ABSTRACT
Aim
Beta-cyclodextrin-based nanosponges were used to augment solubility and dissolution of Biopharmaceutical Classification System (BCS) class II drug Nevirapine.
Materials and Methods
Nanosponges (NS) were prepared using β-cyclodextrin (β-CD) crosslinked with diphenyl carbonate and subjected to spray drying. Binary mixtures of NVP with β-CD, NS, & SDNS were prepared by solvent evaporation. Characterization was done by phase solubility studies and saturation solubility in different media and in vitro dissolution studies. Spectral analysis by Fourier transforms infrared spectroscopy, differential scanning calorimetry, powder X-ray diffraction, field emission scanning electron microscopy & stability studies were performed.
Results
Spectral characterization confirmed the formation of inclusion complexes. The stability constant for the NVP-SDNS complex was found to be 1584m-1, indicating stable complex formulation. Saturation solubility was 2-4 folds more with NVP-SDNS complex in D.W. and 2-10 folds greater in 0.1N HCl. NVP displayed a noteworthy enhancement in solubility in relevant media. Binary mixtures show a 31-65% increase in dissolution in 0.1N HCl, with the highest dissolution rate compared to phosphate buffer pH 6.8, D.W, FaSSIF & FeSSIF. The percentage decrease in crystallinity of NVP-β-CD, NVP-NS, & NVP-SDNS complexes was found to be in the range of 34-59%. Gas chromatography by solvent residue analysis indicated the evaporation of DCM from the NVP-SDNS complex.
Conclusion
The NVP-SDNS binary mixture showed higher saturation solubility & Dissolution rate than other binary mixtures. It was inferred that the solubility of NVP was enhanced by using β-Cyclodextrin nanosponges.
INTRODUCTION
The oral route is one of the most popular routes for drug delivery.1 However, 50% of drugs have high lipophilicity which is a challenge to oral administration. Biopharmaceutical Classification System Class (BCS) II and IV drugs suffer from low bioavailability due to poor water solubility. Consequently drug absorption is compromised.2 Thus increasing drug solubility and oral bioavailability is a challenge to scientists.3 Various approaches to improve solubilization and bioavailability of poorly water-soluble drugs include particle size reduction,4 chemical modification,5 microenvironmental pH modification,6 solid dispersion,7 complex formation,8 co-solvency,9 micellar solubilization,10 hydrotropy,11, 12.
Nanosponges (NS) are a new type of polymer derivatized by crosslinking and comprising colloidal-sized particles with nanosized cavities. The outer surface is usually porous, which allows for sustained drug release and topical drug delivery.13 Lipophilic and hydrophilic substances can be entrapped by these particles, enhancing the solubility of weakly water-soluble substances.14 Solvent evaporation, emulsion solvent evaporation, ultrasound-assisted synthesis, and interfacial phenomenon are some methods for NS preparation.15 NS have been used to improve solubility and stability of telmisartan,16 meloxicam,17 curcumin,18 and efavirenz,19 NSs have effectively masked taste of drugs like oseltamivir phosphate20 and gabapentin.21 Controlled release formulations of drugs like camptothecin,22 tamoxifen,23 and quercetin,24 have been successfully prepared using NS.25, 26
