ABSTRACT
Background
Aegle marmelos, commonly known as bael, is a medicinal plant native to India with well-established use in traditional medicine systems.
Materials and Methods
This study aims to evaluate the antioxidant, anti-inflammatory and Anti-Cancer Properties of Gold Nanoparticles (AuNPs) synthesized from Aegle marmelos, a medicinal plant native to India. Gold nanoparticles were synthesized through a green synthesis method using extracts from Aegle marmelos leaves and fruits as reducing and stabilizing agents. The physicochemical properties of the AuNPs were characterized using UV-vis spectroscopy, Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Fourier-Transform Infrared Spectroscopy (FTIR), X-ray Diffraction (XRD) and energy-dispersive X-ray Spectroscopy (EDX). Antioxidant activity was assessed with DPPH, ABTS, reducing power, Hydrogen Peroxide (H2O2) scavenging and Ferric Reducing Antioxidant Power (FRAP) assays. Anti-inflammatory potential was evaluated through albumin denaturation and heat-induced hemolysis assays. Cytotoxicity against HT-29 cells was investigated using acridine orange staining and Lactate Dehydrogenase (LDH) assay.
Results and Discussion
The synthesized AuNPs demonstrated significant antioxidant activity across all tested assays, indicating effective combat against oxidative stress. Anti-inflammatory tests showed considerable inhibition of albumin denaturation and suppression of hemolysis, reflecting strong anti-inflammatory effects. Notably, the AuNPs exhibited substantial cytotoxicity against HT-29 cells, with concentration-dependent apoptosis and differential effects observed between nanoparticles derived from leaves and fruits.
Conclusion
AuNPs synthesized from Aegle marmelos leaves and fruits possess remarkable antioxidant, anti-inflammatory and anti-cancer properties. These findings highlight their potential as therapeutic agents in the fields of nano medicine and pharmacology, supporting further exploration of Aegle marmelos-derived AuNPs for clinical applications.
INTRODUCTION
Nanotechnology facilitates the fabrication of extremely small particles or devices that can interact with biological systems at the molecular and cellular levels (Danet al., 2023). Nanotechnology improves cancer therapies by increasing treatment specificity, lowering adverse effects and potentially overcoming medication resistance. Thus, the incorporation of nanoparticles into cancer therapy regimens provides hope for more effective, less intrusive and patient-friendly approaches to confronting cancer (Kemp and Kwon, 2021).
The Gold Nanoparticles (AuNPs) have sparked widespread interest in a variety of biomedical fields because of their distinct physicochemical features. These applications include sensors, diagnostics and therapy. Traditionally, AuNPs were synthesized via physical and chemical procedures, frequently involving hazardous substances that risk human health and pollute the environment (Ieloet al., 2021). However, green synthesis approaches have evolved to solve these difficulties in alternative and environmentally acceptable ways.
The utilization of plant metabolites is advantageous for the synthesis of nanoparticles because these are rich in a variety of phytochemicals in plant extracts, including flavonoids, terpenoids, alkaloids and phenolic compounds (Dikshitet al., 2021). These phytochemicals can give the nanoparticles additional functionality, such as increased biodegradability, specificity and the possibility of targeted drug administration. As a result, plant-based nanoparticles show significant promise in a range of areas, including medicine, where they are being studied for uses such as cancer therapy. Recently Prabhukumar Seetharaman et al., (2023) exhibited the promising anticancer activities of plant mediated AuNPs against breast cancer and bacterial infection.
Cancer is a significant public health concern around the world, affecting people from every aspect of life. It is characterized by excessive growth of cells and the ability to infiltrate or spread to other areas throughout the body. Cancer is a complicated category of diseases with variable prognoses and therapeutic obstacles (Maoet al., 2022). Despite breakthroughs in medical science that have improved detection and treatment options over time, cancer is still the leading cause of death worldwide. Mortality rates vary substantially depending on the cancer kind, stage of diagnosis and access to appropriate treatment options. Lifestyle, familial history and environmental factors all influence the prevalence of cancer and its consequences (Debelaet al., 2021).
Human colon cancer, a prevalent kind of malignancy in the gastrointestinal tract, is a major public health concern with serious consequences for patients and healthcare systems. Human colon cancer is usually curable when detected early, but if untreated or in an advanced stage, it can have serious side effects and raise mortality (Hossainet al., 2022). Complications of the condition include intestinal blockage, colon perforation and bleeding, all of which can result in anemia and severe weight loss. Furthermore, colon cancer can spread to other organs, most notably the liver and lungs, complicating treatment and dramatically worsening the prognosis (Katsaounouet al., 2022).
Despite advancements in established therapies such as chemotherapy, surgery and radiation therapy, the desire for better outcomes with fewer side effects has fuelled the search for alternative therapeutic procedures. The rising incidence and complex etiology of colon cancer necessitate novel techniques that not only efficiently target the disease but also work with the body’s natural metabolism to reduce adverse effects (Anandet al., 2023). The need for alternative cancer medicines has prompted developments in nanotechnology, opening new avenues in the fight against this prevalent disease.
