ABSTRACT
Background
Mesalamine produces many side effects through oral route such as stomach cramps, throat irritation, back ache and muscle pain. Furthermore, it failed to yield maximum drug release and also not able to produce the drug release for over a period of time at the target site. To overcome these disadvantages, the current research proposed to develop microspheres with pH-dependent bio-resorbable polymers to obtain site-specific controlled drug release at the target site.
Materials and Methods
Microspheres were prepared by two methods as ionic gelation and solvent evaporation methods using copolymers such as Eudragit S 100 and PLGA.
Results
The prepared formulations were characterized for multiple parameters and the particle size ranges were found in between 5.52 to12.56 μm whereas percentage entrapment efficiency was found in the range of 32 to 98% respectively. Apart from other formulations, PLGA microspheres showed the maximum cumulative percentage drug release through in vitro and ex vivo and the results were found as 96±0.1% and 90.8±0.8% respectively. As compared to Pentasa®, PLGA microspheres showed two folds increase in drug release. The PLGA biodegradability was observed as maximum in the caecal content than other formulations.
Conclusion
Compared to Pentasa®, the prepared PLGA microspheres showed the best results and the obtained result was verified with a p-value which is shown <0.005. Based on the results, it was concluded that the prepared PLGA microspheres showed better-controlled drug release than other test formulations.
INTRODUCTION
Apart from various routes of drug delivery, the oral route is the one which is a safe, convenient and easy route for the administration of many drugs in the treatment of various diseases. In the conventional systems, dosage forms are available to treat several intestinal diseases such as inflammation of Gastrointestinal Tract (GIT), ulceration, Inflammatory Bowel Disease (IBD) etc., apart from these diseases; IBD is one of the deadliest diseases after cancer.1,2 Commonly protein and peptide molecules have been employed in the colon which is degraded by enzymes in the stomach and small intestine.3 Generally, the colon shows higher pH than the upper GI tract and thus a drug-targeting strategy for colonic drug delivery should be developed using this phenomenon. Hence, the ideal polymer should be able to withstand in the stomach region, which shows lower pH and dissolves in the colon.4 The conventional systems failed to attempt a specific site of drug delivery, therapeutic action at site-specific pH region and controlled release of the drug respectively.5
Various pH-dependent polymers are Cellulose Acetate Phthalate (CAP), Hydroxypropyl Methyl-Cellulose Phthalate (HPMCP) 50 and 55, different grades of Eudragit® (S 100, L and FS) and highly biocompatible and biodegradable PLGA (poly lactide-co- glycolide). Based on the viscosity and molar ratio of monomers (LA:GA), these can be available in standard grades (50:50, 75:25, 50:45, 65:35 and 85:15) respectively. Bioresorbable polymers are available with inherent viscosities ranging from 0.2 to 1.0 dL/g. PLGA and PDLLA (poly (D, L-lactide)) are the most commonly used bioresorbable polymers for controlled-release drug delivery systems, due to their predictable degradation rate and amorphous polymer structure. Based on the availability, among these two polymers, we have selected PLGA with L/G ratio of 50:50 and viscosity of 0.4-0.6 dL/g. Currently available novel-controlled release systems are hydrogels, liposomes, nanoparticles, pellets, microspheres, etc., these may overcome the drawbacks produced by conventional systems.6 Hence, the current research work proposed to target the colon by using pH-dependent controlled-release polymers.
Drug entrapped in a matrix and forms a cross-linkage with the pH-dependent biopolymer so that controlled release and site-specific drug delivery may be achieved.7,8 Microspheres are composed of biodegradable polymers like PLGA and eudragit S-100.9,10 Mesalamine is the first-line drug in the treatment of ulcerative colitis. When it is absorbed orally, rapid absorption shows in the upper GIT and it results in producing very little therapeutic effect due to systemic absorption rather than local absorption of the drug and it addresses the failure to reach the colon.9,11
