ABSTRACT
Objectives
The goal of the study was to treat ulcerative colitis by targeting drug delivery to the colon via the novel ingredient’s guar gum and pH-dependent polymer sodium alginate. The drug was released at a high pH in the colon due to the sodium alginates. Guar gum was a novel ingredient that was used as a release retarder, binder, and swelling agent. The galactomannan enzyme naturally degrades the guar gum. Because this enzyme is only found in the human body’s GIT colon, the tablet colon is naturally target-specific.
Materials and Methods
The tablet was prepared through wet granulation using an IPA solution. The formed granules subjected to the pre-formulation studies had free flow ability and a uniform size or shape. After the pre-formulations, the tablets were formed using the ZP-17 machine, and post-compression studies such as the hardness test, friability test, weight variation test, and thickness or diameter test were performed.
Results
The precompression and post-compression studies, including the hardness test, friability test, weight variation test, and thickness or diameter test, were in the specification. The disintegration test was performed on the tablets, but due to the swelling nature of guar gum, they did not disintegrate. The dissolution studies were performed for 24 hr using acidic and basic buffers. The drug releases at a basic pH of 7.4 in the presence of the galactomannose enzyme, which shows its pH dependence and colon targeting. The various kinetic models were used to release kinetic models. According to the kinetic release studies, the R2 explained that formulations were controlled releases and followed the zero order, which is independent of drug concentration.
INTRODUCTION
The most popular and appropriate method of administering drug is the oral route, especially for outpatients. Oral drugs often pass through the liver and gut wall, which both have a number of inactivating enzymes.1 A practical method for treating local colon disorders, site-specific colon drug delivery has various benefits, including a quick commencement of action, a reduction in drug dosage, and a reduction in potentially dangerous side effects.2
Curiosity in colon-specific drug delivery has increased recently due to an increasing number of colonic ailments, such as diverticulosis, polyps, Irritable Bowel Syndrome (IBS), Ulcerative Colitis (UC), local bacterial infections, colorectal cancer, Crohn’s Disease (CD), intestinal wound healing, and fistula.3
By utilizing a variety of techniques, such as incorporating tablets with pH-sensitive polymers, prodrugs, pressure-control systems, osmotic pumps, and gastrointestinal tablets, one can target drug delivery not only for localized colonic diseases but also for protein and peptide delivery.4 The existence of microbial flora in the colon for drug release, the presence of a pH in the range of 6.47, and for improving the oral bioavailability of protein and peptide delivery.5 Polymers that are bacterially degradable and help to keep drugs unaffected at the target location of the colon include polymers such as polysaccharides (guar gum, inulin, pectin, and amylose), azo-cross linked, and pH-dependent polymers like cellulose acetate phthalate, Eudragit® L100, and HPMC phthalate.6
The very first treatment option for mild to severe ulcerative colitis is often mesalamine. Mesalamine’s precise mode of action, however, is still not well understood. It is believed to have a detrimental impact on the lipoxygenase and cyclooxygenase pathways, reducing the formation of prostaglandins and leukotrienes, two substances that encourage inflammation. The peroxisome-triggered receptor-G has also been associated with colonic inflammation and has been discovered as a focus of mesalamine action.7 Mesalamine may also have antioxidant qualities that lessen tissue damage and contribute to the suppression of T cell activation and proliferation. Patients with ulcerative colitis can effectively induce and sustain remission with the help of oral mesalamine drugs. Mesalamine has a therapeutic effect by acting locally on the irritated mucosa through topical localization.8
Guar gum is a seed gum obtained from Cyamopsis tetragonolobus (Leguminosae). As a lipophobic matrix for oral medications with controlled release, guar gum also serves as a binder and dissolves in solid dosage forms.9 Sodium alginate is a polymer that we like for particular pharmaceuticals because of its solubility and pH sensitivity, which make it a desirable biomaterial for drug delivery systems. Sodium alginate prolongs the time that the drug is released from the formulation.10
The aim of this research was to develop a controlled-release pH- dependent tablet that acts locally on the colon. Mesalamine was used as a model drug; the tablets were made up of guar gum polymer in combination with a pH-dependent sodium alginate. The drug release profile was evaluated, and the release kinetics will be obtained.11
MATERIALS AND METHODS
Materials