Aegle marmelos, also known as bael or bael fruit, is a tropical fruit tree aboriginal to India that is grown extensively in South Asia, Southeast Asia and other tropical locations across the world. This tree belongs to the family Rutaceae and can grow 8-10 m high. The fruit of the Aegle marmelos tree is spherical, woody and yellowish-green when ripe. The leaves are enormous, glossy and green-coloured (Sharmaet al., 2022). The Aegle marmelos tree is esteemed for its traditional applications in medicine, cuisine and religious rites. Almost every part of the Bael tree has been employed in traditional Ayurvedic treatments for various health advantages (Subedi and Bashyal, 2022). Various parts of A. marmelos are all thought to have therapeutic characteristics and are used to cure a variety of conditions, including digestive disorders, respiratory issues, skin illnesses and diabetes (Pathiranaet al., 2020). The fruit of the A. marmelos tree is probably the most popular and commonly utilized portion of the plant. The bael fruit is high in critical nutrients, antioxidants and fiber, making it a popular treatment for digestive problems such as constipation and diarrhea. The fruit is typically eaten fresh or as juices and preserves. Hence, literature has documented the wide range of therapeutic benefits of A. marmelos (Sarkaret al.,2020). Aegle marmelos leaf extract showed its anticancer and antitumor effects on various cell lines including breast cancer cell line MCF7 and MDA-MB-231 and SKBR3 also fruit pulp extract against DMBA induced mammary cancer in Charles Foster rats (Lamprontiet al., 2003; Jagetiaet al., 2005; Akhouriet al., 2020). In this study, Gold Nanoparticles (AuNPs) were synthesized from extracts of A. marmelos leaves and fruits. The study aimed to compare the anti-cancer activities of these nanoparticles against the HT-29 cell line.
MATERIALS AND METHODS
Figure 1: Schematic representation of biosynthesis of gold nanoparticles using leaves and fruits of Aegle marmelos extracts and their characterization and comparative analysis followed by in vitro antioxidant, anti-inflammatory and anticancer activities.
Plant material collection and extract preparation
The Aegle marmelos leaves and fruits were freshly collected from in and around the local areas of Virundheeswarar temple, Vadamadurai, Coimbatore, Tamil Nadu, India. The collected leaves and fruits were washed using tap water followed by sterile deionized water. The leaves and fruits were dried separately at room temperature, and it was finely pulverized using the mechanical blender. The resultant powder was stored for further analysis.
Leaf extract preparation
2 g of finely ground leaves were weighed and mixed with deionized water (20 mL) in a beaker and positioned on a hot plate. The mixture was boiled at 70-80ºC for 1 hr and obtained extract was centrifuged at 5000 rpm for 15 min. The resultant supernatant was collected and filtered thoroughly through Whatman filter paper No. 1. The obtained mixture was used for synthesizing gold nanoparticles.
Fruit extract preparation
2 g of finely ground fruits were weighed and mixed with deionized water (20 mL) in a beaker and positioned on a hot plate. The mixture was boiled at 70-80ºC for 1 hr and obtained extract was centrifuged at 5000 rpm for 15 min. The resultant supernatant was collected and filtered thoroughly through Whatman filter paper No. 1. The obtained mixture was used for synthesizing gold nanoparticles (Pattanayak and Nayak, 2013).
Bio-reduction of gold nanoparticles
The aqueous extract of leaves and fruits of Aegle marmelos mixed with 20 mL of Auric chloride solution (1 mM) respectively. The reaction mixtures were incubated for 10 min at 40-60ºC. After around 10 min, the reaction mixture had changed colour to dark violet. This colour shift is the visual confirmation of the formation of Gold Nanoparticles (AuNPs) (Pattanayak and Nayak, 2013). The resultant violet-coloured solution was kept for further characterization tests.
| Concentration (µg/mL) | Percentage of Inhibition (%) | |
|---|---|---|
| Leaf-mediated AuNPs | Fruits mediated AuNPs | |
| 25 | 4.99±0.4 | 0.22±0.005 |
| 50 | 9.13±0.89 | 4.18±0.39 |
| 75 | 10.24±0.99 | 9.73±0.84 |
| 100 | 11.68±1.16* | 11.7±0.98* |
| 250 | 13.62±1.45** | 14.83±1.3* |
| 500 | 19.11±2.00* | 22.35±1.9* |
| 750 | 28.88±2.88* | 35.3±2.84* |
| 1000 | 39.26±3.9* | 39.55±3.12* |
DPPH radical scavenging assay of Aegle marmelos leaf and fruit extract mediated AuNPs.
Characterization of nanoparticles
UV vis Spectra analysis
The UV spectrum of the aqueous extract of Aegle marmelos leaves and fruits and its mediated AuNPs was recorded to examine the bio-reduction activity using UV-vis-NIR, Jasco, V-670. Spectral analysis of test samples was carried out on wavelengths that ranged from 380 to 800 nm.
Scanning Electron Microscopy (SEM)
The size and surface morphology of the resulting AuNPs of Aegle marmelos fruits and leaves were examined using a scanning electron microscope. The SEM data were captured using a ZEISS EVO instrument.
Transmission Electron Microscopy (TEM)
TEM was performed to examine the size, morphology and morphology of the biosynthesized AuNPs. In the present experiment, carbon pre-coated copper grids (200 mesh), acquired from Icon Analytical Equipment Pvt. Ltd., were employed. One drop of the sample solution, which was made up of water and scattered nanoparticles, was left on the grid surface and allowed to air dry at room temperature. The dried grids were analyzed at the necessary magnification using electron microscopes (FEI TECNAI G2 S-Twin TEM and JEOL 2000 FX-II TEM, USA).
Particle size analysis
Particle size, shape and associated Selected Area Electron Diffraction (SAED) were analyzed using TEM and a Japanese JEOL JEM-1200EX microscope with an acceleration voltage of 80 kV respectively.