Few latest literature reports demonstrated that the drug combined with pH-dependent delivery system showed better results. Souza DS et al. developed sustained release risperidone microspheres using low molecular weight PLGA (50:50). They found that risperidone encapsulated microspheres release behaviour was observed up to 20 days in the treatment of schizophrenia.12 Qin Cao et al. reported on pH-dependent drug release in colon. They stated 71% of drug entrapped in phosphate buffer pH 7.4. Compared to suspension the maximum Area Under the Curve (AUC), t0 was obtained with mesalamine microparticles in rats up to 12 hr. Hence, maximum drug concentration was achieved at the target (colon) site.13 Srini Tenjarla test mesalamine drug in various strengths of marketed tablets dissolution in three intestinal pH mediums (0.1 N HCl, phosphate buffer pH 6.4 and phosphate buffer pH 7.2) respectively. They concluded that the controlled release drug was observed from phosphate buffer pH 7.2 than those other two mediums.14 Omar Mady developed microspheres with eudragit RS100 and reported maximum drug absorption obtained from the alkaline region than the acidic region.15 Tapan Kumar Chatterjee reported on site-specific control of colon cancer of 5-Fluoraouracil. They concluded that the use of carrier, i.e., gum katira, to target coons actively with low side effects.16 Recently Dana-Maria Muntean developed curcumin-loaded microspheres to treat ulcerative colitis in rats. They found that curcumin encapsulated in microspheres and showed superior results than encapsulated curcumin.17 Jose et al. studied drugs on the colon for local treatment by using microspheres. They revealed that in the presence of rat caecal contents, the biodegradability of microspheres was attained and from the in vitro caecal content studies, 99.52±0.8% of drug was released upto 12 hr.18
Recently in the year of 2016, Brij Khera et al. developed a patent on stable pharmaceutical compositions on mesalamine. In those compositions, they mentioned about the invention is a capsule dosage form filled with a tablet to develop stable dosage form by differentiating various moisture content percentages of capsule shell. After through reading the patent our idea imposed like the mesalamine had lack of stability as it is in a dosage form. Hence the current research is planned in such a way that the developed microspheres were placed into zero-sized hard gelatin capsules to protect or to stabilize the dosage form.19
MATERIALS AND METHODS
Materials
Mesalamine was obtained from Hetero Drugs Pvt.Ltd., Hyderabad, Eudragit S-100 from Sigma-Aldrich, India, PLGA-ViatelTM DLG 5005 E from Ashland specialties Ireland limited, Ireland as a gift sample. Sodium alginate, dichloromethane, methanol, polyvinyl alcohol was obtained from the stores of CMR College of Pharmacy, Kandlakoya, Medchal, Hyderabad.
Methods
We have selected two methods to prepare microspheres those are ionic gelation and solvent evaporation methods. The excipients were set as three groups as shown in Table 1.
| Ingredients | IF1 | IF2 | IF3 | SF1 | SF2 | SF3 | SPF1 | SPF2 | SPF3 |
|---|---|---|---|---|---|---|---|---|---|
| Mesalamine (g) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Sodium alginate (g) | 1 | 1 | 1 | – | – | – | – | – | – |
| Methanol (mL) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Calcium chloride (g) | 2 | 2 | 2 | – | – | – | – | – | – |
| Eudragit S-100 (mg) | 250 | 350 | 450 | 250 | 350 | 450 | – | – | – |
| PLGA (mg) | – | – | – | – | – | – | 250 | 350 | 450 |
| Dichloromethane (mL) | – | – | – | 10 | 10 | 10 | 10 | 10 | 10 |
| Poly vinyl alcohol (mL) | – | – | – | 100 | 100 | 100 | 100 | 100 | 100 |
| Distilled water (mL) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Composition of formulations of mesalamine microspheres.
Preparation of microspheres
Ion gelation technique
In this method a required amount of sodium alginate and drug were added to 50mL of distilled water and heated on a water bath to get the desired dispersion and upon a thick slurry was obtained then weighed amount of calcium chloride and Eudragit S-100 (250, 350 and 450 mg) are dissolved separately in 50 mL of distilled water in a beaker. Prepared polymer drug slurry was added as dropwise using a syringe at a height of 5 cm to the beaker containing calcium chloride solution. Microspheres are formed which are then washed, filtered, and subjected to drying.11
Solvent evaporation technique
The method of preparation was similar for two different polymers (Eudragit S-100 and PLGA). The method involves organic phase and aqueous phase respectively. Drug-polymers were added to an organic solvent (di-chloro-methane) containing beaker whereas aqueous phase was prepared by dissolving Poly Vinyl Alcohol (PVA) in water. The two phases are subjected to homogenization. The aqueous phase was subjected to stirring using a mechanical stirrer (Remi lab stirrer) at 500 rpm during the process; the organic phase was taken in a syringe and added dropwise to aqueous phase, which resulted in the formation of microspheres as shown in Figure 1. The microspheres were separated by washing and filtration and then subjected to drying.12