Mesalamine was a gift from the local pharmaceutical industry, and guar gum was obtained from a local market and authenticated through FTIR analysis. Avicel PH-102 (microcrystalline cellulose), Magnesium stearate, Aerosil 200, Sodium Alginate, and Galactomannans enzyme were all purchased from Sigma Aldrich. All of the materials used were of laboratory grade and were used in the same manner as they were obtained from the vendor.
Instruments
Tablet compression machine ZP-17, hardness tester machine (Erweka), USP dissolution assembly type 2, friabilator, pH measuring meter, weight measuring balance, UV- Spectrophotometer, tripod stand, vernier calliper, FTIR apparatus, stop watch, DSC apparatus, X-ray diffraction, disintegration apparatus
Method of controlled released tablets preparation
This method was obtained from the article and used with some modifications. The method we used to prepare the colon- specific tablets was the wet granulation method. All ingredients were carefully weighed using the analytical balance, and then the powder passed through the sieve 60 to yield a fine powder. The powder mixture was moistened with a sufficient amount of IPA before being sieved through a sieve 16 to form granules. The moisture content of the granules, as determined by the IR moisture metre, was found to be constrained within 0.85% and 1.2% after the granules were dried at 60°C for two hours. After passing through a sieve 22 mesh screen and being combined with magnesium stearate, the dry granules were compressed into tablets using a ZP-17 compression machine. As shown in Table 1, nine formulations (PF1–PF9) were created by varying the concentrations of guar gum, sodium alginate, and avicel.12
| Additive (mg) | PF1 | PF2 | PF3 | PF4 | PF5 | PF6 | PF7 | PF8 | PF9 |
|---|---|---|---|---|---|---|---|---|---|
| Mesalamine | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| Guar gum | 150 | 200 | 250 | – | – | – | 150 | 100 | 150 |
| Sodium alginate | – | – | – | 150 | 200 | 250 | 100 | 150 | 150 |
| Avicel-102 | 170 | 120 | 70 | 170 | 120 | 70 | 70 | 70 | 25 |
| Aerosil-200 | 75 | 75 | 75 | 75 | 75 | 75 | 75 | 75 | 75 |
| Mg. st./talc (3:2) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Water/IPA | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s |
| Weight of tablet | 650 | 650 | 650 | 650 | 650 | 650 | 650 | 650 | 650 |
Different composition of colon targeted pH dependent formulations.
Preparation of Calibration Curve in Phosphate Buffer Solution pH 7.4
Mesalamine was precisely weighed at 25 mg and then added to a volumetric flask (25 mL). Put the volume up to the mark by dissolving it in a small amount of pH 7.4 phosphate buffer solution. With the help of a pipette, transfer 10 mL from the volumetric flask into a 100 mL volumetric flask and add the remaining volume. The solution’s concentration was 100 g/mL. Take 1 to 10 mL of the prepared stock solution, dilute it in a series of volumetric flasks (10 mL), and then add Phosphate Buffer Solution (PBS) with a pH of 7.4 to the volume to the mark. The dilutions have been studied using a UV spectrophotometer with a maximum wavelength range of 337 nm. In Figure 1, plotting absorbance vs. concentration resulted in the calibration curve’s construction.
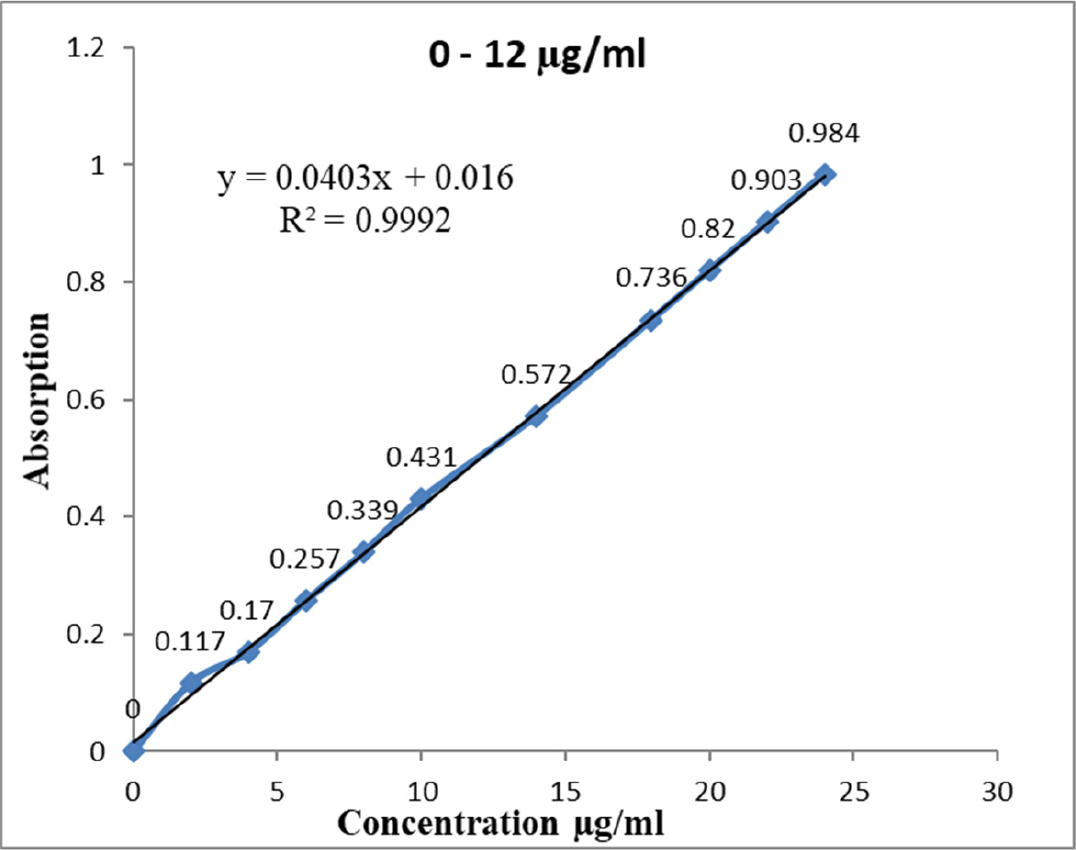
Figure 1:
Calibration curve of mesalamine.
Characterization
Pre-compression Study Bulk Density (Apparent)
Granules are placed in a calibrated measuring cylinder to determine their volume, and then they are weighed on a balance to determine their apparent density.13
Tapped Density
In order to determine the flow characteristics and compressibility of magnesium stearate powders, the Carr’s compressibility index and Hausner’s ratio were determined using the bulk and tapped densities.14
Hausner’s Ratio
The Hausner’s ratio is used to determine the flow characteristics of granular powder by using tap density and bulk density.15
Compressibility Factor
Using the formula below, the compressibility factor can also be determined using tap density and bulk density.15
Angle of repose
The angle of repose is calculated using the rectifying method. A cone is completely filled. The cone height is fixed so that the blend tries to touch the edge of the funnel. The granules height and radius may also be estimated. The formula determines the angle of repose.13
Pre-formulation studies
Analytical Validation
Linearity and Range
The intensity of the acid solution was measured at 302 nm, while the intensity of the basic solution was measured at 331.60 and 337 nanometers, appropriately, at different pH levels (6.8 and 7.4), up to the greatest concentration of mesalamine, until specificity was seen.13
Fourier Transform Infrared Spectroscopy (FTIR) Analysis
To confirm that the pure drug mesalamine and other excipients used to generate optimal dosage forms were compatible, FTIR spectrum analysis was performed.16
Melting point
A digital melting point instrument was used with the capillary. As it approached the anticipated melting point, the equipment reached its maximum temperature at 10°C per minute, then at 1°C per minute. It was recorded at what temperature the medication started to melt.16
Solubility Studies
