ABSTRACT
Background
The classical ayurvedic formulation Bru-satavari after consumption by people can get a restful night’s sleep and feel rejuvenated. The goal of this study is to look at important phytoconstituents which may influence the neuropharmacological activities. Therefore, we made an effort to learn the methanol extract of classical ayurvedic formulation Bru-Satabari having anyneuropharmacological activity.
Materials and Methods
The open field, hole cross, rota-rod, and thiopental sodium-induced sleeping duration tests on mice were used to examine the sedative effect of brusatavari at dosages of 400 mg/kg. The anxiety-relieving effect evaluated using the hole-board test. Compared to the positive control drug diazepam, sedative and anxiolytic effects were seen. Analgesic effect was evaluated by tail flick method.
Results
According to the study, the extract has remarkable neuropharmacological action because it lowers mice’s anxiety and locomotor activity in all instances of hole cross, open field, and rota rot tests when compared to the control. Additionally, compared to the standard and control group, the extract extends sleep time with a rapid onset of effect. Further the Bru-satavari exhibited excellent analgesic activity in comparison to control.
Conclusion
The findings confirm the use of Bru-Satabari in traditional medicine by showing that it exhibits sedative and anxiolytic properties. Best suggested: More research into the drug’s mechanism of action and the isolation of its active compounds.
INTRODUCTION
The research upon neuropharmacology investigates how drugs alter the working of cells in the sensory system and also neurological systems through influencing behaviors and actions.1 Neuropharmacology comprises two main parts: behavioral and molecular. Competence Neuroscience focuses on the effect of medications on the actions of people (neuropsychopharmacology), particularly the study of drug addiction and dependence on the cognitive system.2 The examination of neurons, particularly their combinatorial communications, involves molecular neuropharmacology with the aim of producing medicines that impact neurologic potentials. In stressful conditions, the body goes into “fight or flight” mode to fine-tune cognitive capabilities and actions while also protecting itself from imagined damage. Specific brain chemical substances like endocannabinoids are generated throughout this procedure to aid the physique in its maximum performance. In motor function, cognitive function, emotions, and behavior, endocannabinoids play a significant role. Notably, studies reveal that marijuana, generally referred to as marijuana, comprises endocannabinoids as well as a number of cannabinoids, which can also be favorable for the behaviour of a mouse.3–5 However, chronic marijuana use may lead to dependence and other adverse side effects.6
The ayurvedic drug Bru Satabari contains Satabari– Asparagus racemosus, Sunthi– Gingiber officinalis, Amla– Phyllanthus emblica, Elaichi– Ellettaria cardamomum, Dalchini– Cinnamomum zeylanicum, Labanga– Syzygium aromaticum, Raktachandan– Pterocarpus santalinus, Milk, Sugar, Kesar– Crocus sativus, Madhuri– Foeniculum vulgar, Bhanga– Cannabis sativa. It is stated that Bru-Satabaria a classical ayurvedic medicines has a Bazikaran Dhatu Berdhak constipation relaxation effect. Because of the inclusion of cannabis in the preparation, this medicine may also have implications on the CNS and peripheral nervous system. Tests on the bioactive ingredients and behavioral neuropharmacological activity of conventional ayurvedic medicine in rats were carried out to ensure the quality, purity, and behavioral neuropharmacological effectiveness of the chosen pharmaceutical formulations.
MATERIALS AND METHODS
High performance thin layer chromatography
The HPTLC technique is a sophisticated and automated separation technique that was developed from TLC. Pre-coated HPTLC rated plates and an auto sampler were used to achieve accuracy, sensitivity, and significant separation on both a qualitative and quantitative level. Cannabis is readily soluble in the majority of organic solvents, including methanol, petroleum ether, n-hexane, toluene, chloroform, and solvent blends like methanol:chloroform (9:1). 500 mg of the Bru-Satavari ayurvedic formulation are extracted using the following procedure using 5 ml of methanol: chloroform (9:1 v/v): After centrifugation, there is a 15-minute ultrasonic bath with three additional vortexing periods of 5, 10, and 15. Chromatographic analysis was conducted using 7HPTLC plates that were 10 cm x 20 cm in size. Linomat IV sample applicator was used to apply samples in bands 6 mm wide and 8 mm apart. The sample was put onto the plate at a rate of 160 nL/s. The plates were formed in a previously saturated 20 cm x 10 cm twin-trough glass container using n-hexane, dioxane, and methanol (7:2:1) as the mobile phase under ambient temperature and humidity conditions (25 and 2%, respectively). These plates were allowed to stand at room temperature before being heated to reveal compact bands. Qualitative evaluation was done using CATS 4 and WinCATS software Version 1.2.0 in reflectance mode at wavelengths of 254 and 366 nm.8
FTIR
ATR FTIR spectra were recorded on a Diamond Crystal ATR (Attenuated Total Internal Reflectance) attachment for the Bruker-Alpha II FTIR spectrometer. Vacuum evaporated methanol extract of Bru Satavari was applied enough to coat the crystal with approximately 1 mm thickness of material. The sample was placed under the pressure arm, which then exerted pressure on it. Opus 7.8 software was used to operate the spectrometer. Direct placement of the sample on the diamond crystal plate. At room temperature, spectra covering the wavelength range of 4000 to 650 cm-1. were all captured.9
GC-MS analysis