Cyclodextrins (CDs) have been widely used as a pharmaceutical excipient because of their ability to incorporate or adsorb guest molecules into their central cavity.27 Crosslinking of CDs with crosslinkers such as carbonyl-diimidazole,28 diphenyl carbonate (DPC),29 hexamethylene diisocyanate,30 and pyromellitic anhydride31 have led to the formation of a diverse range of nanosponges which are nontoxic and temperature stable. Unlike plain CDs, NSs can develop supramolecular interactions with drug molecules by entrapping drug within the toroidal structure of CDs and also within the nanochannels created due to crosslinking.32 The most common natural CDs used in the pharmaceutical industry are ά, β, and γ CDs with varying numbers of glucopyranose units.33 NS are usually made from β-CD because it has superior complexing ability and stability with cross-linking agents. The advantage of using B-CD for preparing NS is that it has cavity dimensions, minimal production costs, higher production rates, and is approved by the United States Food and Drug Administration as safe for consumers.34 Van der Waals forces, lipophilic and hydrogen bonds are responsible for complexation.35
Non-nucleoside reverse transcriptase inhibitors (NNRTI) are frequently used along with high-activity antiretroviral therapy (HAART) to improve the lifespan of HIV patients. NNRTIs prevent uninfected cells from becoming infected by inhibiting the reverse transcriptase enzyme. Nevirapine (NVP), dolutegravir, efavirenz, rilpivirine, and etravirine are some of them.36 NVP is classified as BCS class II (low solubility/high permeability). Its low intrinsic water solubility, weak base nature (pKa=2.8) and variable dissolution rates, compromise the drug bioavailability.37 Chemically it comes under diazepin group.38 it prevents various activities of DNA polymerases by binding directly with the allosteric site on reverse transcriptase.39
Solubility and dissolution rate of NVP was evaluated in this study by comparing binary mixtures of NVP with β-CD, NS crosslinked with DPC, and spray-dried NS. Phase solubility studies were performed to compute the stoichiometric ratio and stability constants. The complexes were characterized by solubility profile in different media, Fourier transform infrared spectroscopy, powder X-ray diffraction, differential scanning calorimetry, and field emission scanning electron microscopy, Gas Chromatography for solvent residue, in vitro dissolution and stability studies.
MATERIALS AND METHODS
Materials
NVP was gifted by Cipla Pharmaceuticals Ltd, Mumbai. β-Cyclodextrin (β-CD) gifted from Analab Fine Chemicals, Mumbai. Diphenyl Carbonate (DPC) was bought from Spectrochem Pvt Ltd, Mumbai. All other reagents used were of AR grade and procured locally.
Synthesis of NS
Nanosponges of anhydrous β-CD and DPC in a 1:4 molar ratio prepared by a previously reported procedure.19 Required quantities of β-CD (11.35g) and DPC (8.56g) heated to 100ºC with continuous stirring. The crosslinking reaction results in the generation of phenol crystals separated carefully from the final product. The product was subjected to washings with distilled water (D.W.) and acetone to eliminate unreacted β-CD and phenol. Rinsings’ were checked for phenol residue using a ferric chloride solution 10% w/v. The product was washed and dried for 2h at 60°C in a hot air oven.
Preparation of spray-dried nanosponges (SDNS)
The NS was further subjected to spray drying (Make: LABULTIMA LU-222). Based on the solubility of NS, a 1:1 mixture of ethanol and D.W. was selected as the solvent system. NSs (25 g) dissolved in 200 ml of solvent system and spray dried with an inlet temperature 120ºC and outlet temperature 60ºC. The aspiration flow rate was 60 Nm3/h. The product was collected from the product collection chamber, and the percentage yield was calculated. Malvern Zeta sizer was used to determine the particle size, polydispersity index (PDI) & zeta potential of SDNS and NS (Model no: Nano ZS 90).
Primary Investigation of Binary Mixtures
Determination of stability constants