By putting an excess quantity of the drug in a quartz cuvette with 1 mL of a particular solvent over twelve hours, it was possible to evaluate the drug’s solubility. The vial was then placed aside until the drug that hadn’t yet dissolved settled. The drug content in the sample was determined using spectrophotometry (max 337 nm).16
Post compression study
Hardness Test
Tablets were placed inside the jaws of hardness tester, and the screw was tightened. Reading was noted from the digital screen when the tablet was broken.17
Weight variation test
Select any 20 tablets randomly. Each tablet was weighed on a weighing scale individually to calculate the average weight of the tablets.18
Friability
Tablet strength was determined using a friabilator. Select 20 tablets randomly from the sample. Calculate the averaged mass of the tablets (starting weight) and place them in the friabilator on its shaft. Set the rpm to 25 and ran the machine for 4 min. Then removed the tablet from the drum and again measured its weight (final weight). Calculate the friability of the tablets using the given equation.
Loss of weight should not be greater than one or less than 0.5. The results of the tests were within acceptable limits.
Thickness Test
The vernier caliper is used to determine the tablet thickness. Test results must be within limits, according to USP.
Fourier Transform Infrared Spectroscopy (FTIR) Analysis
To confirm the proper incorporation of a drug and other excipients, FTIR analysis of tablets was performed. The various excipients used to generate optimal dosage forms were compatible; FTIR spectrum analysis was performed.16
Differential Scanning Calorimetry (DSC)
Indium with a purity of 99.98%, a melting point of 156.61°C, and a fusion enthalpy of 28.71 J/g was used to calibrate the Perkin- Elmer Diamond differential scanning calorimeter (Perkin Elmer, Inc., Waltham, MA, USA). Samples were precisely weighed (3-5 mg) and placed into 50-L aluminum pans, which were then covered with a punctured aluminum lid. Measurements were conducted in a pure nitrogen environment at a flow rate of 25 mL/min and a heating rate of 10°C/min. For all samples, the heating temperature ranged from 25 to 350°C. We measured duplicates of each sample.19
Thermal Gravimetric Analysis (TGA)
A sample of about 5 mg was weighed and placed in metal containers. The experiment was run between 25 and 300°C at a heating rate of 10°C/min in a nitrogen environment (20 mL/min).19
X-ray Diffraction (XRD) analysis
The rotating anode X-ray diffractometer (Smart Lab 9kW) was operated at 45 mV of voltage and 20 A of current, and the diffraction patterns over a range of 5 to 10°C/min were expressed in units of 2.19
In vitro Studies
Disintegration Studies
The disintegration test was performed using disintegration apparatus that meets the USP standard. Fill the six tubes of the basket apparatus by placing one tablet into each. used 900 mL of an acidic solution of pH 1.2 and a phosphate buffer having a pH of 7.4 as a dipping intermediate. Disintegration took place at a constant 37°C. The basket structure must be lowered and raised 30 times per minute. It was noted when the tablet had completely broken down and no mass remained in the apparatus.12,18
Dissolution Studies
A USP II apparatus was used to investigate the transfer of mesalamine from solid dispersion. The tablets were examined for 24 hr for the release of the drug in 0.1 M HCl. The three dissolving conditions were developed: two hours at pH 4.5, two hours at pH 6.8 alkaline buffer, and two hours at 37°C with pH 7.4. For 24 hr, (PF1–PF9) formulations were tested for dissolution. After six hours, galactomannans enzyme (from Aspergillus Niger,
2.95 U/mL) (19.65 U/L) was added to a medium having a pH of 7.4 to see how the enzyme affected the final formulation’s capacity to dissolve. The concentration was measured using a spectrophotometric analysis.20
Drug release kinetics
To determine the release kinetics of formulations, different statistical models were used. The models included the zero-order model, the Higuchi model, the Hixon Crowell model, the first- order model and the Korsmeyer peppas model.19
RESULTS
Pre-compression Studies
In Table 2, the formulations from PF1 to PF3 showed poor flow characteristics, according to the results. The pre-compression characteristics of formulations PF4–PPF6 were good but not exceptional. Results from PF7 to PF9 formulations were within range. The bulk density, Hausner’s ratio, tapped density, Carr’s index, and angle of repose are all in the good to excellent range. Of the three tested formulations (PF7–PF9), PF9 performed the best and showed the best fit results, according to the official limits. The cited article showed a similar trend.12
| Product Formulations | Tap density | Bulk density | Hausner’s Ratio | Carr’s Index | Slope angle |
|---|---|---|---|---|---|
| PF1 | 2.50 | 1.25 | 2 | 50 | 33 |
| PF2 | 2.56 | 1.23 | 2.08 | 52 | 33.7 |
| PF3 | 2.52 | 1.21 | 2.08 | 52 | 35.4 |
| PF4 | 0.98 | 0.85 | 1.15 | 13.2 | 27.1 |
| PF5 | 0.98 | 0.84 | 1.16 | 14.2 | 27.5 |
| PF6 | 0.97 | 0.83 | 1.16 | 14.4 | 27.8 |
| PF7 | 0.86 | 0.84 | 1.02 | 2.30 | 18.3 |
| PF8 | 0.87 | 0.84 | 1.03 | 3.44 | 18.5 |