For the GC-MS analysis, 200 gm of BruSatavari were extracted with methanol. The resultant methanol extract was filtered and concentrated at 40°C under decreased pressure in a rotary evaporator. The methanol extract was subjected to the GC-MS analysis. A Chromatography-Mass Spectrometry equipment with a Thermo Trace 1300GC and a Thermo TSQ 800 Triple Quadrupole MS was used to conduct the GC-MS analysis. These are the conditions: The 30 x 0.25mm ID x TG 5MS (30m X 0.25mm, 0.25m) Elite-1 fused silica capillary column was composed of 95% dimethyl polysiloxane and 5% diphenyl. The GC runs in electron impact mode at 70 eV. For the GC-MS analysis, 200 gm of BruSatavari were extracted with methanol. The resultant methanol extract was filtered and concentrated at 40 °C under decreased pressure in a rotary evaporator. The methanol extract was subjected to the GC-MS analysis. A Chromatography-Mass Spectrometry equipment with a Thermo Trace 1300GC and a Thermo TSQ 800 Triple Quadrupole MS was used to conduct the GC-MS analysis. These are the conditions: The 30 x 0.25mm ID x TG 5MS (30m X 0.25mm, 0.25m) Elite-1 fused silica capillary column was composed of 95% dimethyl polysiloxane and 5% diphenyl. The GC runs in electron impact mode at 70 eV. The GC runs for 44 minutes in total. By comparing and evaluating each component part’s average peak area to the total areas, the relative percentage aggregate for each component part was determined. The software used to manage mass spectra and chromatograms is Turbo Mass Version 5.2.0. By percentage peak area, the BruSatavari GC-MS separated components were displayed. By comparing mass spectrum fragmentations and retention indices to values listed in NIST databases, compounds were detected.
Neuropharmacological activity
Utilizing several animal models, the methanolic extract of Bru-Satavari was studied for its neuropharmacological effects. Three groups of six rats each were used in each experiment.
This experimental design is as follows;
Group-I – Normal Control (Tween + Water).
Group-II – Diazepam 1mg/kg.
Group-III –Test (400 mg/kg body wt.).
Sedative activity
Hole cross test methodology was used to test the action of sedatives in mice. A wooden box 30×20×14cm in size was divided in the centre on which the experiment was carried out. In the centre of the box, a 3 cm diameter of the hole drilled with a 7.5 cm height. Following oral administration of the test extract, the number of mice passing through the aperture from one compartment to the other was counted for three minutes at intervals of 0, 30, 60, 120, 180, and 240 minutes.10
Locomotor and behavioural activity
In mice, the free or open space behavioural test is commonly used to assess emotional and motor behavior. Control, positive control, and test groups of animals were separated. The equipment was made of plywood was (72 cm x 72 cm x 36 cm) was composed of plywood. On the board, a half-square-meter open area is divided into a series of squares with contrasting colors (black and white).
The open field equipment’s cardboard floor was separated into 16 squares (18 cm x 18 cm). This experiment was conducted at room temperature in a darkened space. Following oral administration of the test extract for 0, 30, 60, 90, and 120 min, the number of squares each animal from each group had crossed over was counted for 3 min.11
Motor coordination or grip strength activity
After receiving an oral dose of Bru-Satabari methanol extract, each new cohort of rats had their motor performance and coordination tested in a rota-rod apparatus 30 minutes later. Rats were housed on a wooden horizontal rod that rotated at rate of 20 revolutions per minute. Three groups of the animals were created (n = 6). The rats had 180 sec of rota-rod training prior to the trial. Bru-Satabari methanol extract was given orally to the test groups, whereas the control group was given 0.9 percent solvent vehicle. The animals in the positive control group received diazepam (1 mg/kg). The animals were placed in a spinning bar with a diameter of 2.5 cm and a height of 25 cm off the ground. Each rat was observed for 180 sec, which is designated performance time, and rats who failed to stay on the rota-rod more than once were regarded to have passed the test.12
Hypnotic activity
Thiopental sodium-induced sleeping time test was used to determine hypnotic activity. The test groups received Brusatvari methanol extract orally whereas the control group received 0.9% solvent vehicle. Animals in the positive control group received diazepam (1 mg/kg). 30 min later, thiopental sodium (40 mg/kg, i.p.) was administered to each mouse to induce sleep. The animals’ latent period (the amount of time between receiving Thiopental sodium and losing the righting response) and sleep duration were tracked (the time between the loss and recovery of righting reflex).13
Analgesic activity
Also monitored was analgesic action Wistar albino rat tail immersion test using caudal immersion. The tail flick test uses heat stimuli to cause pain, although the heat is different from other tests. To stimulate tail immersion, hot water is employed. The experimental animal was housed in a cage with only one-third of its tail allowed to extend outside. This animal was then submerged in a 55°C hot water bath until the rat withdrew its tail. To avoid injury, the cut-out time is roughly 180 seconds.14
RESULTS
High performance thin layer chromatography
This technique is therefore practical for routine quality control analysis. It produces a phytochemical chromatographic fingerprint that can be used to verify the authenticity and purity of raw medicinal plant materials. The High Performance Thin Layer Chromatography (HPTLC) technology offers the best accuracy and precision with the greatest degree of flexibility for varied steps. It is also the simplest and fastest separation method currently accessible. The outcomes, including the number of peaks and the highest Rf value, are reported in (Tables 1, 2 and Figures 1, 2).The extract of methanolic extract of Bru-Satabari, formulation showed 9 peaks having maximum Rf value 0.09, 0.38, 0.48, 0.51, 0.53, 0.59, 0.62, 0.81, 0.86 at 254 nm. The methanolic extract of Bru-Satabari, showed 2 peaks having maximum Rf value 0.35, 0.45 at 366 nm. The results are reported in with the number of peaks and maximum Rf value (Tables 1 and 2)
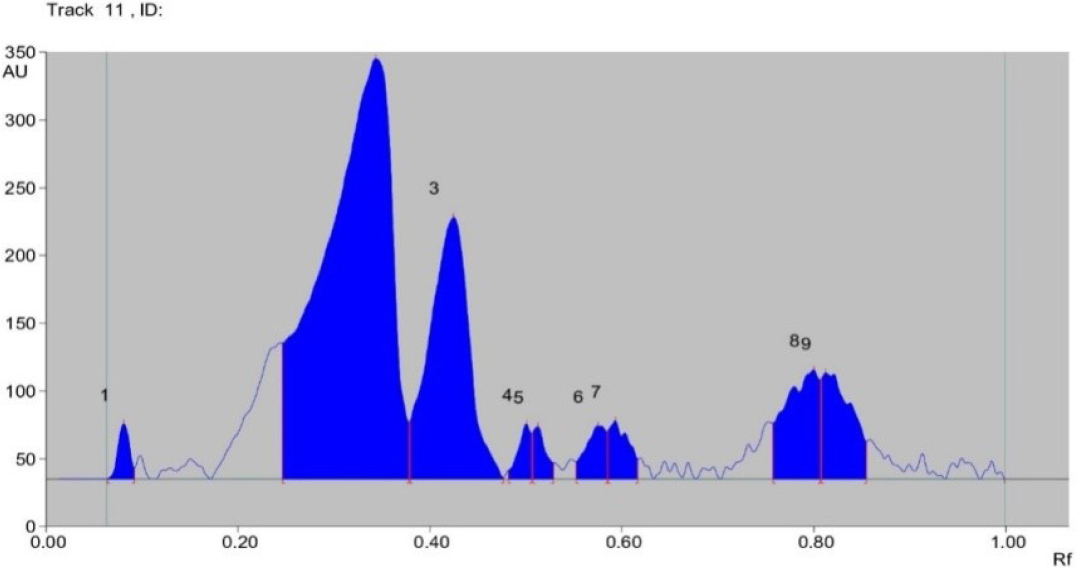
Figure 1:

Figure 2:
| Peak | Start position | Start height | Max position | Max Height | Max % | End position | End position | Area | Area % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.06 Rf | 0.3 AU | 0.08 Rf | 40.7 AU | 4.71% | 0.09 Rf | 7.8 AU | 423.3 AU | 1.29% |
| 2 | 0.25 Rf | 0.1 AU | 0.34 Rf | 110.2 AU | 85.91% | 0.38 Rf | 1.7 AU | 8583.9 AU | 56.63% |
| 3 | 0.38 Rf | 42.6 AU | 0.43 Rf | 93.1 AU | 22.35% | 0.48 Rf | 1.2 AU | 6862.2 AU | 20.91% |
| 4 | 0.48 Rf | 6.3 AU | 0.50 Rf | 40.7 AU | 4.71% | 0.51 Rf | 3.5 AU | 463.4 AU | 1.41% |
| 5 | 0.51 Rf | 34.3 AU | 0.51 Rf | 38.4 AU | 4.45% | 0.53 Rf | 2.0 AU | 441.2 AU | 1.34% |
| 6 | 0.55 Rf | 12.5 AU | 0.58 Rf | 39.1 AU | 4.53% | 0.59 Rf | 4.8 AU | 721.8 AU | 2.20% |
| 7 | 0.59 Rf | 35.7 AU | 0.59 Rf | 42.7 AU | 4.95% | 0.62 Rf | 4.6 AU | 753.1 AU | 2.29% |
| 8 | 0.76 Rf | 41.0 AU | 0.80 Rf | 80.6 AU | 9.33% | 0.81 Rf | 3.1 AU | 2440.9 AU | 7.44% |
| 9 | 0.81 Rf | 74.1 AU | 78.3 Rf | 78.3 AU | 9.06% | 0.86 Rf | 7.5 AU | 2125.7 AU | 6.48% |
High performance thin layer chromatography analysis of Methanolic extract of Bru-Satabari, An ayurvedic classical medicine at 254nm.
| Peak | Start position | Start height | Max position | Max Height | Max % | End position | End height | Area | Area % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.23 Rf | 4.4 AU | 0.32 Rf | 55.9 AU | 63.65% | 0.35 Rf | 4.0 AU | 3375.6 AU | 70.99% |
| 2 | 0.38 Rf | 1.0 AU | 0.42 Rf | 36.2 AU | 36.35% | 0.45 Rf | 3.2 AU | 970.9 AU | 29.01% |
High performance thin layer chromatography analysis of
Methanolic extract of Bru-Satabari
, An ayurvedic classical medicine at 254nm.
ATR FTIR Evaluation
To demonstrate the functionalization of the cannabis present in the ayurvedic preparation Bru Satavari, the ATR FTIR spectra (4000-650 cm-1) of the methanol extract of the formulation were recorded. Figure 3 shows the characteristic bands of cannabinoids around 1635, 1576, 1424, 1161, 1043 cm-1 (Tetrahydrocannabinol) and 1684, 1592, 1515, 1366 cm-1 (cannabidiol).15
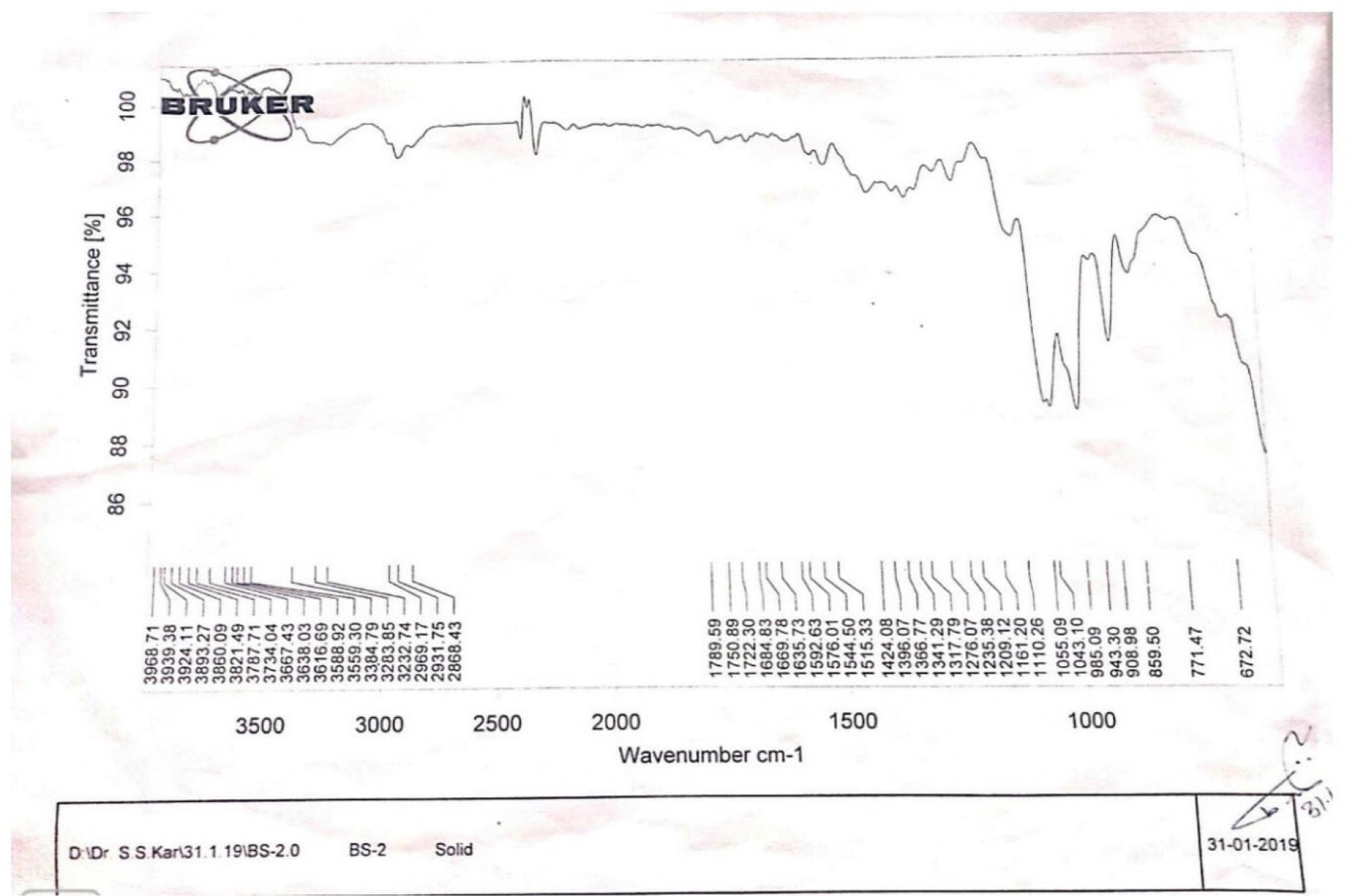
Figure 3:
FTIR analysis of powdered drug of Methanolic extract of Bru-Satabari, a classical ayurvedic formulation.
GC-MS analysis Revision needed
Table 3 and Figure 4 shows the GC-MS analysis of methanolic extracts of Bru-Satabari, a traditional ayurvedic preparation. Cannabidiol, with an RT value of 13.89, was reported to even have functions such as a sleeping aid, pain reduction, and anti-tumor. Cannabidiol inhibits the progression of disorders such as Parkinson’s and Alzheimer’s.16 CBD acts as an inhibitor of anandamide reuptake, an inverse agonist of the CB2 receptor, as well as a non-competitive negative allosteric modifier of the CB1 receptor. DELTA.8-Tetrahydrocannabinol contains antiemetic, anxiolytic, appetite stimulant, analgesic, and neuroprotective effects.17 THC functions as a cannabinoid 1/2 (CB 1/2) receptor partial agonist. It causes the normal effects associated with cannabis, including euphoria, relaxation, and altered perceptions.
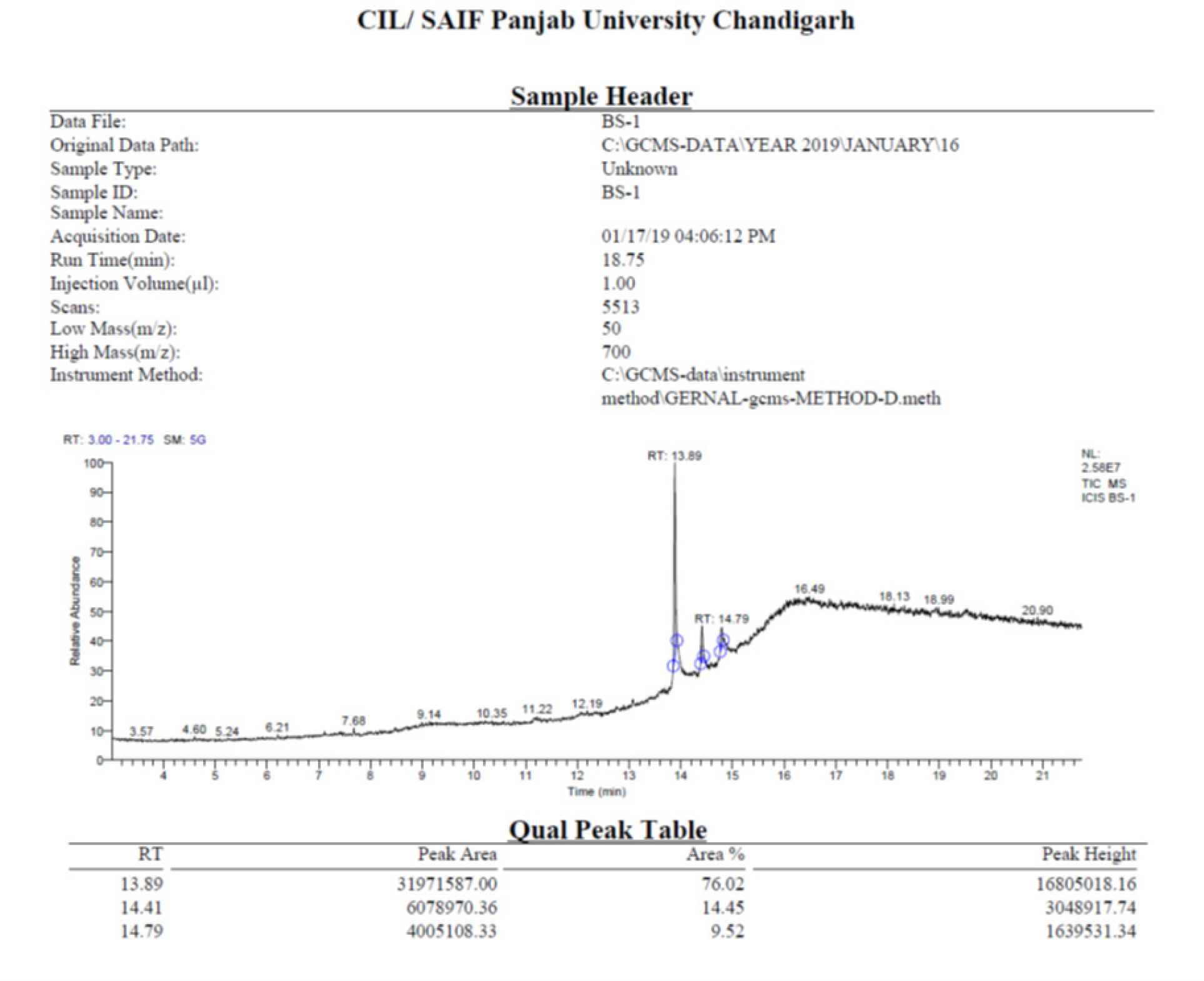
Figure 4:
GC-MS chromatogram of Methanolic extract of Bru-Satabari, a classical ayurvedic formulation.
| Sl. No | Compound name | Peak area% | Retention time in minutes | Molecular formula | Cas no. | Molecular weight | Pharmacological activity |
|---|---|---|---|---|---|---|---|