Figure 1:
Preparation of microspheres.
Characterization of microspheres
All the prepared microspheres were subjected to particle size and shape, percentage entrapment efficiency, in vitro drug release study, drug release kinetics, drug release studies in presence of rat caecal contents, ex vivo drug release study in rat colon and FTIR studies respectively.
Particle size and shape
The shape and size of the optimized mesalamine microspheres were determined using a Scanning Electron Microscope (SEM).11
Percentage Entrapment Efficiency (% EE)
Sufficiently weighed quantities of microspheres were taken and triturated with methanol and kept overnight for extraction of drug. After that, the mixture gets filtered and diluted with methanol. The sample absorbance was measured at 330 nm using UV spectrophotometer.6 % EE calculated by following equation.
In vitro cumulative drug release studies
In vitro cumulative drug release studies can be performed using USP rotating basket method (LABINIDIA DS 8000). Required quantity of microspheres was filled in zero-size hard gelatin capsules and placed in the basket containing 900 mL dissolution medium. Initial 4 hr the study was conducted in pH 1.2 HCl buffer followed by 3 hr in pH 6.8 phosphate buffer and the remaining hours in phosphate buffer pH 7.4. The samples were collected at predetermined time intervals of 0, 1, 2, 3, 4, 6, 8, 10, 12, and 24 hr and the collected samples were filtered and analysed at 330 nm by UV spectrophotometer.11
Release kinetics
All the test formulations were fitted to zero-order, first-order, Higuchi-model and Korsemeyer-peppas model to ascertain pattern of drug release and to know the mechanism of drug release respectively. The zero-order kinetic model was plotted against cumulative % drug released vs. time, first-order kinetic model was plotted against log cumulative % drug remaining vs. time, Higuchi-model plotted against cumulative % drug released vs. square root of time and Korsemeyer-peppas model plotted against log cumulative % drug released vs. log time respectively.15
In vitro release studies in the presence of rat caecal contents
The experiment protocol was accepted by the CMR institutional ethical committee (IAEC) and approved the protocol with ethical clearance No: (CPCSEA/1657/IAEC/CMRCP/COL-21/65). Three Wistar male rats weighing around 200-250 g were selected and the animals were provided with a normal diet for the entire drug treatment. Total treatment period was seven days, and every day 1 mL of prepared microspheres in solution form was injected into rats, the same procedure was continued for every 24 hr up to seven days. After the treatment period, the animals were sacrificed and immediately placed in phosphate buffer pH 7.2. The caecal bag was opened; the contents were weighed to justify the desired caecal content. Later, the content was placed in a dissolution basket as described under the in vitro drug release procedure. The collected samples were filtered and the drug content was determined by UV spectrophotometer.11
Ex vivo drug release studies