| PF9 | 0.86 | 0.84 | 1.07 | 6.97 | 18.9 |
Pre-compression studies.
Pre-formulation studies
Analytical Validation
Results revealed that the validation parameters for formulations PF1–PF3 were in a poor range. The formulations PF4–PF6 showed a wide range of validation conditions. The validation parameters for formulations PF7 to PF9 were good, and PF9 was the best of these three formulations (PF7–PF9) in terms of validation parameters.
Melting Point
Mesalamine M.P. was discovered to be 282°C. However, the drug began to degrade at this temperature, and this is according to the specification.16
Solubility Studies
After the solubility analysis of Mesalamine in different solutions, it shows excellent solubility in dissolving agents.16
FTIR
FTIR of mesalamine
During FTIR analysis of mesalamine, the first peak appeared at 1645 cm-1 which represents the N-H bending with medium intensity. Another peak was observed at 1615 cm-1 which represents C=C bonding with medium intensity. At 1446 cm-1 a peak appeared that showed C-H bending with strong intensity. The last peak appeared at 1351 cm-1 which showed C-N bending with strong intensity. The same pattern was explained.21
FTIR of Sodium Alginate
During FTIR of sodium alginate, the first peak was observed at 2883 cm-1 which showed the aldehyde group with weak intensity. Another broad peak was observed at 3285 cm-1 with medium intensity, which showed the OH group. The last peak was observed at 1405 cm-1 which indicated the bending of the CH group. The same pattern was explained.22
FTIR study of Aerosil
During FTIR analysis of the aerosol, the first peak appeared at 805.1 cm-1 which represents the Si-O bending with medium intensity. Another peak was observed at 1075.3 cm-1 which indicated the Si-o-Si stretching. The same pattern was explained.23
FTIR study of Avicel
During FTIR of avicel, the first peak was observed at 3334 cm-1 which showed the alcoholic group with medium intensity. Another peak appeared at 2890 cm-1 which showed the aldehyde group with weak intensity. At 1425 cm-1 a peak appeared that showed C-H bond with weak intensity. Another peak was observed at 1159 cm-1 that showed the C-O group with strong intensity. The last peak appeared at 1105 cm-1, which showed the C-O group with strong intensity. The same pattern was explained.24
FTIR of Guar Gum
During FTIR analysis of guar gum, we identified the first peak at wave number 2912 cm-1 which demonstrated the stretching of the CH2 group. At 1636 cm-1, a peak appeared, indicating that the ring was stretching. Another peak that explained the deformation of the CH2 group was observed at 1375 cm-1. A peak induced by the primary OH group and C-OH was noticed at 1146 cm-1. The peak at 1023 cm-1 was generated by the CH2 group’s twisting vibration, and the peak at 659.7 cm-1 showed linkage between galactose and mannose. The cited article gave an explanation for the similar pattern of peaks.25
FTIR Analysis of Colon targeted Matrix tablet
In Figure 2, FTIR analysis of the mesalamine shows many characteristic bands in the FTIR spectrum. Figure 7 shows the mesalamine FTIR spectrum. Mesalamine showed many bands in FTIR. Mesalamine exhibits a band at 1647.5 cm-1, 1448.1 cm-1, 1487.2 cm-1, and 1354.29 cm-1 due to the C=O stretching bond, C=C bond, and CH2 bond. This peak offered strong evidence that the sample presented was pure mesalamine. The excipient and mesalamine had no interaction, according to the FTIR.19
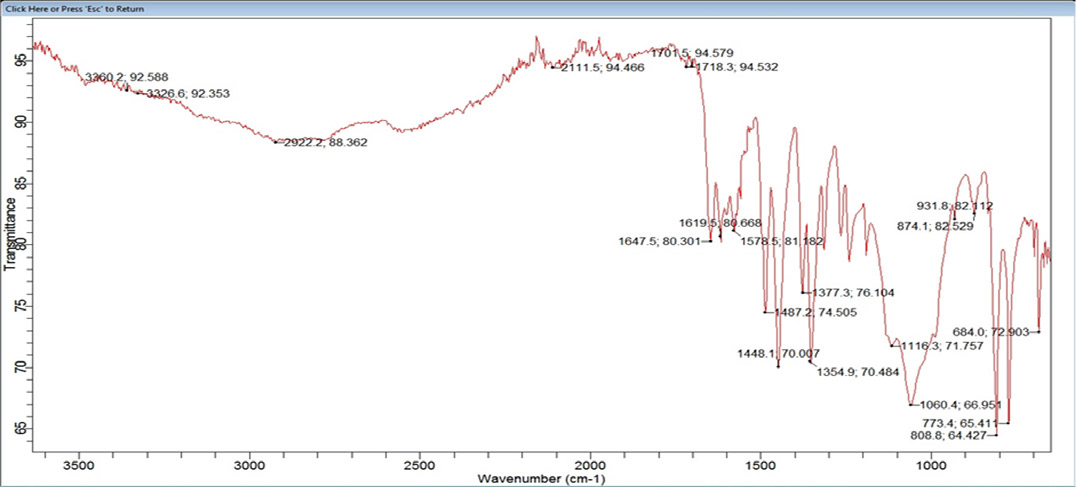
Figure 2:
FTIR Analysis of Colon targeted Matrix tablet.
Post compression studies
The tablets were round and properly inspected in accordance with internal and pharmacopoeia standards Multiple formulation of tablets were used. As the results show in Table 3, the friability, weight variation, diameter, hardness, and thickness followed and continued under high pressure for physical evaluation. The results showed that the hardness and friability tests for PF1 to PF3 were out of range. The hardness test and friability findings for PF4 to PF9 were within the acceptable range. The formulation PF9 was the best formulation among them. According to the results of thickness, diameter, and weight variation of formulations (PF1– PF9), they are within a limit to USP specification.12
| Formulations | Hardness | Friability (%) | Thickness | Diameter | Average weight |
|---|---|---|---|---|---|
| PF1 | 3.1 | 1.69 | 6.001 | 12 ± 0.001 | 650.01 |
| PF2 | 3.5 | 1.56 | 6.004 | 12 ± 0.002 | 650.32 |
| PF3 | 2.0 | 1.61 | 6.002 | 12 ± 0.005 | 650.45 |
| PF4 | 5.0 | 0.94 | 6.001 | 12 ± 0.001 | 650.12 |
| PF5 | 5.7 | 0.92 | 6.009 | 12 ± 0.003 | 650.78 |
| PF6 | 6.0 | 0.97 | 6.002 | 12 ± 0.003 | 650.87 |
| PF7 | 8.2 | 0.52 | 6.001 | 12 ± 0.007 | 650.19 |
| PF8 | 8.5 | 0.58 | 6.005 | 12 ± 0.001 | 650.01 |
| PF9 | 8.7 | 0.51 | 6.001 | 12 ± 0.005 | 650.07 |
Post compression studies.
Differential scanning calorimetry