As proposed by Higuchi and Connors,40 phase solubility studies were conducted to study supramolecular interactions between host and guest and determine stability constants. Excess amounts of the drug were added to 20 ml of DW with higher amount of β-CD, NS, and SDNS for binary mixtures of NVP with β-CD, NS, and SDNS. The mixture was swirled in orbital shaker (Remi CIS-24BL) for 48 h at 26°C, filtered using Whatman filter paper (0.45), and quantified at 280 nm using UV-Spectrophotometer (Make Model: Jasco V-730).
Drug loading
The solvent evaporation method was used to form binary mixtures (1:1) of NVP with β-CD, NS, and SDNS by dispersing in dichloromethane (1:1) and triturating until the solvent evaporated. To remove traces of dichloromethane, the moist SDs was dried at 50°C in an oven for 6h (Lab oven Biomedica). The complexes were characterized by saturation solubility studies, in vitro dissolution, thermal analysis, powder X-ray diffraction, gas chromatography for solvent residue, field emission scanning electron microscopy and stability studies.
Evaluation and Characterization of Complexes
Saturation Solubility Studies
Saturation solubility studies were carried out by adding excess drug/binary mixtures to multiple media (20 ml each), including distilled water (D.W.), 0.1N HCl, phosphate buffer pH 6.8, biorelevant media.41 The dispersions were subjected to swirling motion in an orbital shaker (Remi CIS-24BL) at 37±0.5°C for 24h at 100 rpm and then filtered by membrane filter paper (0.45), and the filtrate was quantified for NVP content by UV spectrophotometry at 280 nm to determine the amount of NVP in the filtrates. GraphPad InStat [DATASET1.ISD] was used to apply the Statistical p-test.
Fourier Transform Infrared Spectroscopy (FESEM)
IR spectra of drug and binary mixtures were examined by SHIMADZU FTIR (version 2.26) spectrophotometer to assess the interaction between the NVP, β-CD, NS, and SDNS and to ensure the formation of the mixtures. The samples were studied from a frequency range of 3600-650 cm-1.
Differential Scanning Calorimetry (DSC)
Thermograms for NVP and binary mixtures were recorded by differential scanning calorimeter (Instrument name: Mettler Toledo DSC 1), by exposing samples to 30-300°C at increments of 10°C/min in a nitrogen atmosphere (40–60 ml/min).
Field Emission Scanning Electron Microscopy (FESEM)
The surface characteristics of plain NS, NVP loaded NS & NVP loaded SDNS were analyzed by Field Emission Scanning Electron Microscopy (FESEM) (Instrument name: FEI Nova Nano SEM 450). The magnification power of NS and NS-NVP was 30,000x, and SDNS and NVP-SDNS were 60,000x at a pressure of 1.5-4pa.
Powder X-ray Diffraction (PXRD)
The PXRD spectra of NVP, NVP-β-CD, NVP-NS & NVP-SDNS were examined using a high-power powder X-ray diffractometer (Rigaku Analytical XRD, India) using a K-beta filter at voltage of 40 kV and current of 40 mA. The scanning mode of 2θ/θ was used. The degree of crystallinity was evaluated by below equation:
Where, c= crystalline peak intensities
a= amorphous peak intensities
Gas chromatography (GC) for solvent residue
For the NVP-SDNS complex, a solvent residue test for DCM was performed using gas chromatography (Shimadzu GCMS QP2020). The sample was evaluated over a temperature range of 320/350ºC using an SH-Rxi-5Sil column and Mass Spectroscopy detector.
In vitro dissolution studies
The rate and extent of dissolution of plain NVP and binary mixtures of NVP with β-CD, NS, and SDNS were studied. Samples (equal to 100 mg of NVP) were subjected to dissolution studies using a USP class II Paddle apparatus [Lab India Dissolution Test Apparatus, DISSO 2000, and India] at 37±0.5ºC and paddle speed of 50 rpm with 900 ml of 0.1N HCl, phosphate buffer pH 6.8, FaSSIF and FeSSIF separately as dissolution media. Samples (10 ml) were taken from each dissolution vessel at 15, 30, 45, 60, 90, and 120 min intervals,39 filtered using Whatman filter paper (0.45) and quantified by UV spectrophotometer (Make model: Jasco V-730) at λmax 280 nm.