The studies were conducted by the Everted gut sac method as the first time described by Wilson and Wiseman. The rat intestine was separated and the intestinal part was filled with 1 mL of drug solution. The beaker containing luminal pH medium was perfused under a magnetic stirrer at a speed of 500 rpm at room temperature 37°C. The drug release in the luminal pH was determined by UV spectrophotometer.20
Correlation between in vitro and ex vivo studies
Correlation coefficient value was found by taking in vitro and ex vivo percentage cumulative drug release; a graph was plotted on two y-axis’s.
FTIR studies
FTIR (Fourier Transform Infrared Spectroscopy) identifies the functional group alterations between individual drugs and excipients and the formulations prepared. The common use of FTIR is the identification of unknown materials, confirmation, and checking of drug-excipient compatibility. FTIR spectra of pure drug and physical mixture of drug with polymers were obtained with FTIR (BRUKER) instrument. The samples were mixed with KBr pellets.20
RESULTS
Colonic inflammation, ulcerative colitis, and IBDs are the most severe diseases of the colon, to treat such diseases; colonic absorption of the drug is required. The mesalamine microspheres were successfully prepared and evaluated for various parameters. Mesalamine solubility (mg/mL) was performed using different buffers and from Table 2, it was observed that it was shown pH-dependent solubility. Maximum solubility was found in pH 1.2 buffers and increasing the pH from 4.5 to 7.4, the solubility was observed to increase from 0.9 mg/mL to 6 mg/mL respectively. The highest dose solubility volume is 1500 mL (1200/0.80=1500), hence this drug would be considered as a very slightly soluble drug.
| Sl. No. | Media | pH | Solubility (mg/mL) | |||
|---|---|---|---|---|---|---|
| 1 | 0.1N HCl | 1.2 | 7.96 | 8.02 | 8.17 | 8.43 |
| 2 | Acetate Buffer | 4.5 | 0.89 | 0.89 | 0.91 | 0.94 |
| 3 | Acetate buffer | 6.8 | 3.64 | 3.80 | 3.68 | 3.74 |
| 4 | Phosphate buffer | 7.2 | 4.65 | 4.67 | 4.77 | 4.89 |
| 5 | Phosphate buffer | 7.4 | 5.48 | 5.49 | 5.67 | 5.65 |
| 6 | Water | – | 0.80 | 0.82 | 0.82 | 0.86 |
Solubility studies of mesalamine in various buffers.
The drug content values for all microspheres were found to be in the range of 32% and 98%. The results are within IP limits and the results are tabulated in Table 3. The % EE was found to be in the range of 27.9 to 98.9%, respectively. The maximum % EE was observed for SPF2 (98±0.8%). Comparative values of % EE and % DC for all prepared microspheres are shown in Figure 2.
| Optimized Batch | Model | Coefficient of determination (r2) | Mechanism of release |
|---|---|---|---|
| IF1 | Zero order First order Higuchi Peppas | 0.9725 0.9951 0.9785 0.9931 | First order |
| SF1 | Zero order First order Higuchi Peppas | 0.9783 0.9963 0.9885 0.9831 | First order |
| SPF2 | Zero order First order Higuchi Peppas | 0.9814 0.9928 0.9993 0.9989 | First order |
In vitro drug release kinetics of microspheres.
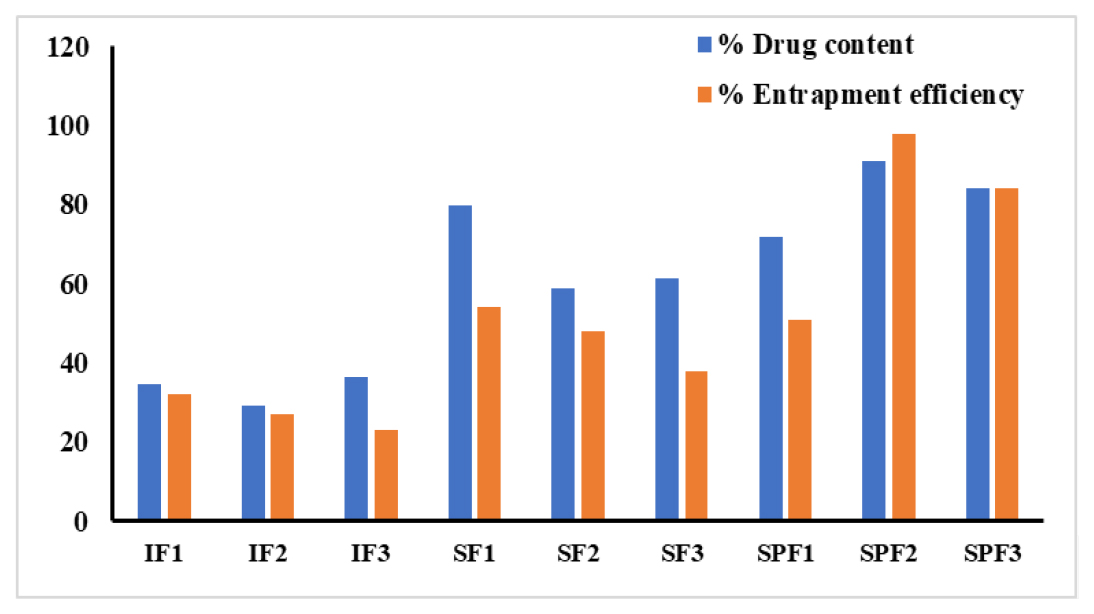
Figure 2:
Graphical representation of %EE and % DC.