In the DSC results, the graph shown in Figure 3 indicated that the exothermic behaviour started at 182°C due to the phase transition of mesalamine. At this temperature, mesalamine starts melting and is converted into a liquid by evolving energy from the surrounding environment. The exothermic process was deflected by this phenomenon and behavior. In the same way, a sharp peak was observed at 80°C, which showed the endothermic phenomenon. In the same way, a sharp peak was observed at 80°C, which showed the endothermic phenomenon. Endothermic processes explain the heat absorption from the surrounding environment. The reason behind this phenomenon was the breaking of a double bond in the aromatic structure present in mesalamine.
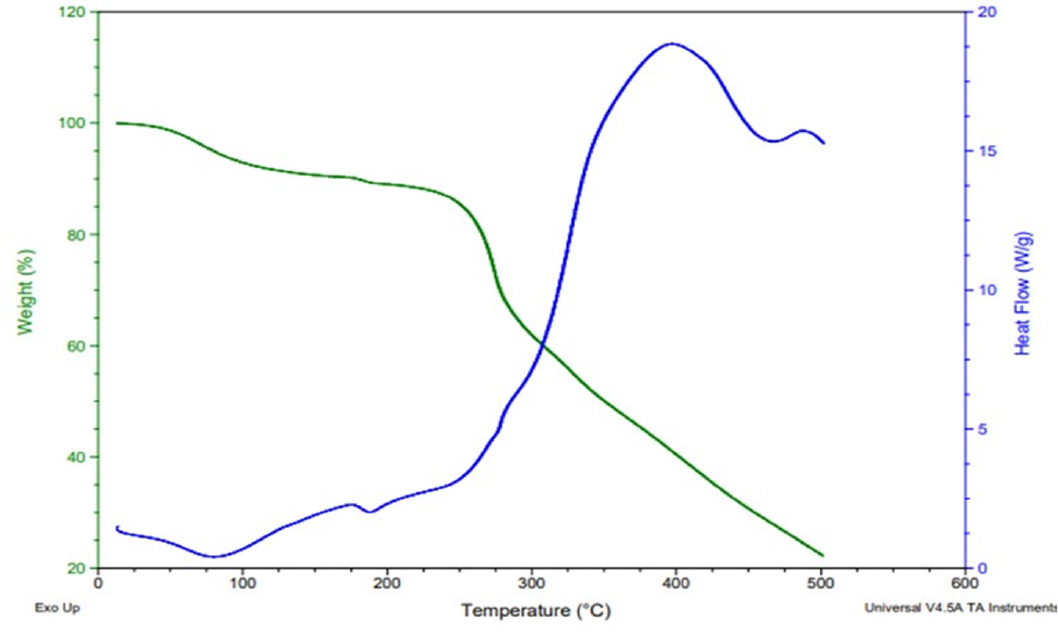
Figure 3:
Thermogram of DSC and TGA.
Thermal gravimetric analysis
In Figure 3, the TGA results showed that from 0°C to 80°C, there was no change in the initial weight of the sample, which showed the stability of the sample. At temperatures above 80°C, there was some degradation of the sample and weight variation was observed, but the sample remained stable. When the temperature was raised above 282°C, (which is the melting point of mesalamine), a sharp deflection peak was observed, which indicated the degradation of the sample. The degradation of the sample continued as the temperature rose. As a result, the sample remained stable until it reached 282°C.19
X- ray Diffraction
The spectra of X-ray diffraction showed many sharp and strong peaks, which describe the pattern of crystallinity in a pure mesalamine powder. In Figure 4, diffraction peaks were observed at 2θ; 7.12, 10, 15.32, 16.16, and 21.4. These peaks showed that the excipient reduced the crystallinity of the mesalamine and it indicated that mesalamine had been incorporated into the tablet.19
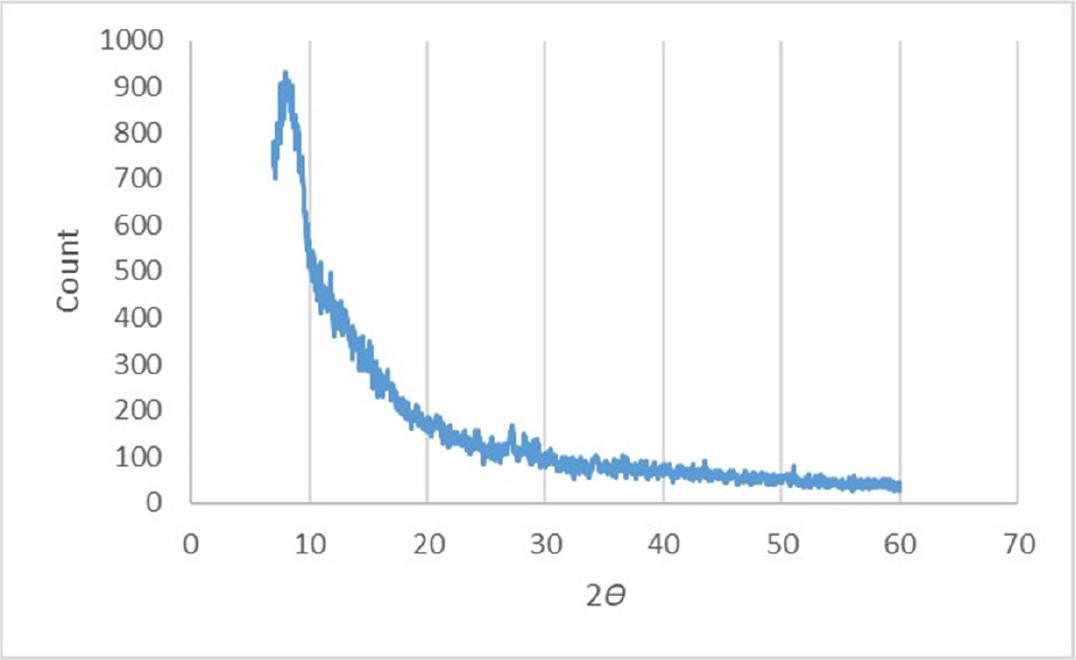
Figure 4:
XRD Diffractogram of Mesalamine containing controlled released tablets.
In vitro Testing
In vitro Disintegration Test
According to the results, the presence of guar gum or sodium alginate, which are controlled-release novel ingredients or pH- dependent polymers, respectively, causes none of the product formulations (PF1 to PF9) to disintegrate under the given conditions. The same behaviors was explained by the cited article.26
In vitro Dissolution Studies
Guar gum is susceptible to microbial destruction in the large intestine. It can carry drugs to the colon. There are several ways to simulate colonic situations, such as adding galactomannans enzyme or rat cecal content to the dissolving medium. In our research, it was noted that a tablets containing guar gum released 4.99% of mesalamine over 0.5 hr. The release of mesalamine from the matrix tablet was enhanced after adding an enzyme substance (galactomannans enzyme at 19.6 U/L level) to the dissolution media. The total amount of mesalamine release 24 hr later under the influence of the galactomannan enzyme was found to be 99.67%. Because, the galactomannan enzyme enhanced the release of mesalamine by degrading the guar gum polymer. The pattern of the drug’s percentage drug release is demonstrated in Figure 5.12
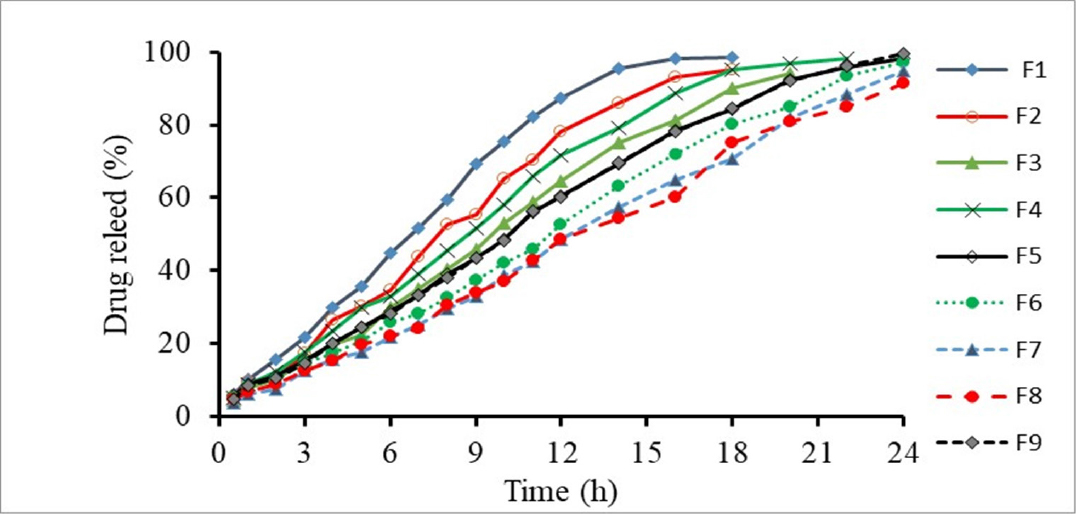
Figure 5:
Graph of Dissolution rate of formulation PF1 – PF9.
Release kinetic studies
The ability to correlate drug dissolution data to appropriate logical representations is a critical aid that not only makes it possible to better understand and interpret the processes involved in the drug dissolution method but also makes it possible to manage the release properties to meet specific therapeutic requirements. The coefficients of several mathematical models were computed and used to indicate the in vitro dissolution rate patterns as shown in Table 4. Table 4 illustrates the R2 values for each model, and it can be seen that the matrix tablet was more closely fitted to the zero- order kinetics.19
| Formulation | Zero Order | First Order | Higuchi | Korsmeyer-peppas | Crowell | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| K0 | R2 | K1 | R2 | kH | R2 | kKP | n | kHC | R2 | |
| PF1 | 6.679 | 0.9388 | 0.125 | 0.9369 | 22.281 | 0.8833 | 11.450 | 0.954 | 0.035 | 0.9710 |
| PF2 | 5.994 | 0.9773 | 0.101 | 0.9334 | 19.796 | 0.8574 | 7.954 | 0.887 | 0.029 | 0.9892 |
| PF3 | 5.056 | 0.9912 | 0.080 | 0.9302 | 17.443 | 0.8384 | 5.764 | 0.950 | 0.023 | 0.9884 |
| PF4 | 6.263 | 0.9606 | 0.094 | 0.938 | 19.238 | 0.8800 | 8.512 | 0.822 | 0.027 | 0.9715 |
| PF5 | 4.602 | 0.9747 | 0.079 | 0.9359 | 17.537 | 0.8693 | 6.692 | 0.866 | 0.023 | 0.9699 |
| PF6 | 4.727 | 0.9935 | 0.068 | 0.9191 | 16.122 | 0.8320 | 4.671 | 0.968 | 0.020 | 0.9545 |
| PF7 | 3.9730 | 0.9965 | 0.060 | 0.9163 | 14.899 | 0.8088 | 3.582 | 1.037 | 0.081 | 0.9502 |
| PF8 | 3.903 | 0.9941 | 0.059 | 0.9252 | 14.683 | 0.8186 | 3.855 | 1.004 | 0.017 | 0.9561 |
| PF9 | 4.609 | 0.9837 | 0.079 | 0.9321 | 17.534 | 0.8638 | 6.411 | 0.882 | 0.023 | 0.9672 |
Release kinetic studies.
DISCUSSION