Stability studies
The International Council for Harmonization guidelines were followed for accelerated stability studies.42 The NVP-NS and NVP-SDNS were kept in a stability chamber (CHM-65 REMI) at 40°C and 70% RH. The complexes were evaluated for 3 months for their physical appearance and drug content with a sampling frequency of 1 month.43
RESULT AND DISCUSSION
Characterization of NS and SDNS
The practical yield of SDNS was 60% w/w yield of plain NS. The particle size of plain NS was 1589 nm and PDI was 0.493. Zeta potential was found to be -26.0 mV. Spray drying resulted in reduction in particle size and improved the particle size distribution by reducing the polydispersity. Particle size of SDNS was 601.7 nm with a PDI of 0.002. Zeta potential was found to be -23.7 mV. Spray drying is an energy-intensive process for producing free-flowing powders with well-defined particle sizes.44 During spray drying, the droplet diameter is 10-100 µm, which enables faster drying due to rapid heat and mass transfer.45–47
Determination of stability constants
Phase solubility studies aid to determine the stoichiometry of complexation and stability of the inclusion mixtures. Phase solubility diagrams of NVP with all complexing agents showed AL-type curves (Table 1), as suggested by Higuchi and Connors’ model. Inclusion complex formation was seen in a 1:1 stoichiometric ratio. The slope of linear part of phase solubility plotwas calculated to determine the stability constant (Kc). Kc values greater than 150 m-1 indicate significant complexation activity. SDNS had the strongest interaction with NVP among all the complexes.40 The stability constants reflect the strength of the interaction between host and guest. This will indicate the tendency for leakage of drugs from the complexes. Host-guest interactions involved various forces, like hydrogen bonding and van der Waal forces. The stability constant was with SDNS non-inclusion complexes due to the cross-linking, hence a greater degree of van der Waals forces of attraction.35 It can be presumed between NVP and NS. The smaller particle size of SDNS also provides a greater interfacial area for interaction between NVP and NS. The increments in stability constants can be attributed to these factors.40
| Sl. No. | Binary Mixtures | Type of curve | Stability constant (Kc)±S.D. |
|---|---|---|---|
| 1 | NVP-β-CD | AL | 1169M-1±25.7 |
| 2 | NVP-NS | AL | 1312M-1±33.1 |
| 3 | NVP-SDNS | AL | 1584M-1±46.4 |
Stability constants (Kc) for binary mixtures [Mean ± SD, n=2]
Evaluation and Characterization of Binary Mixtures
Saturation Solubility Studies
Maximum solubility of NVP, NVP-β-CD, NVP-NS, and NVP-SDNS mixture in D.W. was found in the range of 6.4 to 45µg/ml, respectively. Solubility of NVP binary mixture with NS and SDNS (p<0.01) was increased by 2 to 4-fold, respectively as compared to NVP. The binary mixture with β-CD indicated the same solubility in D.W as plain NVP. In phosphate buffer pH 6.8, the solubility of binary mixtures with NS and SDNS (p<0.01) was enhanced by 3-3.5 fold, respectively, compared with plain NVP. Compared to plain NVP in FaSSIF (pH 6.5), the solubility of binary mixtures with β-CD, NS, and SDNS (p<0.001) were increased by 2-8 folds, respectively. When compared to plain NVP in FeSSIF (pH 5), the solubility of binary mixtures with β-CD, NS, and SDNS (p<0.001) was enhanced by 2-8folds, respectively. Compared to plain NVP in 0.1N HCl, binary mixtures of SDNS (p<0.001) obtained a solubility increase of 10-fold while binary mixtures of β-CD and NS showed a solubility increase of 2 to 7fold, respectively. The solubility of NVP is pH-dependent, as noted by the observation that it was most soluble in 0.1 N HCl (Figure 1). NVP has a pKa of 2.8, suggesting it is weakly basic.48 According to studies, NVP has high solubility at pH 3.49