| 1 | Cannabidiol | 31971587.00 | 13.89 | C21H30O2 | 13956-29-1 | 314.464 g/mol | Can relieve pain, reduce anxiety and depression, can alleviate cancer related symptoms, reduce acne, have neuroprotective properties, could benefit heart health. |
| 2 | DELTA.8-Tetrahydro cannabi-nol | 6078970.36 | 14.41 | C21H30O2 | 5957-75-5 | 314.5 g/mol | Antiemetic, anxiolytic, appetite-stimulating, analgesic, and neuroprotective properties. |
| 3 | Cannabinol | 4005108.33 | 14.79 | C21H26O2 | 521-35-7 | 310.437 g/mol | CBN has strong sedative and relaxing properties, provide effective pain relief, CBN may stimulate the growth of bone marrow cells, used to stimulate appetite, is an anticonvulsant, and is anti-bacterial. |
Compounds identified by GC-MS analysis of Sukumaram Kashayam.
Additionally, dysphoria, anxiety, and psychotic symptoms have all been connected to THC. THC is employed to alleviate chronic pain, as an appetite stimulant, and to ease nausea and vomiting brought on by chemotherapy. THC is employed to alleviate chronic pain, as an appetite stimulant, and to ease nausea and vomiting brought on by chemotherapy.18Cannabinol with an RT value of 14.79 has sedative, anti-convulsant, and anti-bacterial properties.19–21
Sedative activity
From 0 to 120 minutes, mice in the control group opened the same number of chambers before moving on to the next. In the hole-cross test, methanolic extract of aclassical ayurvedic formulation- Bru Satabari at dose given at 400 mg kg-1 demonstrated a diminishing in velocity in mice from second observation period (30 min.) and was sustained up to third observation (60 min.) as appears by the decrease in multitude of holes crossed by the treated mice compared with the control group. The outcome was exactly equivalent to control group and was not statistically significant for the Bru-Satavari 400mg/kg at 0th min to 120 min. The positive control diazepam reduced locomotion behavior in the test animal as well. The results and experimental data were shown in Table 4.
| Groups (n=6) | Treatment | Dose | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|---|---|
| I | Control | 1% CMC | 17.00 ± 0.83 | 18.80 ± 0.66 | 20.80 ± 0.97 | 19.00 ± 1.30 | 17.40 ± 0.51 |
| II | Diazepam | 1mg/kg | 9.9±0.64 | 8.20±0.80** | 7.8± 0.67*** | 6.20± 0.57*** | 3.66± 0.41*** |
| III | Methanol extract | 400mg/kg | 10.80 ± 1.22 | 10.80 ±0.49** | 9.80 ± 2.33*** | 7.00 ± 1.81*** | 4.00 ± 0.31*** |
Effect of methanolic extract of
Bru-Satabari
on Hole Cross Test in Mice.
Locomotor and behavioural activity
It has been demonstrated experimentally that, in the absence of a special task to undertake, a given animal’s behavior tends to sustain that inner activation level, which is sometimes inconsistent with the animals’ actual level of activation. The open field test was carried out in order to get the most precise picture of the drug’s effect on exploration. Methanolic extract of a classical ayurvedic formulation-Bru-Satabari 400 mg/kg reduced exploration in the open field test. However, after half an hour of the drug administration of Brusatavari produced a mark reducing effect on the exploration. The drug significantly decreased the animal on exploration (p > 0.01)by experimental animals in 90 and 120 min. when compared to control group. The outcome of this test was presented in Table 5.
| Groups | Treatment | Dose | No. of square crossed | ||||
|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | |||
| I | Control | 1% CMC | 102.80 ± 2.92 | 98.00 ± 2.81 | 97.40 ± 2.27 | 82.40 ± 2.69 | 72.00 ± 2.55 |