Figure 1:
Schematic representation of biosynthesis of gold nanoparticles using leaves and fruits of Aegle marmelos extracts and their characterization and comparative analysis.
X-ray diffraction studies
The X-ray diffraction studies were performed for the AuNPs of Aegle marmelos leaves and fruits. All samples in the current study were characterized using the Rigaku Miniflex 600 X-ray diffractometer, Japan. Cu K radiation (1.5406) was used as the X-ray source, resulting in diffraction patterns in the 10-80º range with a step value of 0.02o at ambient temperature.
The average size was determined using the Debye–Scherrer equation:
Where D=thickness of the nanocrystal, k is a constant, λ= wavelength of X-rays, β= width at half maxima at Bragg’s angle 2θ.
Energy-Dispersive X-Ray Analysis
The EDAX analysis of AuNPs of Aegle marmelos leaves and fruits were carried out with an EDS X-ray spectrophotometer normally integrated with the current SEM (ZEISS EVO Scanning Electron Microscope, Germany).
Fourier Transform Infrared Spectrophotometer analysis
The FTIR analysis of the AuNPs of Aegle marmelos leaves and fruit was performed using Fourier-Transform Infrared (FTIR) Spectroscopy (SHIMADZU, IRSpirit-X, Germany) to validate the existence of functional groups. The transmittance spectrum was recorded in the wave number range of 4000-600 cm-1 at a resolution of 16 cm−1.