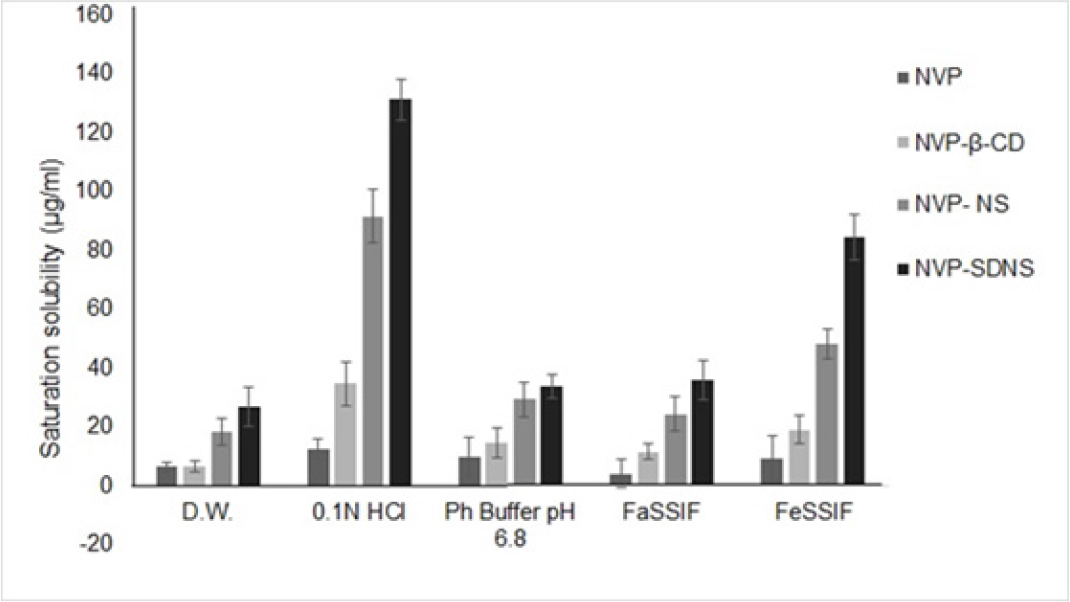
Figure 1:
Saturation solubility.
FTIR
FTIR spectra of NVP showed stretching vibrations of characteristic functional groups like -NH at 3310-3360 cm-1, -CH at 2862-2882 cm-1, C=N at 1250-1340 cm-1, and C=O at 1705-1725 cm-1.31 Peaks at 3311, 2925, 1019, and 1642 cm-1 in the FTIR spectra of β-CD correspond to the stretching vibrations of –OH, –CH2, C–O, and C=O, respectively.50 The spectra of NVP-NS and NV-SDNS displayed a C=O stretching peak at 1643 cm-1. FTIR studies confirmed the complexation between NVP, NS, and SDNS (Figure 2). The specific peaks observed in the IR spectra of plain NS and NVP were dislodged in complexes. NVP was compatible in drug-excipient compatibility studies with both β-CD and NS.
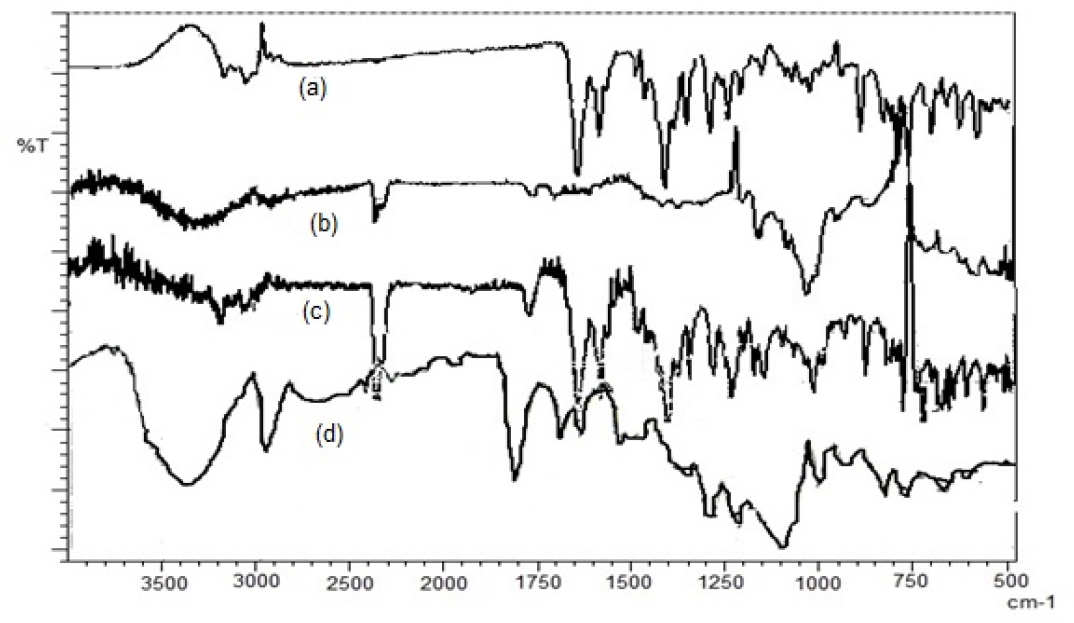
Figure 2:
FTIR Spectra of (a)-NVP, (b)-NVP- β-CD, (c)-NVP-NS, (d)-NVP-SDNS
DSC
DSC Thermogram of NVP displayed a sharp endotherm at 249°C, and β-CD showed at 300ºC,51 which correlates to their melting point (Figure 3). The absence of endotherm’s in binary mixtures showed that NVP is distributed in the NS in the molecular form. At temperatures between 140-200°C, the thermogram of binary mixtures showed broadened and shallow peaks. We can hence conclude that drug is molecularly distributed in the NS, with hydrophobic functional groups of NVP entrapped within lipophilic cavities.
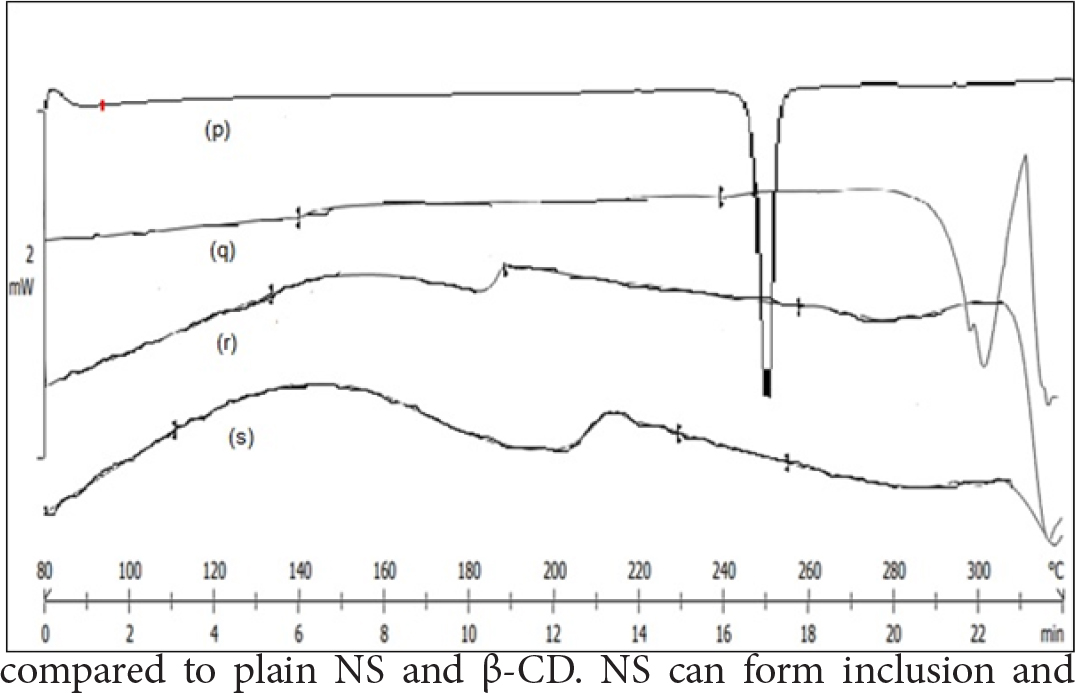
Figure 3:
DSC thermogram of (p)-NVP, (q)-NVP-β-CD, (r)-NVP-NS, (s)-NVP-SDNS
Field Emission Scanning Electron Microscopy (FESEM)
Using FESEM, the surface characteristics of plain and drug-loaded NS and SDNS with NVP was determined. According to FESEM results, NVP-NS and NVP-SDNS exhibit NVP crystals entrapped in channels inside the NS structure. SEM analyses revealed that the form was flat plate-like crystals (Figure 4).
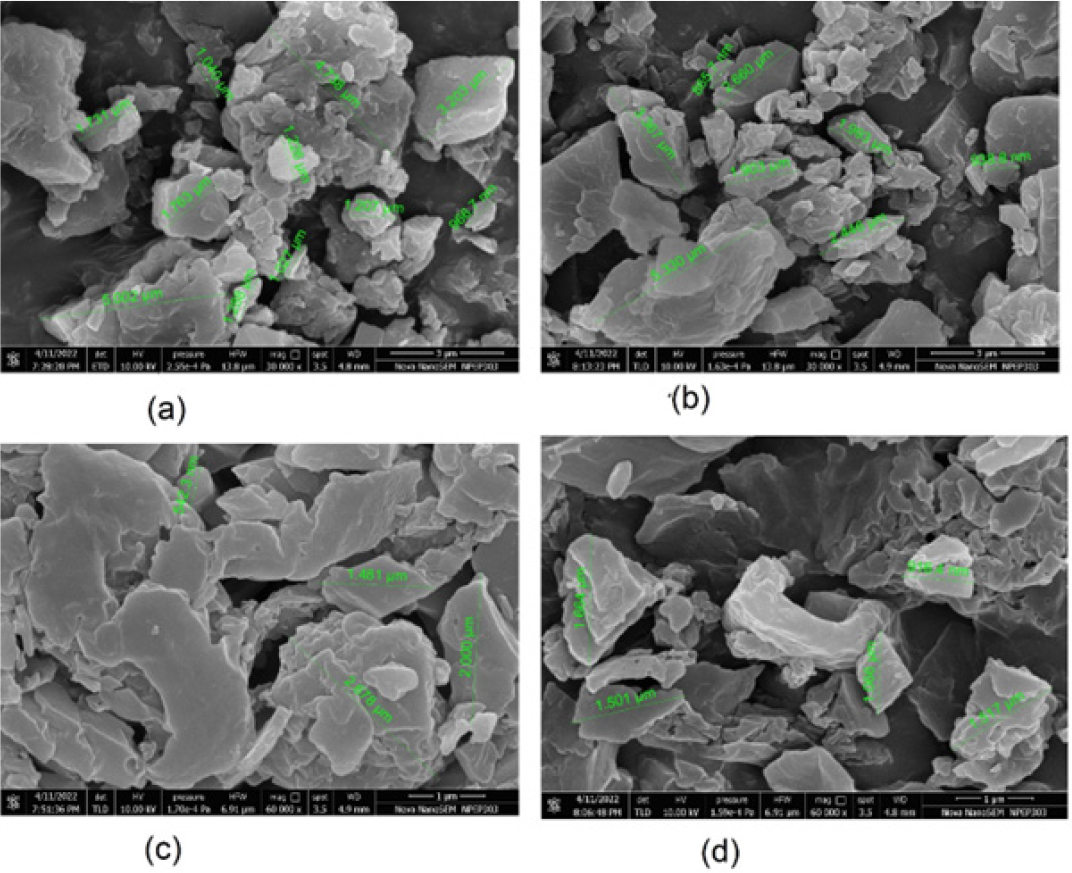
Figure 4:
FESEM: (a) Plain NS (30,000x), (b) NVP-NS (30,000x), (c) Plain SDNS (60,000x), (d) NVP-SDNS(60,000x).
Powder X- ray Diffraction (PXRD)
PXRD of NVP exhibited sharp peaks at 2θ angles of 13.1°, 25.5°, 19°, and 9.2°, confirming its crystalline nature (Figure 5). Decreased peak heights and the loss of few peaks in the diffractograms of binary mixtures with β-CD, NS, and SD-NS confirmed formation of inclusion complexes. Compared to plain NVP, the crystallinity of NVP in the binary mixtures was significantly reduced. The degree of crystallinity of plain NVP was 50.04% (Figure 6). A percent decrease in crystallinity was found in 34-59% of NVP-β-CD, NVP-NS, and NVP-SDNS complexes. We can infer from these findings that a reduction in peak sharpness and height in the diffractogram indicate inclusion complexation. During the process, guest molecules entrapped in CD cavities may undergo partial amorphization. Partial amorphization of the drug may be caused by the complexifying agent’s presence which restricting the crystal building phenomena.52 Poorly soluble drugs show improved solubility due to amorphization. Thus, we may conclude that NVP has undergone amorphization, which could be an additional factor responsible for solubility enhancement.2, 41
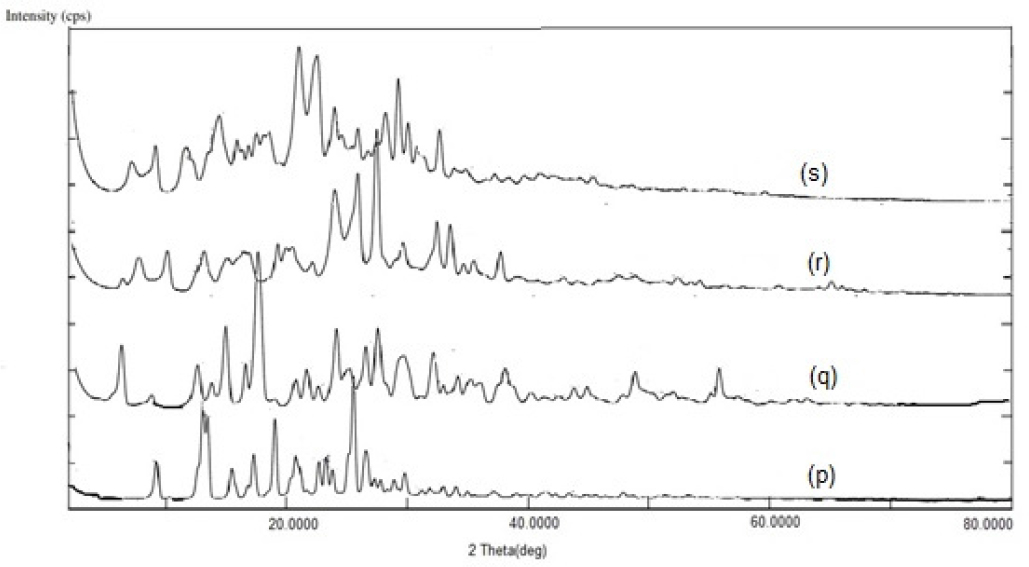
Figure 5:
PXRD Spectra (p): NVP, (q): NVP-β-CD, (r): NVP-NS, (s): NVP-SDNS.
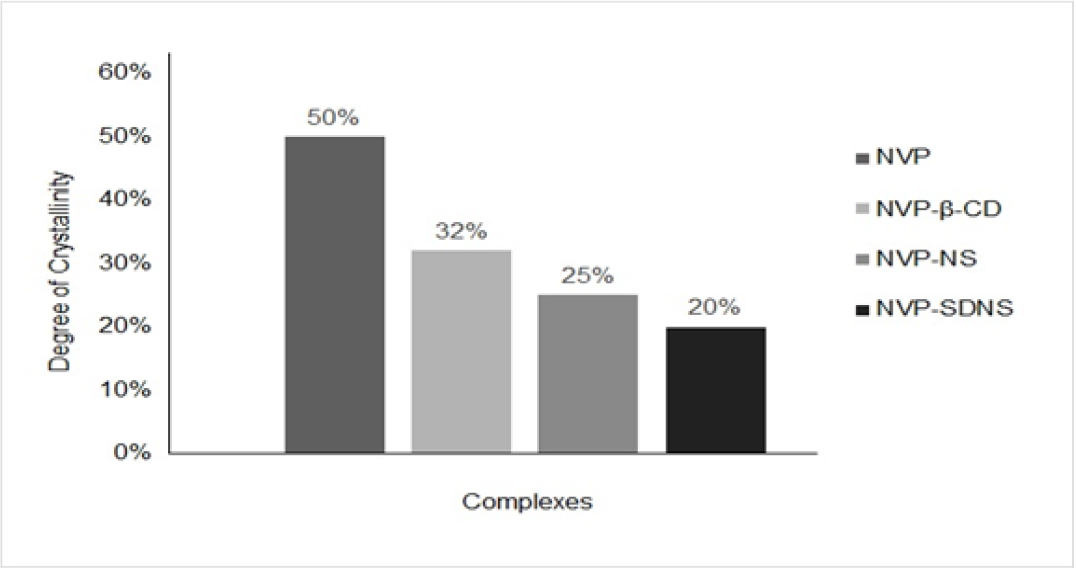
Figure 6:
Degree of Crystallinity
In vitro dissolution Studies