| II | Diazepam | 1mg/kg | 94.00 ± 1.74 | 26.60 ± 1.81*** | 21.00 ± 1.20*** | 11.60 ± 0.51*** | 4.20 ± 0.56*** |
| III | Methanol extract | 400mg/kg | 99.80 ± 2.43 | 87.00 ± 2.58** | 84.20 ± 1.15*** | 21.40 ± 2.11*** | 15.60 ± 2.31** |
Effect of methanolic extract of
Bru-Satabari
on Open Field Test in Mice.
Motor coordination or grip strength activity
It is well accepted that several benzodiazepines, such as diazepam, produce muscle relaxants, ambulatory mobility decrease, and drowsiness, lowering rat efficiency on the rota-rod. The effect of Bru-Satabari after 30 min. of oral administration at 400 mg/kg on the rota-rod test was statistically very significant (p < 0.001) in increasing the multitude of falls while decreasing the performance time. Muscle relaxant effects were comparable to the favourable benefits. The rota-rod test is a popular method for assessing muscle relaxant influence in animals. Treatment with Bru-Satabari increased the frequency of rats falling and lowered the time they spent performing on the revolving rod, according to our findings (Table 6). It was also discovered that diazepam promoted muscle relaxant influence in the animal’s, which resulted in a longer period for them to fall in the rota-rod.
| Groups | Treatment | Dose | Performance time (s) | Number of falls |
|---|---|---|---|---|
| I | Control | 1% CMC | 175.00 ± 1.89 | 1.30 ± 0.64 |
| II | Diazepam | 1mg/kg | 151.00 ± 1.54*** | 13.60 ± 0.40*** |
| III | Methanol extract | 400mg/kg | 136.00 ± 1.22*** | 14.20 ± 0.73*** |
Effect of methanolic extract of
Bru-Satabari
on Rota Rod Test in Mice.
Thiopental sodium-induced sleeping
Significant (p<0.001) decrease in the onset of sleep was observed in the thiopental sodium-induced sleeping time test. Furthermore, a significant (p<0.001) increase in total sleeping time was observed in mice treated with Bru-Satabari methanolic extract (400 mg/kg) mg/kg when compared to the control group. The positive control group (diazepam 1 mg/kg) had a marked impact, as did the ayurvedic formulation (Table 7).
| Groups | Treatment | Dose | ||
|---|---|---|---|---|
| I | Control | 1% CMC | 13.57 + 0.45 | 87.80 + 1.85 |
| II | Diazepam | 1mg/kg | 6.72 + 0.06*** | 187.40 + 1.12*** |
| III | Methanol extract | 400mg/kg | 8.50 + 0.22*** | 163.00 + 1.37*** |
Effect of methanolic extract of
Bru-Satabari
on thiopental sodium-induced sleeping time test in mice.
This is a standard method in cognitive and behavioural pharmacology for investigating sedative properties. In our study, the herbal remedies formulation significantly reduced sleep latency while also increasing sleep duration (Table).
Tail-immersion Test
Treatment with the Methanolic extract of a classical ayurvedic formulation-BruSatabari (400 mg/kg) showed a critical and portion subordinate antinociceptive movement in the tail immesrsion test. The 400 mg/kg of the Methanolic extract of a classical ayurvedic formulation-Bru Satabari expanded an antinociceptive movement in 0 min and 30 min after infusion that was equivalent to the control gathering. Under comparative conditions, treatment with morphine 5% mg/kg altogether expanded dormancy to warm incitement 45 min after organization and the antinociceptive impact was kept up amid the whole time of assessment. Treating the experimental rats with the methanolic extract of a classical ayurvedic formulation-Bru-Satabari at portion 400 mg/kg modifies rats inactivity to excruciating warm upgrade in tail flick tests. These discoveries recommend that central and peripheral systems are engaged with the antinociceptive movement of the extract. The Methanolic extract of a classical ayurvedic formulation-Bru Satabari acts at the most extreme portion (400 mg/kg) could ease the torment in all time of tail flick test. The results and experimental data were shown in Table 8.