Figure 2:
Changes in colour after adding Auric chloride solution in the aqueous extracts of Aegle marmelos Leaf and fruit.

Figure 3a:
UV spectra of Aegle marmelos fruits mediated AuNPs.
| Concentration (µg/mL) | Leaf-mediated AuNPs | Fruits mediated AuNPs |
|---|---|---|
| 25 | 0.28±0.02 | 0.44±0.02 |
| 50 | 3.39±0.31 | 0.53±0.04 |
| 75 | 6.85±0.65* | 0.92±0.09 |
| 100 | 9.27±0.89* | 1.01±0.21 |
| 250 | 16.42±1.55* | 1.38±0.13* |
| 500 | 26.68±2.59* | 1.37±0.24* |
| 750 | 34.9±3.5* | 2.05±0.18* |
| 1000 | 40.51±4.0* | 2.54±0.25* |
FRAP assay of Aegle marmelos leaf and fruit extract mediated AuNPs.
Antioxidant activity
DPPH radical scavenging assay
The DPPH (1,1-Diphenyl-2-picrylhydrazyl) radical scavenging activity of the AuNPs of Aegle marmelos leaves and fruits was assessed according to the protocol of Shimadaet al., 1992. Various aliquots of AuNPs of Aegle marmelos leaves and fruits were taken in individual test tubes and 0.1mM of freshly prepared DPPH was added. The reaction mixture was incubated at 37ºC for 30 min. The discolouration of purple colour was recorded for each reaction mixture at λ=517 nm.
| Concentration (µg/mL) | % of inhibition | |
|---|---|---|
| Leaf-mediated AuNPs | Fruits mediated AuNPs | |
| 25 | 3.67±0.29 | 0.18±0.02 |
| 50 | 5.93±0.49 | 0.8±0.08 |
| 75 | 8.37±0.78 | 1.24±0.17 |
| 100 | 13.91±1.41 | 4.56±0.39 |
| 250 | 18.75±1.77* | 7.04±0.68* |
| 500 | 22.52±2.19* | 11.16±0.21* |
| 750 | 31.32±3.09* | 19.31±0.20* |
| 1000 | 38.95±3.76* | 23.96±0.22* |
H2O2 radical scavenging assayof Aegle marmelos leaf and fruit extract mediated AuNPs.
FRAP assay
The antioxidant potential of the AuNPs of Aegle marmelos was evaluated using FRAP assay. Different aliquots of biosynthesized AuNPs were mixed with FRAP reagent (3 mL). The test tubes containing the reaction mixture were incubated at 37ºC for 10 min. The test tubes were examined for the presence of an apparent blue colour, which indicates the reduction of Fe2+ ions to Fe3+ ions. The FRAP values were determined by measuring the absorbance at 593 nm (Benzie and Strain, 1996).