Mesalamine microspheres shape and surface morphology were determined using SEM. The particle size of all prepared microspheres was found to be in the range of 5.52 μm-12.56 μm. The obtained particle size was within the range of standard microsphere particle size (1 μm- 500 μm). Their shape was spherical-like and the surface was partly rough due to drying. The image is shown in Figure 3.
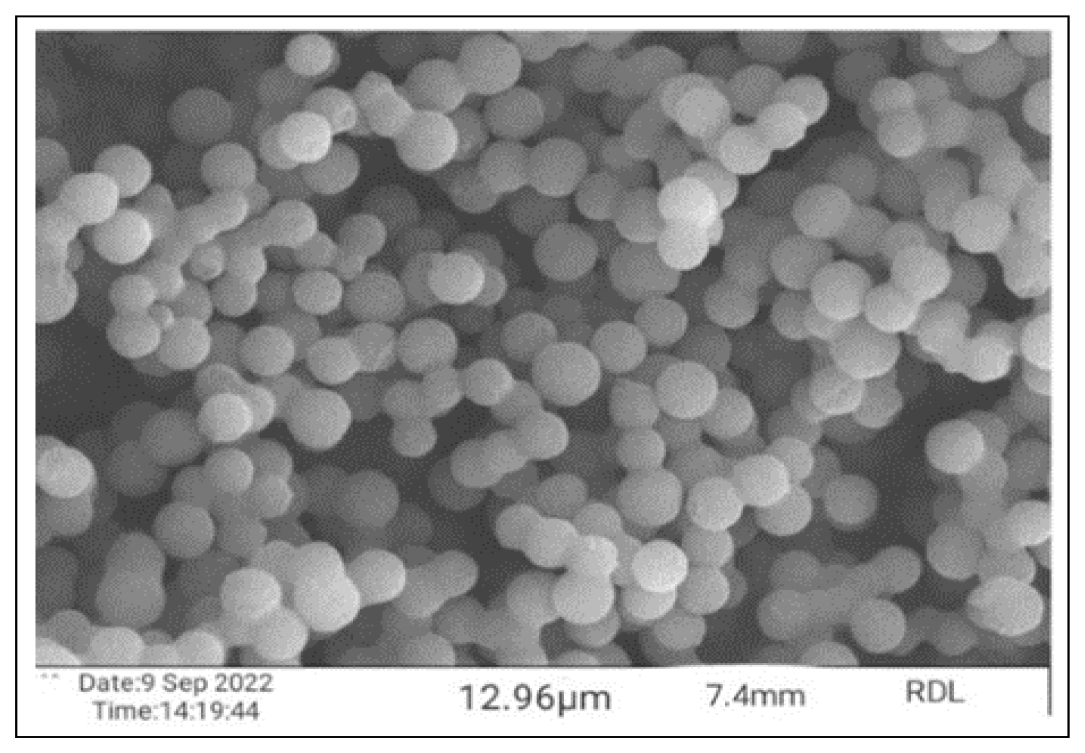
Figure 3:
SEM image of optimized formulation (SPF2).
From the in vitro drug release studies, it was observed that IF1, SF1, SPF2 formulations exhibited better drug release as compared to other formulations. Apart from IF1, SF1, SPF2 (96±0.1%) has shown maximum drug release than IF1 (58±0.7%) and SF2 (87±0.4%) formulations respectively. The cumulative drug release profiles of all prepared formulations are shown in Figure 4.
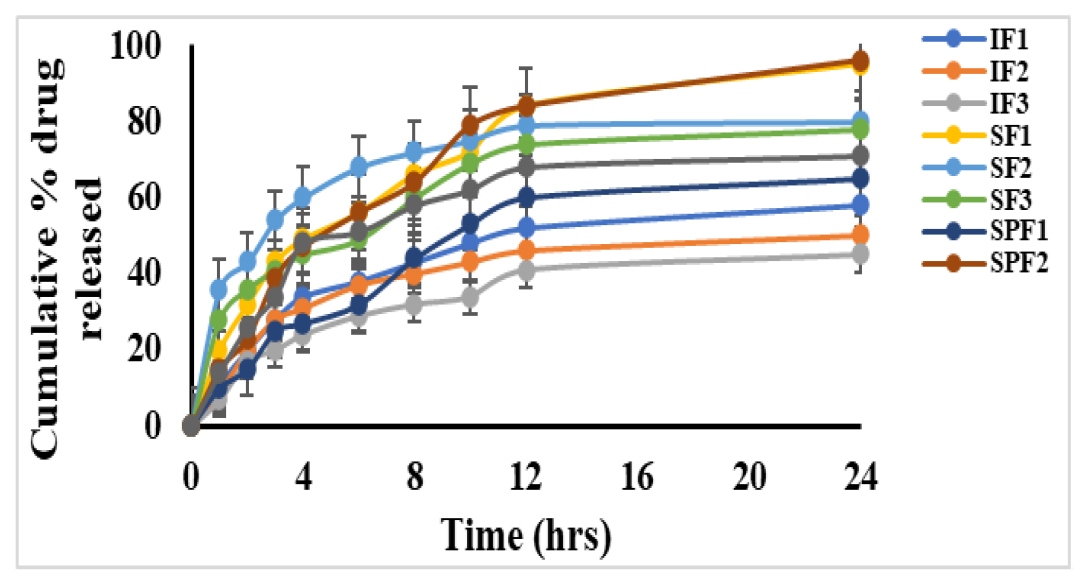
Figure 4:
Cumulative percentage drug release profiles of microspheres
The pattern of drug release and mechanism of test formulations was studied by using zero, first, Higuchi and Peppas kinetic models. The values of all the kinetic models are shown in Table 3.
The compatibility between drug and excipients used in the formulation can be determined by FTIR. The drug peak due to the strong (C-H) stretch of the aromatic group, medium (C=C) stretch of the aromatic group, strong (C-C) stretching mode, strong (O-H) deformation of the hydroxyl groups, medium (C-O) stretching mode, strong (C-H) bond out of plane bending mode; ring deformation of the aromatic group, strong (O-H) stretching mode associated with the hydroxyl groups. It was observed that there were no changes in these main peaks in the IR spectra of a mixture of drug and excipients. The results are tabulated in Table 4.
| Compound | Functional group | Peak length |
|---|---|---|
| Mesalamine | -NH2-Bending C=O-Stretching C-O-Stretching | 1615-1700 cm-1 1615-1700 cm-1 1215 cm-1 |
| Eudragit S-100 | OH-Bending CH2 Stretching O=H- Stretching | 931.8-1060 cm-1 1485 cm-1 1351 cm-1 |
| PLGA | OH-Bending C-H-Stretching C=O-Stretching C-O-Stretching C-H-Bending | 3450-3500 cm-1 2885-3010 cm-1 1762.6 cm-1 1186-1089 cm-1 150-850 cm-1 |
| Optimized formulation | C-H, -NH2-Bending C=O, C-O- Stretching OH, O=H-Stretching | 500-2000 cm-1 1865.6 cm-1 1435 cm-1 |
FTIR studies of test products.
Comparison of in vitro drug release studies of SPF2 against PENTASA®
Pentasa®, available as different strengths in the market in the current study we have used 500 mg tablets for the comparison against test formulation. Microspheres of SPF2 formulation were also weighed to equivalent dose as Pentasa® and conducted in vitro cumulative drug release studies. From Figure 5, it was observed that prepared microspheres showed better drug release over Pentasa®.
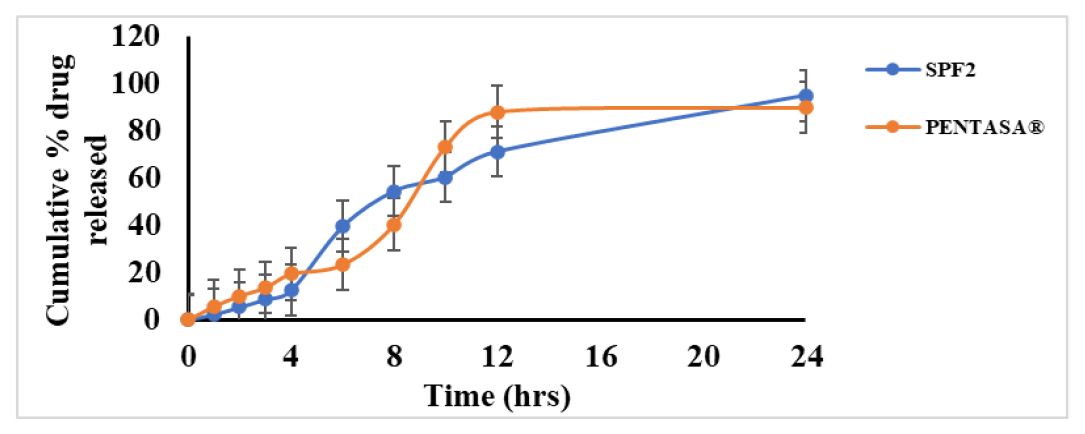
Figure 5:
Comparative in vitro drug release studies of Pentasa® and SPF2.
In vitro drug release with caecal contents
To ensure the biodegradability of PLGA microspheres by colonic bacteria, we have performed caecal content estimation by in vitro as shown in Figure 6.