The dissolution profiling of plain NVP and binary mixtures of NVP-β-CD, NVP-NS, and NVP-SDNS were carried out in various dissolution media. The percent release of NVP in 0.1N HCl and phosphate buffer pH 6.8 was observed to be 29.63% and 27.04%, respectively, in 120 min (Figure 7(a)). Percent drug release of NVP-β-CD, NVP-NS, and NVP-SDNS complexes in 0.1N HCl was found in the range of 42.97% to 86.33%, respectively, in 120 min. Cumulative percent drug release was enhanced by approximately 31-60% for NVP-β-CD and NVP-NS complexes, respectively, and approximately 65.67% for the NVP-SDNS complex in 0.1N HCl at 120 min,. Percent drug release of NVP in NVP-β-CD, NVP-NS, and NVP-SDNS complexes in phosphate buffer pH 6.8 were found between 45 to 68%, respectively, in 120 min (Figure 7(b)). Percent drug release increased by approximately 40 to 57% for NVP-β-CD and NVP-NS complexes, respectively, and approximately 60% for the NVP-SDNS complex at 120 min in phosphate buffer pH 6.8 than plain NVP.
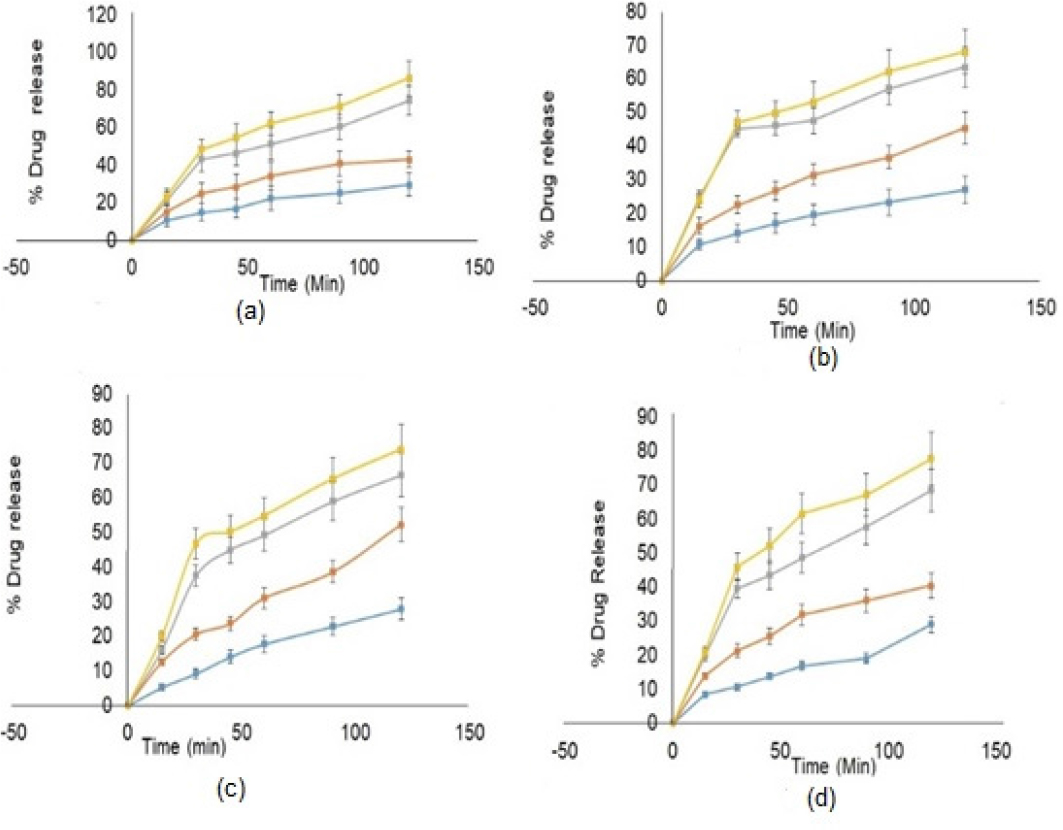
Figure 7:
In vitro dissolution Studies (a) NVP, (b) NVP-β-CD, (c) NVP-NS, (d) NVP-SDNS
The percent drug release from NVP-β-CD, NVP-NS, and NVP-SDNS complexes in FaSSIF ranged from 52 to 73 % in 120 min (Figure 7(C)). Compared to plain NVP in FaSSIF, the percent drug release was enhanced by 40-58 % for NVP-β-CD and NVP-NS complexes, respectively, and by 62 % for the NVP-SDNS complex at 120 min. Percent drug release of NVP-β-CD, NVP-NS, and NVP-SDNS complexes in FeSSIF was found in the range of 40 to 77%, respectively, in 120 min (Figure 7(d)). Compared to plain NVP in FeSSIF, the percent drug release was increased by approximately 28-57% for NVP-β-CD and NVP-NS complexes, respectively, and approximately 62% for the NVP-SDNS complex at 120 min. An increase in dissolution is associated with an increase in saturation solubility. This could be attributed to loading of drug molecules in the lipophilic cavity of β-CD in the NS and the interstitial spaces created due to crosslinking.53
Gas Chromatography for solvent residue analysis
Solvent selection for preparing solid dispersions depends upon their solubility in various solvents. In present study DCM was sued. As per ICH guidelines Q3C, it belongs to the Class 2 solvents (R6). From these guidelines, the permissible daily exposure (PDE) is 6 mg/day, and the concentration limit is 600 ppm.54 Solvent residues also harm drug stability. The spray-drying and freeze-drying methods ensure complete removal of solvents.55 In current study, the SDs were dried in an oven, therefore samples were analyzed for solvent residues. The retention time of DCM is 1.62 min evident in the chromatogram. The absence of peak at 1.62 min in the chromatogram of sample is indicative of absence of solvent residues. (Figure 8).
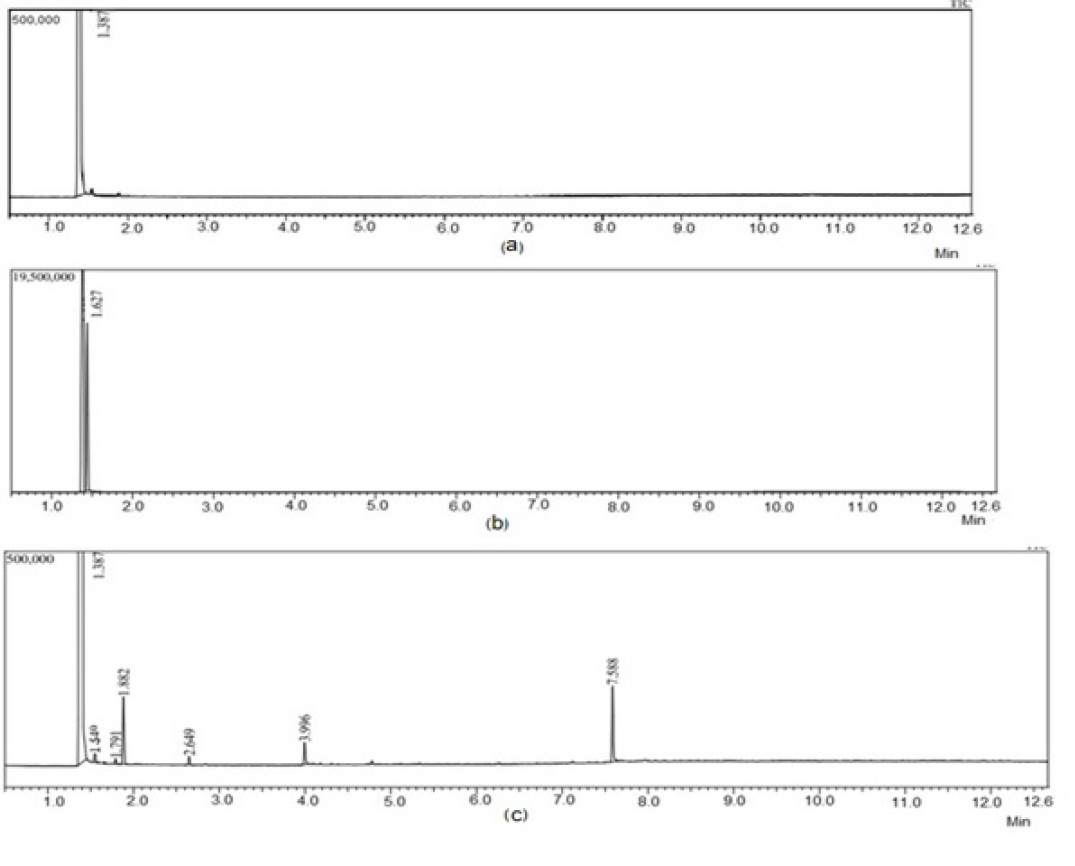
Figure 8:
Gas Chromatograms (a-D.W., b-DCM, c-NVP-SDNS). Graphical Abstract
Stability studies
There were no significant changes in the complexes’ physical appearance and drug loading after three months under accelerated conditions (40ºC/75%RH). These results were confirmed by evaluating the nanosponges’ stability at in both acidic and basic
environments. Compared to the basic environment, which did not affect the nanosponges’ physical appearance and drug content, which is 90% of NVP-SDNS and 80.1% of NVP-NS, acidic conditions (0.1 N HCl) caused a restricted release from cyclodextrin units after 2h.56
CONCLUSION
Beta-cyclodextrin-based nanosponges are newer carriers used for solubility and dissolution enhancement of many poorly soluble drugs. Spectral studies of SDs of nevirapine with β-CD, NS, and SDNS demonstrated stability of inclusion complexes. Phase solubility plots indicated a linear relationship between aqueous solubility of nevirapine and concentrations of nanosponges. The binary mixtures of nevirapine with SDNS showed maximum saturation solubility and in vitro drug release. The enhanced solubility can be connected to the presence of molecularly dispersed drug in the nanocarrier.
References
- Nasr A, Gardouh A, Ghorab M.. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics. 2016;8(3):20 [Google Scholar]