Figure 3b:
UV spectra of Aegle marmelos leaf mediated AuNPs.
| Concentration (µg/mL) | % of inhibition | |
|---|---|---|
| Leaf-mediated AuNPs | Fruits mediated AuNPs | |
| 25 | 18.64±1.8 | 4.66±0.38 |
| 50 | 23.42±2.21 | 8.03±0.91 |
| 75 | 28.52±2.90 | 13.79±1.29 |
| 100 | 36.26±3.19 | 19.78±1.81 |
| 250 | 44.28±4.50* | 25.43±2.65 |
| 500 | 49.07±4.87* | 29.41±2.9* |
| 750 | 53.34±5.19* | 36.18±3.58* |
| 1000 | 65.86±6.5* | 39.68±4.00* |
Reducing power assayof Aegle marmelos leaf and fruit extract mediated AuNPs.
H2O2 radical scavenging assay
The potential of the AuNPs of Aegle marmelos leaves and fruits to quench the hydrogen peroxide radicals was examined using the method of Ruchet al., 1983. The biosynthesized AuNPs of varying concentrations were added to the test tubes containing 10 mM hydrogen peroxide solution and kept for 10 min at 37ºC. The radical scavenging potency was assessed by taking the absorbance at the wavelength of 230 nm.
Reducing power assay
The Reducing power assay was carried out for the biosynthesized AuNPs according to Yen and Chen, 1995 with slight modifications. The test samples were added to the test tube containing 1% potassium ferrocyanide and 1% phosphate buffer. The reaction mixture was incubated at 50ºC for 20 min, followed by the addition of 10% trichloroacetic acid. The mixture was centrifuged for 10 min at 3000 rpm and the supernatant was collected. 1.5 mL of deionized water was added to the obtained supernatant (1.5 mL) and then 0.1% of ferric chloride was added and the absorbance was read using a UV vis spectrophotometer at 700 nm.
ABTS assay
Different concentrations of AuNPs of Aegle marmelos leaves and fruits were added to 950 μL of ABTS working solution followed by the addition of Phosphate buffered saline to make a final volume of 1 mL. After incubating the reaction mixture at room temperature for 4 min, the optical density was obtained using a UV-vis Spectrophotometer at 734 nm (Auddyet al., 2003).
| Concentration (µg/mL) | % of inhibition | |
|---|---|---|
| Leaf-mediated AuNPs | Fruits mediated AuNPs | |
| 25 | 9.83±0.9 | 0.08±0.0079 |
| 50 | 14.48±1.21 | 0.51±0.004 |
| 75 | 19.26±1.87 | 0.93±0.10 |
| 100 | 23.02±2.5 | 3.26±0.29 |
| 250 | 29.15±2.79* | 7.38±0.81* |
| 500 | 36.17±3.88* | 10.58±0.98* |
| 750 | 42.75±4.19* | 14.36±1.36* |
| 1000 | 61±6.0* | 19.61±2.01* |
ABTS assay of Aegle marmelos leaf and fruit extract mediated AuNPs.
| Concentration (µg/mL) | % of inhibition | |
|---|---|---|
| Leaf-mediated AuNPs | Fruits mediated AuNPs | |
| 25 | 2.04±0.18 | 0.0135±0.00009 |
| 50 | 8.07±0.78 | 15.321±1.49 |
| 75 | 13.1±1.19 | 20.41±2.11* |
| 100 | 17.12±1.71 | 28.162±2.67* |
| 250 | 24.22±2.22* | 31.439±2.99* |
| 500 | 31.49±3.09* | 36.296±3.62* |
| 750 | 39.62±3.76* | 40.774±3.91* |
| 1000 | 48.78±4.8* | 43.104±4.27* |
Inhibition of albumin denaturation assay of Aegle marmelos leaf and fruit extract mediated AuNPs.

Figure 4a:
SEM images of Aegle marmelos fruits mediated AuNPs.

Figure 4b:
SEM images of Aegle marmelos leaf-mediated AuNPs.

Figure 5a:
TEM images ofAegle marmelos fruits mediated AuNPs.

Figure 5b:
TEM images ofAegle marmelos leaf-mediated AuNPs.

Figure 6a:
Particle size analysis of Aegle marmelos fruits mediated AuNPs.

Figure 6b:
Particle size analysis of Aegle marmelos leaf-mediated AuNPs.

Figure 7a:
XRD analysis of Aegle marmelos fruits mediated AuNPs.