Mesalamine is a common anti-inflammatory medication used to treat a variety of disorders, including colorectal cancer, ulcerative colitis, and Crohn’s Disease (CD). As a result, current research focuses on the regulated release of mesalamine in the colon to treat ulcers. The controlled-release tablet was produced using sodium alginate and guar gum. By adjusting the amounts of sodium alginate and guar gum, several formulations might be synthesized. In comparison to the remaining eight formulations, PF9 had the best results in pre- and post-formulation testing and kinetic release studies. At lower concentrations of guar gum and sodium alginate, the drug was released from the tablet before it reached the colon due to the breakdown of guar gum by enzymes, whereas at higher concentrations, the drug was not released from the tablet. Because the higher concentration of guar gum did not get degraded by an enzyme in the colon, the drug was not released. Therefore, product formulation PF9 demonstrated the precise concentration needed. The bulk density, tapped density, Hausner’s ratio, compressibility factor, and angle of repose were all studied as part of pre-compression investigations on all Product Formulations (PF1-PF9). The result of the first three formulations (PF1–PF3) was not within a specific limit. The formulations PF4– PF9 showed results within the official limit, and the formulation PF9 showed excellent results. Pre-formulation studies were also performed before product formulation. According to the solubility test, mesalamine was not soluble in water. In DSMO and acetone, mesalamine is freely soluble. FTIR studies were conducted on an individual ingredient that showed the peaks as described in the official range. Mesalamine exhibits a band at 1647.5 cm-1, 1448.1 cm-1, 1487.2 cm-1, and 1354.29 cm-1 due to the C=O stretching bond, C=C bond, and CH2 bond. This peak offered strong evidence that the sample tablet contained pure mesalamine. The excipient and mesalamine had no interaction, according to the FTIR. A DSC test was performed, which shows the phase transitions, i.e., exothermic at 182°C and endothermic at 80°C, and these endothermic peaks due to the phase transition and breaking of the double bond present in the aromatic ring of mesalamine. TGA is used to explain drug stability, as it began to degrade above a high temperature of 282°C. According to X-ray diffraction, bend appeared at 2θ; 7.12, 10, 15.32, 16.16, and 21.4 with very low reproducibility because of the presence of an excipient that caused a decrease in the crystallinity of the mesalamine and mesalamine incorporated into the tablet. In in vitro evaluation, disintegration and dissolution tests were performed. The tablets did not disintegrate in the acidic or basic medium within the official limit, and it is due to the presence of guar gum. In the dissolution study, findings demonstrated the ability of the guar gum tablets to dissolve in the presence of the enzyme galactomannans and, consequently, their capacity to release the content of the matrix tablet into the colon. The quantity and form of alginate were discovered to have an impact on drug release. Sodium alginate is a polymer that is pH-dependent. The amount of drug released was enhanced in the basic medium. At pH 1.2 and pH 4.5, it was found that the drug dissolved from the tablets in distinct ways throughout a 4-hr period. The matrix tablet with the highest sodium alginate content indicated a drug release of 17.40% at pH 1.2 and 4.5, whereas the tablet with the lowest sodium alginate content revealed a drug release of 15.61% over 4 hr. It was observed that the drug release improved by 97.12% and exceeded 99.64% during the course of 24 hr at pH 6.8 and 7.4, respectively. In the literature, a variety of pH-dependent polymers have been used to achieve colon-specific drug release. Mesalamine was transported or properly released at the colon using sodium alginate, a polymer that is pH-dependent. During dissolution testing, the formulation PF9 demonstrated the highest drug release of approximately 99.97% in 24 hr. According to the release kinetic, the product formulation was showing zero order, which confirmed the controlled release nature of the product and confirmed that the amount of drug released from the product does not depend on concentration. During formulation data modelling, we concluded that the PF9 formulation was best fit to zero order as the R2 value is near 1. So, it was shown that the release of drugs does not depend on concentration, which explained the zero-order behavior of the formulation and controlled release kinetics. According to the Higuchi model and Crowell, the formulation showed a controlled release pattern because the value of R2 was the best fit to the values, that is, 0.8638 and 0.9672, respectively.
CONCLUSION
This study concludes that adding the appropriate polymer (guar gum) delays the release, and a pH-dependent polymer (sodium alginate) releases the mesalamine into the lower GI tract, allowing it to reach its target in the colon. According to in vitro drug evaluation experiments, the F9 formulation was the optimal one for the colon’s targeted site due to its pH dependence and controlled release behavior. Mesalamine releases 99.67% of its entire quantity after 24 hr in the presence of the galactomannanase enzyme. The drug release rate of these colon-specific formulations was aligned with zero-order kinetics, indicating that released activity was controlled.
References