Figure 6:
In vitro analysis of rat caecal contents.
The drug release from SPF2 in the presence of rat caecal contents and the absence of rat caecal contents were compared. The study showed that, in the presence of rat caecal contents, the cumulative % drug release was improved two folds, i.e., 98.52±0.2% in 24 hr respectively.
Ex vivo drug release studies
The procedure of extracting the colon and the way of everything was done as shown in Figure 7. The amount of mesalamine transported through colon and its release through the colon was estimated.
Figure 7:
Ex vivo studies using the everted sac technique.
From Table 5, at the end of 24 hr, the maximum amount (492±0.6 mg) and drug release (90.6±0.2 %) was obtained, this result may be the impact of PLGA in microspheres, it may get transported and degraded rapidly without disturbing the controlled release pattern than in vitro studies using dissolution apparatus.
| Time (hrs) | Amount of mesalamine (mg) | Cumulative drug release (%) |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 28±0.4 | 5.7±0.7 |
| 2 | 68±0.6 | 13.6±0.5 |
| 3 | 98±0.5 | 19.5±0.1 |
| 4 | 117±0.4 | 23.4±0.4 |
| 5 | 235±0.1 | 42.9±0.1 |
| 6 | 292±0.6 | 49.0±0.2 |
| 8 | 368±0.5 | 55.7±0.3 |
| 9 | 441±1.1 | 69.1±0.9 |
| 10 | 468±0.2 | 74.2±0.6 |
| 12 | 470±0.8 | 88.5±0.8 |
| 24 | 492±0.6 | 90.6±0.2 |
Amount and cumulative % drug release of SPF2 through colon.
We also determined the correlation coefficient value (0.984) between in vitro and ex vivo cumulative percentage of drug which is released up to 24 hr and illustrated in Figure 8.
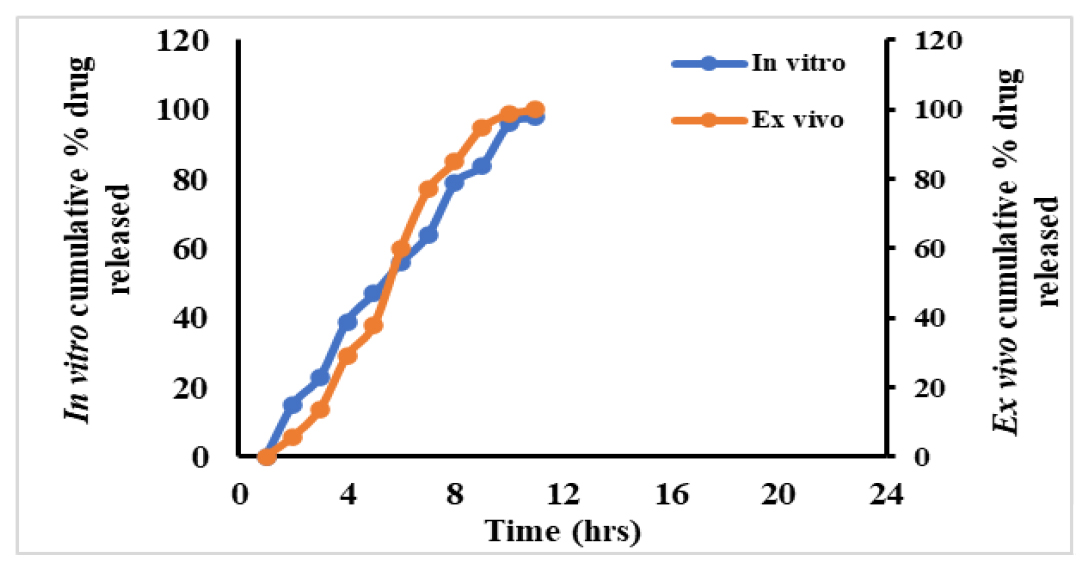
Figure 8:
Correlation between in vitro-ex vivo studies.
DISCUSSION
Mesalamine microspheres were successfully prepared for the treatment of IBD by using biodegradable polymer. The drug solubility checked in various buffer mediums and the maximum solubility was found in 7.4 pH phosphate buffer the drug showed a pH dependent solubility which is an important key parameter to develop the dosage form to the colon. The drug content of all the formulations was within the limits. Hence, we may ensure the uniform distribution of drug and polymers in the formulation. The SPF2 formulation shown maximum % EE. The obtained result may be due to ViatelTM DLG 5005 E grade PLGA which shows lactide to glycolide (L/G ratio) as 50:50 in that LA monomer shows less hydrophobicity and GA monomer shows faster degradation.5 Thus, the drug encapsulation is more with PLGA 50:50 ratio and obtained microspheres were shown uniform distribution and enhances the ease of absorption at a specific region. The particle size and size distribution of optimized formulation were found within the range and there will be an absence of agglomeration was observed.
The in vitro drug release studies of SPF2 showed maximum drug release (96±0.1%) in 24 hr than other test formulations and this result may be due to pH-dependent bioresorbable copolymer (PLGA) and it also achieves controlled drug release in phosphate buffer pH 7.4 (which resembles colonic pH) thereby it bypasses mesalamine absorption in the stomach or intestine and promotes the release respectively. The biodegradation of PLGA occurred by cleavage of lactic acid and glycolic acid monomers by hydrolysis process through ester