | [Google Scholar]

- Ajazuddin A, Alexander A, Qureshi A, Kumari L, Vaishnav P, Sharma M, et al. Role of herbal bioactives as a potential bioavailability enhancer for active pharmaceutical ingredients. Fitoterapia. 2014;97:1-14. [PubMed] | [CrossRef] | [Google Scholar]

- Atal N, Bedi KL. Bioenhancers: revolutionary concept to market. J Ayurveda Integr Med. 2010;1(2):96-9. [PubMed] | [CrossRef] | [Google Scholar]

- Tatiraju DV, Bagade VB, Karambelkar PJ, Jadhav VM, Kadam V. Natural Bioenhancers: an overview. J Pharmacogn Phytochem. 2013;2:55-60. [PubMed] | [CrossRef] | [Google Scholar]

- Mao QQ, Xu XY, Cao SY, Gan RY, Corke H, Beta T, et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods. 2019;8(6):185 [PubMed] | [CrossRef] | [Google Scholar]

- Schalkwijk HH, Snoeck R, Andrei G. Acyclovir resistance in herpes simplex viruses: prevalence and therapeutic alternatives. Biochem Pharmacol. 2022;206:115322 [PubMed] | [CrossRef] | [Google Scholar]

- Chaudhary B, Verma S. Preparation and evaluation of novel in situ gels containing acyclovir for the treatment of oral herpes simplex virus infections. ScientificWorldJournal. 2014;2014:280928 [PubMed] | [CrossRef] | [Google Scholar]

- Nair AB, Attimarad M, Al-Dhubiab BE, Wadhwa J, Harsha S, Ahmed M, et al. Enhanced oral bioavailability of acyclovir by inclusion complex using hydroxypropyl-β-cyclodextrin. Drug Deliv. 2014;21(7):540-7. [PubMed] | [CrossRef] | [Google Scholar]

- Shojaei AH, Berner B, Xiaoling L. Transbuccal delivery of acyclovir: I. In vitro determination of routes of buccal transport. Pharm Res. 1998;15(8):1182-8. [PubMed] | [CrossRef] | [Google Scholar]

- Koland M, Charyulu RN, Vijayanarayana K, Prabhu P. In vitro and in vivo evaluation of chitosan buccal films of ondansetron hydrochloride. Int J Pharm Investig. 2011;1(3):164-71. [PubMed] | [CrossRef] | [Google Scholar]

- Semalty M, Semalty A, Kumar G. Formulation and characterization of mucoadhesive buccal films of glipizide. Indian J Pharm Sci. 2008;70(1):43-8. [PubMed] | [CrossRef] | [Google Scholar]

- Ammar HO, Ghorab MM, Mahmoud AA, Shahin HI. Design and in vitro/in vivo evaluation of ultra-thin mucoadhesive buccal film containing fluticasone propionate. AAPS PharmSciTech. 2017;18(1):93-103. [PubMed] | [CrossRef] | [Google Scholar]

- Semalty A, Semalty M, Nautiyal U. Formulation and evaluation of mucoadhesive buccal films of enalapril maleate. Indian J Pharm Sci. 2010;72(5):571-5. [PubMed] | [CrossRef] | [Google Scholar]

- Lodhi M, Dubey A, Narayan R, Prabhu P, Priya S. Formulation and evaluation of buccal film of ivabradine hydrochloride for the treatment of stable angina pectoris. Int J Pharm Investig. 2013;3(1):47-53. [PubMed] | [CrossRef] | [Google Scholar]

- Bahri-Najafi R, Tavakoli N, Senemar M, Peikanpour M. Preparation and pharmaceutical evaluation of glibenclamide slow release mucoadhesive buccal film. Res Pharm Sci. 2014;9(3):213-23. [PubMed] | [Google Scholar]

- Haque SE, Sheela A. Development of polymer-bound fast-dissolving metformin buccal film with disintegrants. Int J Nanomedicine. 2015;10(Suppl 1):199-205. [PubMed] | [CrossRef] | [Google Scholar]

- Satishbabu BK, Srinivasan BP. Preparation and evaluation of Buccoadhesive films of atenolol. Indian J Pharm Sci. 2008;70(2):175-9. [PubMed] | [CrossRef] | [Google Scholar]

- Ansari M, Sadarani B, Majumdar A. Optimization and evaluation of mucoadhesive buccal films loaded with resveratrol. J Drug Deliv Sci Technol. 2018;44:278-88. [CrossRef] | [Google Scholar]

- Ashri LY, Abou El Ela AESF, Ibrahim MA, Alshora DH, Naguib Mj. Optimization and evaluation of chitosan buccal films containing tenoxicam for treating chronic periodontitis: in vitro and in vivo studies. J Drug Deliv Sci Technol. 2020;57:101720 [CrossRef] | [Google Scholar]

- Yehia SA, El-Gazayerly ON, Basalious EB. Fluconazole mucoadhesive buccal films: in vitro/in vivo performance. Curr Drug Deliv. 2009;6(1):17-27. [PubMed] | [CrossRef] | [Google Scholar]

- Raghunath I, Koland M, Vadakkepushpakath AN, Kumar L, Shenoy SCS. Herbal Bioenhancers with Nanocarriers: a Promising Approach for Oral Peptide Delivery. Int J Pharm Investigation. 2022;13(1):7-13. [CrossRef] | [Google Scholar]

- Kaushal N. Influence of piperine on transcutaneous permeation of repaglinide in rats and on tight junction proteins in HaCaT cells: unveiling the mechanisms for enhanced permeation. Sci Pharm. 2009;77:877-98. [CrossRef] | [Google Scholar]