- Sardo HS, Saremnejad F, Bagheri S, Akhgari A, Garekani HA, Sadeghi F., et al. A review on 5-aminosalicylic acid colon-targeted oral drug delivery systems. Int J Pharm. 2019;558:367-79. [Google Scholar]

- Kotla NG, Rana S, Sivaraman G, Sunnapu O, Vemula PK, Pandit A, et al. Bioresponsive drug delivery systems in intestinal inflammation: state-of-the-art and future perspectives. Adv Drug Deliv Rev. 2019;146:248-66. [PubMed] | [CrossRef] | [Google Scholar]

- Patel MM. Cutting-edge technologies in colon-targeted drug delivery systems. Expert Opin Drug Deliv. 2011;8(10):1247-58. [PubMed] | [CrossRef] | [Google Scholar]

- Rubinstein A.. Colonic drug delivery. Drug Discov Today Technol. 2005;2(1):33-7. [PubMed] | [CrossRef] | [Google Scholar]

- Advankar A, Maheshwari R, Tambe V, Todke P, Raval N, Kapoor D, et al. Specialized tablets: ancient history to modern developments. Drug Deliv Syst.. 2019:615-64. [PubMed] | [CrossRef] | [Google Scholar]

- Ham M, Moss AC. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev Clin Pharmacol. 2012;5(2):113-23. [PubMed] | [CrossRef] | [Google Scholar]