Figure 7b:
XRD analysis of Aegle marmelos leaf-mediated AuNPs.
Anti-inflammatory activity
Inhibition of albumin denaturation assay
The ability of the biosynthesized AuNPs to inhibit the denaturation of albumin was using the method of Govindappaet al., 2018 with some changes. 5% of bovine albumin solution was added to the varying aliquots of biosynthesized AuNPs. The reaction mixture was incubated for 20 min at 37ºC and then kept in a boiling water bath for 20 min at 50ºC. The reaction mixture was allowed to cool at room temperature and the absorbance was measured at the wavelength of 660 nm.
Heat-induced hemolysis assay
Blood (2 mL) was drawn from a healthy volunteer and the 10%v/v RBC suspension (10% v/v) was prepared using normal saline (Sakatet al., 2010; Sadique et al., 1989).
The inhibitory activity of the biosynthesized AuNPs of Aegle marmelos leaves and fruits against heat-induced hemolysis was done using the method of Anwaret al., 2021 with slight modification. 3 mL of RBC suspension was added to the biosynthesized AuNPs and incubated at 55ºC for 30 min. The test tubes containing the reaction mixture were centrifuged for 10mins and the absorbance of the obtained supernatant was measured at the wavelength range of 560 nm spectrophotometrically.
Anti-cancer activity
MTT assay
The anti-cancer potential of biosynthesized AuNPs of Aegle marmelos leaves and fruits against the HT-29 (Human colorectal cancer) cell line, which was procured from the National Centre for Cell Sciences (NCCS), Pune, India. The cultured cell line was propagated in MEM medium enhanced with Fetal Bovine Serum and kept in an incubator at 37ºC with 5% CO2. After 24 hr, the original growth medium was swapped out with a fresh batch containing various concentrations of biologically synthesized AuNPs and then the cell line was incubated for an additional 24 hr. Following this incubation, the entire plate underwent evaluation with an Inverted Phase Contrast Microscope and images were taken to document the findings. Notable alterations in cell morphology, such as cell rounding or shrinking, the formation of cytoplasmic vacuoles and granulation, were considered signs of cytotoxic effects. Subsequently, the well contents were discarded and 10 µL of MTT reagent (mg/mL) was added to each experimental and control well. The supernatant was then extracted and 200 µL of DMSO, which serves as an MTT solubilizer, was thoroughly combined to break down any undissolved formazan crystals. The optical density was gauged with a microplate reader at a wavelength of 570 nm (Van de Loosdrechtet al., 1994).
| Concentration (µg/mL) | % of inhibition | |
|---|---|---|
| Leaf-mediated AuNPs | Fruits mediated AuNPs | |
| 25 | 0.32±0.03 | 0.08±0.0079 |
| 50 | 2.32±0.31 | 16.16±1.54 |
| 75 | 6.43±0.65 | 24.54±2.61* |
| 100 | 8.76±0.79* | 35.37±3.49* |
| 250 | 10.46±0.99* | 40.85±3.99* |
| 500 | 14.76±1.51* | 56.26±5.78* |
| 750 | 17.65±1.69* | 67.65±7.10* |
| 1000 | 19.54±2.11* | 79.08±8.10* |
Heat induced hemolysis assay of Aegle marmelos leaf and fruit extract mediated AuNPs.
The percentages of viability (%V) and inhibition (%I) were calculated as:
Where, at-absorbance of treated cells; Ac-absorbance of control cells.
Detection of Apoptosis using Fluorescence Dyes
Apoptosis in the HT-29 cells treated with AuNPs of Aegle marmelos leaves and fruits were detected by staining with fluorescence dyes. The treated cells were washed with cold PBS and stained with 20 µg/mL of Acridine orange (20 µg/mL in PBS). A 10 µL aliquot of the gently mixed suspension was placed on microscope slides, covered with glass slips and examined under an inverted fluorescent microscope using a blue filter and photographed with a digital camera (Behzadet al., 2016).

Figure 8:
EDAX analysis of Aegle marmelos fruits mediated AuNPs.
| Concentration (µg/mL) | Leaf AuNPs | ||
|---|---|---|---|
| Cytotoxicity (%) | Cell Viability (%) | Cytotoxic Reactivity | |
| 20 | 28 | 72 | Slight |
| 40 | 42 | 58 | Mild |
| 60 | 49 | 51 | Moderate |
| 80 | 64* | 36 | Severe |
| 100 | 71* | 29 | Severe |
MTT assay of Aegle marmelos Leaf AuNPs.