| Groups | Treatment | Dose | 0 min | 30 min | 60 min |
|---|---|---|---|---|---|
| I | Control | 1% CMC | 3.9±0.1 | 3.7±0.2 | 4.1±0.2 |
| II | Diazepam | 1mg/kg | 3.9±0.1 | 9.4±0.1** | 13.1±0.1** |
| III | Methanol extract | 400mg/kg | 4.1±0.2 | 8.0±0.2** | 12.1±0.2** |
Effect of methanolic extract of
Bru-Satabari
on Tail-immersion Test in Mice.
At all time points except 60 minutes, the methanol extract Bru-Satabari exhibited significant analgesic activity by increasing the reaction time of the rats compared to the control (saline treated rats). Diazepam produced the most significant antinociception effect in comparison to the control during all observation times, followed by the extract. The tail-flick method is based on the discovery that morphine-like compounds selectively prolong the reaction time of rats’ typical tail-withdrawal effect.
DISCUSSION
This HPTLC methodology may be beneficial for both identifying and assessing the quality of methanolic extract-containing preparations of Bru-Satabari-An ayurvedic classical medicine. From the study the extract showed two constituents which was found that to have Rf value of 0.38 and 0.48 at 254 nm which indicates the presence of Tetrahydrocannabinol and cannabidiol respectively present in cannabis as per the previous literature.22
The FTIR result conclude that methanol extract of Bru-Satavari include Cannabidiol as well as tetrahyrocannabinol component, having psychoactive properties. From the literature it was found that Cannabidiol does not appear to have any intoxicating effects such as those caused by THC.23
The presence of compounds such as Cannabidiol, DELTA. 8-Tetrahydrocannabinol, and Cannabinol in the formulation confirms the presence of cannabis. Furthermore, the presence of cannabis demonstrates neuropharmacological action.
GABA is the most important inhibitory neurotransmitter in the central nervous system. Distinctive anxiolytic, sedative hypnotic drug elucidate their activity through GABAA receptor. Its sedative effect observed here could be due to a interaction with a chemical agent that adheres to a CNS receptor.
Therefore it is possible that methanolic extract of a classical ayurvedic formulation-Bru-Satabari may act by potentiating GABA restraint in the CNS or might be because of the initiation of the GABAergic receptor by the extract. GC-MS examination on the extract showed the presence of THC etc. which prompted the conclusion that phytoconstituents like THC present in the extract is responsible for sedative activity.24 The decline number of holes crosses by diazepam treated mice contrasted with control might be because of the portion 1mg kg-1 utilized in the standard that can deliver sedation in experimental animals.25
The potential of Bru-satavari to suppress locomotor activity implies that it has central nervous system depressant activity, which was confirmed by GCMS analysis of the extract.26