bonds20. From the prepared formulations, we have observed that in the presence of acid medium and alkaline medium (pH 6.5 to 6.8), no desirable drug release was observed due to the monomer (LA/GA) ratio and Molecular Weight (MW) in the PLGA and these properties may also affect the drug release kinetics of PLGA microspheres. The drug release kinetics of SPF2 batch follows zero order and the result stated that the drug was obtained at a constant rate with prolonged effect in the colon (specific site). All the test formulations follow Higuchi’s type of mechanism as the process indicated by diffusion. FTIR studies confirmed that there will be no functional group deviation and the absence of incompatibility was observed. The obtained result shows compatibility among the materials used for the formulation. The cumulative drug release comparative results between SPF2 and PENTASA® showed that microspheres with PLGA showed better-controlled release than the PENTASA®. This may be due to the presence of pH-dependent bioresorbable polymers.21 To verify the biodegradability of test product in the colon, the drug release studies were performed with caecal contents and the results proved that the release from the colon was twofold more than that of in vitro by lab scale. The ex vivo drug release through the everted sac method indicated that the PLGA microspheres showed maximum drug release through colon the statement was exactly correlated with the objective of the study.
CONCLUSION
Potential colonic drug delivery system based on pH sensitivity was developed for delivering mesalamine to the colon. Mesalamine microspheres were formulated and evaluated for drug release in simulated physiological conditions. The optimized formulation was compared with the marketed formulation and observed that the polymer content alters the physicochemical parameters and drug release pattern of the microspheres. Best results were obtained with PLGA among other polymers, which effectively controlled drug release up to 24 hr and bypassed the drug release in non-specific sites. Hence, the present research work concluded that the prepared PLGA based microspheres are efficiently delivering the drug in a controlled manner to a specific site. However, a more detail in vivo study is necessary to ensure its benefits.
Cite this article
Madhavi N, Divya B, Sudhakar B, Rao TR. Effect of Bioresorbable Copolymers on Mesalamine Loaded Microspheres for Colon-Specific Drug Delivery. Int. J. Pharm. Investigation. 2024;14(2):428-35.
ACKNOWLEDGEMENT
The authors thank CMR College of Pharmacy, Kandlakoya, Medchal for providing a source to complete our work. Authors special thanks to Dr. Sundeep Chaurasia, who assist us to get the PLGA from Ashland specialities Ireland limited, Ireland. Authors also acknowledge our institution, pharmacology department, HOD Dr. V.V Rajesham, who guided us in animal studies work.
ABBREVIATIONS
| IBD | Inflammatory bowel disease |
|---|---|
| CAP | Cellulose acetate phthalate |
| HPMCP | Hydroxypropyl methyl-cellulose phthalate |
| PLGA | Poly lactide-co-glycolide |
| GIT | Gastrointestinal tract |
| AUC | Area under the curve |
References
- Bayan MF, Bayan RF. Recent advances in mesalamine colonic delivery systems Futur. J Pharm Sci. 2020;6(1):43 [Google Scholar]