- Baker DE. MMX mesalamine. Rev Gastroenterological Disord.. 2006;6(3):146-52. [PubMed] | [Google Scholar]

- George A, Shah PA, Shrivastav PS. Guar gum: versatile natural polymer for drug delivery applications. Int J Pharm. 2019;112:722-35. [CrossRef] | [Google Scholar]

- Hariyadi DM, Islam N. Current status of alginate in drug delivery. Adv Pharmacol Pharm Sci. 2020 [PubMed] | [CrossRef] | [Google Scholar]

- Souza DFd, Goebel K, Andreazza IF. Development of enteric coated sustained release minitablets containing mesalamine. Braz J Pharm Sci.. 2013;49(3):529-36. [CrossRef] | [Google Scholar]

- Mehmood Y, Yousaf UFH, Riaz H, Saleem N, Hassan MB, Mahmood RK, et al. Formulation development using different natural and semi synthetic polymers, evaluation of colon targeted sulfasalazine tablets for ulcerative colitis. I.J.B. 2019;15(1):42-55. [CrossRef] | [Google Scholar]

- Banerjee A, Pathak S, Subramanium VD, G D, Murugesan R, Verma RS, et al. Strategies for targeted drug delivery in treatment of colon cancer: current trends and future perspectives. Drug Discov Today. 2017;22(8):1224-32. [PubMed] | [CrossRef] | [Google Scholar]

- Shah RB, Tawakkul MA, Khan MA. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech. 2008;9(1):250-8. [PubMed] | [CrossRef] | [Google Scholar]

- Shah RB, Tawakkul MA, Khan MA. Comparative evaluation of flow for pharmaceutical powders and granules. A.A.P.S. PharmSciTech. 2008;9(1):250-8. [PubMed] | [CrossRef] | [Google Scholar]

- Deshmukh R, Kumar RK. Preformulation considerations development and evaluation of mesalamine loaded polysaccharide-based complex mucoadhesive beads for colon targeting. IJPER. 2021;55(1):95-106. [CrossRef] | [Google Scholar]

- Chaturvedi H, Garg A, Rathore US. Post-compression evaluation parameters for tablets-an overview. E.J. P.M. R.. 2017;4(11):526-30. [CrossRef] | [Google Scholar]

- Maad AH, Ali Shayoub ME, Elnima EI, Osman Z, Magbool FF. Formulation and evaluation of colon targeted matrix tablets containing extract of (Hargel). Univ J Pharm Res.. 2019;4(4):33-8. [CrossRef] | [Google Scholar]

- Gandhi H, Rathore C, Dua K, Vihal S, Tambuwala MM, Negi P, et al. Efficacy of resveratrol encapsulated microsponges delivered by pectin-based matrix tablets in rats with acetic acid-induced ulcerative colitis. Drug Dev Ind Pharm.. 2020;46(3):365-75. [PubMed] | [CrossRef] | [Google Scholar]

- Ye B, van Langenberg DR.. Mesalazine preparations for the treatment of ulcerative colitis: are all created equal?. World J Gastrointest Pharmacol Ther.. 2015;6(4):137-44. [PubMed] | [CrossRef] | [Google Scholar]

- Deshmukh P, Upadhyaya N, Patidar S, Pawar R.. Probiotic-assisted colon-specific delivery of anti-inflammatory Drug-5 ASA. I.J.P.S.H. IJPSM.. 2022;7(10):119-35. [CrossRef] | [Google Scholar]

- Bhatia S, Al-Harrasi A, Al-Azri MS, Ullah S, Bekhit AE-DA, Pratap-Singh A, et al. Preparation and physiochemical characterization of bitter orange oil loaded sodium alginate and casein based edible films. Polymers (Basel).. 2022;18(18):3855 [PubMed] | [CrossRef] | [Google Scholar]

- Shokri B, Firouzjah MA, Hosseini SI. FTIR analysis of silicon dioxide thin film deposited by metal organic-based PECVD. In: Proceedings of the 19th international symposium on plasma chemistry society. 2009 [PubMed] | [CrossRef] | [Google Scholar]

- Islam MN, Hossain SMM, Khatton A, Rahman MM, Sarker J, Sikder HA, et al. Microcrystalline cellulose from jute fiber: A bright prospect for pharmaceutical industry. Sch Int J Chem Mater Sci. 2022;5(6):100-4. [CrossRef] | [Google Scholar]

- Thombare N, Mishra S, Shinde R, Siddiqui MZ, Jha U.. Guar gum based hydrogel as controlled micronutrient delivery system: mechanism and kinetics of boron release for agricultural applications. Biopolymers.. 2021;112(3):e23418 [PubMed] | [CrossRef] | [Google Scholar]

- Tuğcu-Demiröz F, Acartürk F, Takka S.. Investigation of colon-specific dosage forms of ondansetron prepared with natural polymers. Pharmazie. 2006;61(11):916-9. [PubMed] | [Google Scholar]