- Rao MR, Chaudhari J, Trotta F, Caldera F.. Investigation of cyclodextrin-based nanosponges for solubility and bioavailability enhancement of rilpivirine. AAPS Pharmscitech. 2018;19(5):2358-69. [Google Scholar]

- Kumar A, Sahoo SK, Padhee K, Kochar PS, Sathapathy A, Pathak N., et al. Review on solubility enhancement techniques for hydrophobic drugs. PharmacieGlobale. 2011;3(3):1-7. [Google Scholar]

- Mandić Z, Gabelica V. Ionization, lipophilicity and solubility properties of repaglinide. J. Pharm. Biomed.. 2006;41(3):866-71. [Google Scholar]

- Mohamed SA, El-Shishtawy RM, Al-Bar OA, Al-Najada AR. Chemical modification of curcumin: Solubility and antioxidant capacity. Int. J. Food Prop. 2017;20(3):718-24. [Google Scholar]

- Almotairy A, Almutairi M, Althobaiti A, Alyahya M, Sarabu S, Alzahrani A, et al. Effect of pH modifiers on the solubility, dissolution rate, and stability of telmisartan solid dispersions produced by hot-melt extrusion technology. J. Drug Deliv.Sci.Technol. 2021;65:102674 [Google Scholar]

- Rao M, Sapate S, Sonawane A.. Pharmacotechnical evaluation by SeDem Expert system to develop Orodispersible tablets. AAPS Pharm Sci Tech. 2022;23(133):1-14. [Google Scholar]

- Loh GO, Tan YT, Peh KK. Enhancement of norfloxacin solubility via inclusion complexation with β-cyclodextrin and its derivative hydroxypropyl-β-cyclodextrin. Asia Journal Of Pharmaceutical Sciences.. 2016;11(4):536-46. [Google Scholar]

- Thakkar V, Dhankecha R, Gohel M, Shah P, Pandya T, Gandhi T, et al. Enhancement of solubility of artemisinin and curcumin by co-solvency approach for application in parenteral drug delivery system. Int. J. Drug Deliv. 2016;8:77-88. [Google Scholar]

- Vinarov Z, Katev V, Radeva D, Tcholakova S, Denkov ND. Micellar solubilization of poorly water-soluble drugs: effect of surfactant and solubilizate molecular structure. Drug Dev.Ind. Pharm. 2018;44(4):677-86. [Google Scholar]

- Madan JR, Pawar KT, Dua K.. Solubility enhancement studies on lurasidone hydrochloride using mixed hydrotropy. Int.J.Pharm.Investig. 2015;5(2):114 [Google Scholar]

- Vemula VR, Lagishetty V, Lingala S. Solubility enhancement techniques. Int.J.Pharm. Sci.. 2010;5(1):41-51. [Google Scholar]

- Swaminathan S, Vavia PR, Trotta F, Cavalli R.. Nanosponges encapsulating dexamethasone for ocular delivery: formulation design, physicochemical characterization, safety and corneal permeability assessment. J.Biomed. nanotech. 2013;9(6):998-1007. [Google Scholar]

- Guineo-Alvarado J, Quilaqueo M, Hermosilla J, González S, Medina C, Rolleri A, et al. Degree of crosslinking in β-cyclodextrin-based nanosponges and their effect on piperine encapsulation. Food Chem. 2021;340:128132 [Google Scholar]

- Pawar S, Shende P, Trotta F.. Diversity of β-cyclodextrin-based nanosponges for transformation of actives. Int.J. Pharm.. 2019;565:333-50. [Google Scholar]

- Rao M, Bajaj A, Khole I, Munjapara G, Trotta F.. Array. J.Incl.Phenom. Macrocycl.Chem.. 2013;77(1):135-45. [Google Scholar]

- Shende PK, Gaud RS, Bakal R, Patil D.. Effect of inclusion complexation of meloxicam with β-cyclodextrin-and β-cyclodextrin-based nanosponges on solubility, release and stability studies. Colloids and surfaces B: biointerfaces.. 2015;136:105-10. [Google Scholar]

- Darandale SS, Vavia PR. Cyclodextrin-based nanosponges of curcumin: formulation and physicochemical characterization. J.IncPhenom.Mcrocycl.Chem. 2013;75(3):315-22. [Google Scholar]

- Rao MR, Shirsath C.. Enhancement of bioavailability of non-nucleoside reverse transciptase inhibitor using nanosponges. AAPS PharmSciTech. 2017;18(5):1728-38. [Google Scholar]

- Sevukarajan M, Bachala T, Nair R.. Novel Inclusion complexs of oseltamivir phosphate-with [beta] cyclodextrin: physico-chemical characterization. J. Pharm. Sci.. 2010;2(9):583 [Google Scholar]

- Rao MR, Bhingole RC. Nanosponge-based pediatric-controlled release dry suspension of Gabapentin for reconstitution. Drug Dev Ind Pharm.. 2015;41(12):2029-36. [Google Scholar]

- Swaminathan S, Pastero L, Serpe L, Trotta F, Vavia P, Aquilano D, et al. Cyclodextrin-based nanosponges encapsulating camptothecin: physicochemical characterization, stability and cytotoxicity. Eur. J. of Pharm.Biopharm.. 2010;74(2):193-201. [Google Scholar]

- Argenziano M, Gigliotti CL, Clemente N, Boggio E, Ferrara B, Trotta F, et al. Improvement in the anti-tumor efficacy of doxorubicin nanosponges in and in mice bearing breast tumor models. Cancers.. 2020;12(1):162 [Google Scholar]

- Lockhart JN, Stevens DM, Beezer DB, Kravitz A, Harth E.. Dual drug delivery of tamoxifen and quercetin: regulated metabolism for anticancer treatment with nanosponges. Journal of Controlled Release. 2015;220:751-7. [Google Scholar]