- Gohel MC, Parikh RK, Nagori SA, Dabhi MR. Design of a potential colonic drug delivery system of mesalamine. Pharm Dev Technol. 2008;13(5):447-56. [PubMed] | [CrossRef] | [Google Scholar]

- Kadam NR, Suvarna V. Microsphere: A brief review. AJB. PS. 2015;47(5):14-7. [PubMed] | [CrossRef] | [Google Scholar]

- Lee SH, Bajracharya R, Min JY, Han JW, Park BJ, Han HK, et al. Strategic approaches for colon targeted drug delivery: an overview of recent advancements. Pharmaceutics. 2020;12(1):68 [PubMed] | [CrossRef] | [Google Scholar]

- Essa D, Kondiah PPD, Choonara YE, Pillay V. The design of poly (lactide-co-glycolide) nanocarriers for medical applications. Front Bioeng Biotechnol. 2020;8:48 [PubMed] | [CrossRef] | [Google Scholar]

- Sarlesh R, Ashish P, Nikhil S. A review on microspheres: methods of preparation and evaluation. WJPPS. 2012;1(1):422-38. [PubMed] | [CrossRef] | [Google Scholar]

- Patil A, Pawar P, Gharge V, Doltade U, Doijad R. Mesalamine-loaded mucoadhesive microsphere for colon drug delivery system: effect of process variables and in vitro characterization. Int J Pharm Investig. 2018;8(2):74-82. [CrossRef] | [Google Scholar]

- Rai G, Yadav AK, Jain NK, Agrawal GP. Eudragit-coated dextran microspheres of 5-fluorouracil for site-specific delivery to colon. Drug Deliv. 2016;23(1):328-37. [PubMed] | [CrossRef] | [Google Scholar]

- Ham M, Moss AC. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev Clin Pharmacol. 2012;5(2):113-23. [PubMed] | [CrossRef] | [Google Scholar]

- Arévalo-Pérez R, Maderuelo C, Lanao JM. Recent advances in colon drug delivery systems. J Control Release. 2020;327:703-24. [PubMed] | [CrossRef] | [Google Scholar]

- Patole VC, Pandit AP. Mesalamine-loaded alginate microspheres filled in enteric coated HPMC capsules for local treatment of ulcerative colitis: in vitro and in vivo characterization. J Pharm Investig. 2018;48(3):257-67. [CrossRef] | [Google Scholar]

- D’Souza S, Faraj JA, Giovagnoli S, Deluca PP. Development of risperidone PLGA microspheres. J Drug Deliv. 2014;2014:620464 [PubMed] | [CrossRef] | [Google Scholar]

- Jin L, Ding YC, Zhang Y, Xu XQ, Cao Q. A novel pH-enzyme-dependent mesalamine colon-specific delivery system. Drug Des Dev Ther. 2016;10:2021-8. [PubMed] | [CrossRef] | [Google Scholar]

- Tenjarla S. Dissolution of commercially available mesalamine formulations at various pH levels. Drugs R D. 2015;15(2):211-5. [PubMed] | [CrossRef] | [Google Scholar]

- Mady O. Ibuprofen encapsulation by eudragit RS100 as microspheres: preparation and drug release. MOJ Bioequiv Availab. 2017;4(1):193-9. [CrossRef] | [Google Scholar]

- Karan S, Choudhury H, Chakra BK, Chatterjee TK. Polymeric microsphere formulation for colon targeted delivery of 5-fluorouracil using biocompatible natural gum Katira. Asian Pac J Cancer Prev. 2019;20(7):2181-94. [PubMed] | [CrossRef] | [Google Scholar]

- Hales D, Muntean DM, Neag MA, Kiss B, Ştefan MG, Tefas LR, et al. Curcumin-loaded microspheres are effective in preventing oxidative stress and intestinal inflammatory abnormalities in experimental ulcerative colitis in rats. Molecules. 2022;27(17):5680 [PubMed] | [CrossRef] | [Google Scholar]

- Jose S, Dhanya K, Cinu TA, Aleykutty NA. Multiparticulate system for colon targeted delivery of ondansetron. Indian J Pharm Sci. 2010;72(1):58-64. [PubMed] | [CrossRef] | [Google Scholar]

- Khera Brij, Kulkarni Sushrut Krishnaji, Kiran Hothur R. 2016 stable pharmaceutical compositions of mesalamine US2016/0045442. ;A1 [PubMed] | [CrossRef] | [Google Scholar]

- French DL, Mauger JW. Evaluation of the physicochemical properties and dissolution characteristics of mesalamine: relevance to controlled intestinal drug delivery. Pharm Res. 1993;10(9):1285-90. [PubMed] | [CrossRef] | [Google Scholar]

- Wong JM, Wei SC. Efficacy of Pentasa tablets for the treatment of inflammatory bowel disease. J Formos Med Assoc. 2003;102(9):613-9. [PubMed] | [Google Scholar]