The fact that Bru-Satabari and diazepam have similar effects led experiment to believe that Bru-Satabari can have sedative effects in experimental mice, reducing both overall activity and motor coordination.27 Our previous evidence supports the experimental data found in the thiopental sodium-induced sleeping period observation test. The sedative characteristics of a substance are investigated using this test, which is a standard procedure in behavioural pharmacology.
As anticipated, diazepam administration produced similar effects. Substantial evidence suggests that CNS depressants, such as medicinal phytoconstituents, bind to the gamma aminobutyric acid type A (GABAA) receptor complex and end up causing
postsynaptic neuron hyperpolarization. 28 Based on the findings of this study, cannabidiol (CBD) found in the formulation and endorsed by GC-MS data may have greater therapeutic potential for the treatment of insomnia.
Centrally acting analgesics raise the pain threshold of animals in response to pressure and heat.29 As a result, the extract’s onset of action on this pain-state model suggests that it may be acting centrally due to the presence of cannabinol. At all time points, the extract’s tail-flick latency was less than that of the reference drug. According to the investigation, BruSatabari may be a better natural alternative for mild pain relief.
CONCLUSION
The analytical data presented here makes it possible the key phytoconstituents spotted in the Ayurvedic formulation to be ascertained. Cannabinoids’ identification, isolated from or reveal in ayurvedic therapies would be facilitated by expressing all analytical measurements taken under standardised conditions. The presence and number of substances in the herbal formulation were ascertained using HPTLC. The presence of cannabidiol substances in the Ayurvedic formulation was affirmed by the FTIR result obtained. The FTIR result conclude that methanol extract of BruSatavari include Cannabidiol as well as tetrahydrocannabinol component. Finally, the use of GCMS made It is possible to identify all of the tests performed phytoconstituents especially cannabinoids in one single analysis, even in the low ng/mL concentration range. The sedative effect of the formulation is due to the presence of cannabinoids, methanolic extract BruSatabari may act by potentiating GABA restraint in the CNS or might be because of the initiation of the GABAergic receptor by the extract. Bru-satavari’s ability to suppress locomotor activity suggests that it has a central nervous system depressant activity due to the presence of cannabinoids as confirmed in GCMS analysis of the extract. The fact that Bru-Satabari effects led us to believe that the ayurvedic drugs can have sedative effects in experimental mice, reducing both overall activity and motor coordination. The outcome or results found in the thiopental sodium-induced sleeping period observation test have been further supported by our above-mentioned evidence. The formulation’s onset of action on this pain-state model suggests that it may be going to act centrally due to the presence of cannabinol. According to the study, Bru-Satabari may be a better natural solution for fairly benign pain relief. However, additional research can be conducted to determine the phytoconstituents responsible for the mechanism of action involved.
References
- Yeung AWK, Tzvetkov NT, Atanasov AG. When neuroscience meets pharmacology: A neuropharmacology literature analysis. Front Neurosci. 2018;12:852 [PubMed] | [CrossRef] | [Google Scholar]