Figure 9a:
FTIR spectrum of Aegle marmelos fruit extract mediated AuNPs.
Lactate Dehydrogenase (LDH Assay)
The LDH test kit was utilized in compliance with the guidelines provided by the manufacturer. First, 96-well plates were seeded with 1×104 HT-29 cells per well and left to adhere overnight. The cells were exposed to various quantities of AuNPs synthesized from the leaves and fruits of Aegle marmelos and then incubated at 37ºC for 24 hr. After incubation, the culture plate was centrifuged for 10 min at 1000 rpm and 50 µL of the cell culture supernatant from per well was cautiously moved to a fresh 96-well plate. Following the addition of 100 μL of freshly made LDH reaction mixture to each well, the plate was left to incubate at room temperature in the dark for 30 min and the absorbance was measured at 490 nm (Wypijet al., 2020).
Statistical analysis
Results are expressed as mean±SD for each experimental performed. All in vitro data were obtained from at least three independent experiments. Comparisons were made between the control and treated groups by one-way ANOVA test (SPSS 20) (SPSS Inc., Chicago, IL, USA) followed by Duncan’s test. p values less than 0.05 (P V 0.05) were considered statistically significant.
RESULTS
Bio-reduction of gold nanoparticles
The Aegle marmelos leaves and fruit extract effectively synthesized gold nanoparticles within a very short span of time. A. marmelos leaves and fruits extracts were incipiently green and pale-yellow coloured respectively and it was transmuted to vivid violet which indicated the formation of AuNPS (Figure 2). Therefore, the A. marmelos leaves and fruit extract can serve as electron shuttles for the reduction ofAu3+ ions to Au nanoparticles.
Characterization of nanoparticles
UV vis Spectra Analysis
The Analysis Of UV-vis Spectra for Gold Nanoparticles (AuNPs) synthesized with Aegle marmelos fruits and leaves revealed clear absorption peaks indicating the formation of nanoparticles. AuNPs synthesized with A. marmelos fruit extract exhibited a prominent peak at approximately 598 nm (Figure 3a), indicative of the presence of gold nanoparticles displaying characteristic Surface Plasmon Resonance (SPR) absorption. On the contrary, AuNPs of Aegle marmelos leaf extract showed a distinct absorption peak around 559 nm (Figure 3b), suggesting the formation of gold nanoparticles with a slightly varied size or structure, resulting in a shifted SPR peak. These spectroscopic features demonstrate the successful synthesis of gold nanoparticles utilizing A. marmelos fruit and leaf extracts as environmentally friendly reducing and stabilizing agents.
Scanning Electron Microscopy (SEM)
Figure 4a and 4b show the SEM photograph of AuNPs of Aegle marmelos fruits and leaves respectively. Both AuNPs produced at room temperature have an average size of 80 nm and a spherical form, as shown in the SEM image. A. marmelos fruits-mediated AuNPs were highly agglomerated whereas, the A. marmelos leaves-mediated AuNPs were clustered and dispersed.
Transmission Electron Microscopy (TEM)
TEM images of the synthesized AuNPs revealed distinctive morphologies. Interestingly, the majority of the nanoparticles exhibited hexagonal forms (Figure 5a and 5b), indicating the influence of the Aegle marmelos extracts on the shape-controlled synthesis of AuNPs. The hexagonal morphology observed in the TEM images suggests a high degree of crystallinity and uniformity in the synthesized nanoparticles. The average size of the A. marmelos fruits and leaf-mediated AuNPs were observed to be 50 nm and 100 nm.
Particle size analysis
Particle size analysis of the synthesized AuNPs revealed a broad size distribution, ranging from 8 nm to 35 nm. The broad distribution suggests the presence of polydispersity in the synthesized nanoparticles. The median diameter considered the average particle diameter, was found to be approximately 15 nm (Figure 6a and 6b). The observed variation in particle sizes could be attributed to several factors, including the composition of the plant extracts, reaction conditions and kinetics of nucleation and growth during synthesis.
X-ray diffraction studies