- Safwat MA, Soliman GM, Sayed D, Attia MA. Gold nanoparticles capped with benzalkonium chloride and poly (ethylene imine) for enhanced loading and skin permeability of 5-fluorouracil. Drug Dev. Ind. Pharm.. 2017;43(11):1780-91. [Google Scholar]

- Swaminathan S., Cavalli R, Trotta F.. Cyclodextrin-based nanosponges: a versatile platform for cancer nanotherapeutics development. Wiley Interdisciplinary Reviews: NANOMED-NANOBIOTECHNOL. 2016;8(4):579-601. [Google Scholar]

- Sherje AP, Dravyakar BR, Kadam D, Jadhav M.. Cyclodextrin-based nanosponges: a critical review. Carbohydr.Polym. 2017;173:37-49. [Google Scholar]

- Desai D, Shende P.. Drug-free Cyclodextrin-based nanosponges for antimicrobial activity. J. Pharm. Innovation. 2021;16(2):258-68. [Google Scholar]

- Rezaei A, Varshosaz J, Fesharaki M, Farhang A, Jafari SM. Improving the solubility and cytotoxicity (anticancer activity) of ferulic acid by loading it into cyclodextrin nanosponges. Int.J.Nanomed.. 2019;14:4589 [Google Scholar]

- Chin YP, Mohamad S, Abas MR.. Removal of parabens from aqueous solution using β-cyclodextrin cross-linked polymer. Int J. Mol.Sci. 2010;11(9):3459-71. [Google Scholar]

- Kozieł K, Łagiewka J, Girek B, Folentarska A, Girek T, Ciesielski W, et al. Synthesis of New Amino—β-Cyclodextrin Polymer, Cross-Linked with Pyromellitic Dianhydride and Their Use for the Synthesis of Polymeric Cyclodextrin Based Nanoparticles. Polymers. 2021;13(8):1332 [Google Scholar]

- Pushpalatha R, Selvamuthukumar S, Kilimozhi D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery-Physicochemical characterization, drug release, stability and cytotoxicity. J. Drug Deliv. Sci.Technol.. 2018;45:45-53. [Google Scholar]

- Shelley H, Babu RJ. Role of cyclodextrins in nanoparticle-based drug delivery systems. J.Pharm.Sci.. 2018;107(7):1741-53. [Google Scholar]

- Garrido B, González S, Hermosilla J, Millao S, Quilaqueo M, Guineo J, et al. Carbonate-β-cyclodextrin-based nanosponge as a nanoencapsulation system for piperine: physicochemical characterization. Journal of Soil Science and Plant Nutrition. 2019;19(3):620-30. [Google Scholar]

- Gidwani B, Vyas A.. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed Res. Int.. 2015:1-15. [Google Scholar]

- Hayashida T, Hachiya A, Ode H.. Rilpivirine resistance mutation in HIV-1 reverse transcriptase predisposed by prevalent polymorphic mutations. J AntimicrobChemother.. 2016;71(10):2760-6. [Google Scholar]

- Lokamatha KM, Bharathi A, Shanta Kumar SM, Rama Rao N. Preparation, characterization and evaluation of nevirapine-β cyclodextrin solid complexes. Res J Pharm Bio chem. 2010;1(2):372-284. [Google Scholar]

- Datta A, Ghosh Ns, Ghosh S, Samanta T, Das R.. Enhancement of solubility and dissolution profile of nevirapine by solid dispersion technique. Int. J. Chem. Res.. 2011:53-8. [Google Scholar]

- Nalte YK, Arsul VA, Shep SG, Bothara SB. Solubility enhancement of nevirapine by cocrystallisation technique. J. Pharm. Res.. 2015;9(8):556-61. [Google Scholar]

- Kim DH, Lee SE, Pyo YC, Tran P, Park JS. Solubility enhancement and application of cyclodextrins in local drug delivery. J. Pharm.Investig. 2020;50(1):17-27. [Google Scholar]

- Bajaj A, Rao MR, Pardeshi A, Sali D.. Nanocrystallization by evaporative antisolvent technique for solubility and bioavailability enhancement of telmisartan. AAPS Pharm. Sci Tech. 2012;13(4):1331-40. [Google Scholar]

- [2022 Aug 15];The guidelines of International Council for Harmonization. 1993 [Google Scholar]

- Shende P, Deshmukh K, Trotta F, Caldera F.. Novel cyclodextrin nanosponges for delivery of calcium in hyperphosphatemia. Int. J.Pharm. 2013;456(1):95-100. [Google Scholar]

- Santos D, Mauricio A, Sencadas V, Santos J, Fernandes M, Gomes P., et al. Spray Drying: an Overview. Biomaterials. IntechOpen. [CrossRef] | [Google Scholar]

- Both EM, Boom RM, Schutyser MA. Particle morphology and powder properties during spray drying of maltodextrin and whey protein mixtures. Powder Technology. 2020;363:519-24. [CrossRef] | [Google Scholar]

- Singh A, Van den Mooter G. Spray drying formulation of amorphous solid dispersions. Advanced drug delivery reviews.. 2016;100:27-50. [CrossRef] | [Google Scholar]

- [CrossRef] | [Google Scholar]

- Marques MR, Loebenberg R, Almukainzi M.. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011;18(3):15-28. [CrossRef] | [Google Scholar]

- [20 Aug 2022];Nevirapine Drug Information. [CrossRef] | [Google Scholar]

- Zhou K, Li Y, Li Q, Du Q, Wang D, Sui K, et al. Kinetic, isotherm and thermodynamic studies for removal of methylene blue using β-cyclodextrin/activated carbon aerogels. J. Polym. Environ. 2018;26(8):3362-70. [CrossRef] | [Google Scholar]

- [25 aug 2022];Beta-Cyclodextrin, Chemical book. [CrossRef] | [Google Scholar]

- Jójárt-Laczkovich O, Szabó-Révész P.. Amorphization of a crystalline active pharmaceutical ingredient and thermoanalytical measurements on this glassy form. J. Therm.Anal. Calorim. 2010;102(1):243-7. [CrossRef] | [Google Scholar]

- Chintalapudi R, Murthy TE, Lakshmi KR, Manohar GG. Formulation, optimization, and evaluation of self-emulsifying drug delivery systems of nevirapine. Int J. Pharm. Investig.. 2015;5(4):205 [CrossRef] | [Google Scholar]

- [28 Aug 2022];ICH guideline Q3C (R6) on impurities: guideline for residual solvents, European Medicines Agency. 2009 [CrossRef] | [Google Scholar]

- Janssens. S, Van den Mooter G. Review: Physical chemistry of solid dispersions. J. Pharm. Pharmaco. 2009;61:1571-86. [CrossRef] | [Google Scholar]

- Trotta F, Zanetti M, Cavalli R.. Cyclodextrin-based nanosponges as drug carriers. Beilstein journal of organic chemistry. 2012;8(1):2091-9. [CrossRef] | [Google Scholar]