- EverittB J, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481-9. [PubMed] | [CrossRef] | [Google Scholar]

- Andre CM, Hausman JF, Guerriero G.. Cannabis sativa: the Plant of the Thousand and One Molecules. FrontPlant Sci.. 2016;7:19 [PubMed] | [CrossRef] | [Google Scholar]

- [PubMed] | [CrossRef] | [Google Scholar]

- Fride E, Perchuk A, Hall FS, Uhl GR, Onaivi ES. Behavioral methods in cannabinoid research. Methods Mol Med.. 2006;123:269-90. [PubMed] | [CrossRef] | [Google Scholar]

- Grant BF, Pickering R.. The relationship between cannabis use and DSM-IVcannabis abuse anddependence: results from the National Longitudinal alcohol Epidemiologic Survey. J Subst Abuse. 1998;10(3):255-64. [PubMed] | [CrossRef] | [Google Scholar]

- Daharwal SJ, Shrivastava S.. Preliminary Phytochemical Screening and HPTLC Fingerprinting of Extracts of . Res J Pharm Technol. 2019;12(10):4782-4. [CrossRef] | [Google Scholar]

- Alam P, Alajmi MF, Siddiqui NA, Al-Rehaily AJ, Basudan OA. Determination of bioactive marker glycyrrhizin in Glycyrrhizaglabraroot and commercial formulation by validated HPTLC-densitometric method. J Coast Life Med.. 2014;2(11):882-87. [CrossRef] | [Google Scholar]

- Valh JV, Peršin Z, Voňcina B, Vrezner K, Tušek L, FrasZemljiˇc L., et al. Microencapsulationo f cannabidiol in liposomes as Coatingfor cellulose for potential advanced sanitary material. Coatings.. 2021;11(1):1-18. [CrossRef] | [Google Scholar]

- Subhan N, Alam MA, Ahmed F, Shahid IJ, Nahar L, Sarker SD, et al. Bio activity of . Rev bras farmacogn.. 2008;18(4):521-6. [CrossRef] | [Google Scholar]

- Shahed-Al-Mahmud Md, Lina SMM. Evaluation of sedative and anxiolytic activities of methanol extract of leaves of in mice. Clin Phytosci.. 2017;3(1):20 [CrossRef] | [Google Scholar]

- Chatterjee M, Verma P, Maurya R, Palit G.. Evaluation of ethanol leaf extract of in experimental models of anxiety and depression. PharmBiol. 2011;49(5):477-83. [PubMed] | [CrossRef] | [Google Scholar]

- Moniruzzaman M, Rahman MA, Ferdous A.. Evaluation of sedative and hypnotic activity of ethanolic extract of Linn. Evid Based Complement Alternat Med. 2015:873954 [PubMed] | [CrossRef] | [Google Scholar]

- Sumanta M, Debjit G, Seru G, Onkar M, Venkata RM, Vankayalpati R, et al. Evaluation of analgesic, antipyretic and anti-inflammatory effects of ethanol extract from fern species (Gaudich.) Aerial parts. Pahrmacognosy Commun.. 2016;6(2):57-63. [CrossRef] | [Google Scholar]

- [CrossRef] | [Google Scholar]

- Fernández-Ruiz J, Sagredo O, Pazos MR, García C, Pertwee R, Mechoulam R, et al. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid?. Br J ClinPharmacol.. 2013;75(2):323-33. [PubMed] | [CrossRef] | [Google Scholar]

- [PubMed] | [Google Scholar]

- Kleckner AS, Kleckner IR, Kamen CS, Tejani MA, Janelsins MC, Morrow GR, et al. Opportunities for cannabis in supportive care in cancer. TherAdv Med Oncol.. 2019;11:1-29. [PubMed] | [CrossRef] | [Google Scholar]

- Pickens JT. Sedative activity of cannabis in relation to its delta ‘-trans-tetrahydrocannabinol and cannabidiol content. Br J Pharmacol. 1981;72(4):649-56. [PubMed] | [CrossRef] | [Google Scholar]

- Karler R, Cely W, Turkanis SA. The anticonvulsant activity of cannabidiol and cannabinol. Life Sci.. 1973;13(11):1527-31. [PubMed] | [CrossRef] | [Google Scholar]

- Van Klingeren B, Ten Ham M.. Antibacterial activity of delta9-tetrahydrocannabinol and cannabidiol. Antonie Leeuwenhoek. 1976;42(1-2):9-12. [PubMed] | [CrossRef] | [Google Scholar]

- [PubMed] | [CrossRef] | [Google Scholar]

- [PubMed] | [CrossRef] | [Google Scholar]

- Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012;2(6):241-54. [PubMed] | [CrossRef] | [Google Scholar]

- Takeda H, Tsuji M, Matsumiya T.. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. EurJPharmacol. 1998;350(1):21-9. [PubMed] | [CrossRef] | [Google Scholar]

- Iversen L. Cannabis and the brain. Brain. 2003;126(6):1252-70. [PubMed] | [CrossRef] | [Google Scholar]

- Tirumalasetti J, Patel M, Shaikh U, Harini K, Shankar J.. Evaluation of skeletal muscle relaxant activity of aqueous extract of flowers in Albino rats. Indian J Pharmacol. 2015;47(4):409-13. [PubMed] | [CrossRef] | [Google Scholar]

- Anisuzzman Md, Hasan M, Acharzo AK, Das AK, Rahman S.. Array. Evid Based Complement Alternat Med.. 2017;2017:5034827 [PubMed] | [CrossRef] | [Google Scholar]

- Fan SH, Ali NA, Basri DF. Evaluation of analgesic activity of the methanol extract from the galls of (Olivier) in rats. Evid Based Complement Alternat Med.. 2014;2014:976764 [PubMed] | [CrossRef] | [Google Scholar]