XRD analysis of the AuNPs of Aegle marmelos fruits and leaves revealed distinct peaks in the XRD pattern respectively, indicating the crystalline lattice of the nanoparticles (Figure 7a and 7b). The prominent peaks observed at 2θ angles of 44.23º, 37.25º and 88.23º aligns with the (111), (200) and (220) crystallographic planes of gold, respectively. These peaks are characteristic of Face-Centered Cubic (fcc) gold structure, confirming the crystallinity of the synthesized AuNPs. The absence of impurity peaks in the XRD pattern further supports the purity of the synthesized nanoparticles.
Energy-Dispersive X-ray Analysis
For elements with atomic numbers (Z) greater than 3, Energy Dispersive X-ray Spectroscopy (EDX) was used to gather data on the chemical makeup of samples. EDX analysis of the synthesized AuNPs revealed characteristic absorption peaks in the spectra. Notably, each AuNPs spectrum displayed a prominent absorption peak at 2.2 keV, which is typical of gold absorption Figure 8. This peak corresponds to the characteristic X-ray emission line of gold (Au), confirming the presence of gold in the synthesized nanoparticles.
FTIR analysis
In A. marmelos fruit extract-mediated AuNPs, the IR absorption peak at 1646.81 cm-1 corresponds to the C=O stretching vibration of carboxylic acids, slightly shifted compared to the fruit extract peak (Figure 9a). The IR spectrum at 3179.26 cm-1, 3350.80 cm-1, 3436.57 cm-1 and 3550.93 cm-1 indicate the presence of -OH stretching vibrations, suggesting the involvement of hydroxyl groups in the stabilization of AuNPs. In the context of AuNPs (Figure 9b), the peak 1646.81 cm-1could be related to the capping agents or stabilizers used during nanoparticle synthesis. The absorption band at 3299.34 cm-1 in fruit-mediated AuNPs indicated the O-H stretching. Hence, most of the absorption peaks present in the A. marmelos fruits and leaves have also existed in the AuNPs. The slight deviation in the IR absorption peaks in the FTIR spectrum of fruits and leaves extract may be due to the formation of AuNPs.
| Concentration (µg/mL) | Fruit AuNPs | ||
|---|---|---|---|
| Cytotoxicity (%) | Cell Viability (%) | Cytotoxic Reactivity | |
| 20 | 11 | 89 | Slight |
| 40 | 22 | 78 | Mild |
| 60 | 37 | 63 | Moderate |
| 80 | 42 | 58 | Moderate |
| 100 | 64 | 36 | Severe |
MTT assay of Aegle marmelos fruits AuNPs.
Antioxidant activity
DPPH radical scavenging assay
The inhibition of DPPH radical by the biosynthesized AuNPs was in a concentration-related manner (Table 1). Both the Aegle marmelos leaves and fruits mediated AuNPs have exhibited equivalent DPPH scavenging activity. The highest percentage of DPPH inhibition was recorded for fruit-mediated AuNPs (39.55%) at the concentration of 1000 µg/mL, it was 39.26% for leaves-mediated AuNPs at the same concentration. The lowest percentage of inhibition was found to be 4.99% and 0.33% at the concentration of 25 µg/mL for leaves and fruits mediated AuNPs respectively. The IC50 value was 1408.36 µg/mL for leaves-mediated AuNPs and 1214.94 µg/mL for fruits-mediated AuNPs.
FRAP assay
The FRAP assay revealed that the leaf-mediated AuNPs have more significant ferric-reducing antioxidant power than the fruit-mediated AuNPs. The maximum FRAP value of leaves and fruits mediated AuNPs were recorded as 40.51% and 2.54% respectively at the concentration of 1000 µg/mL (Table 2).
Each result represents the mean±Standard Deviation (SD) and each of these was performed in triplicate. The results were considered statistically significant at ‘*’ p<0.05.
H2O2 radical scavenging assay
The leaves and fruits mediated AuNPs quenched the hydrogen peroxide radicals effectively. The highest inhibition activity was obtained for leaves and fruits mediated AuNPs were 38.95% and 23.96% respectively. The lowest inhibitory percentage recorded was 3.67% for leaves-mediated AuNPs and 0.18% for fruits-mediated AuNPs at the concentration of 25 µg/mL (Table 3). The biosynthesized AuNPs showed concentration-related hydrogen peroxide radical scavenging activity.

Figure 9b:
FTIR spectrum of Aegle marmelos leaf extract mediated AuNPs.
| Concentration (µg/mL) | % of LDH Leakage | ||
|---|---|---|---|
| Standard | AuNPs of Aegle marmelos leaves | AuNPs of Aegle marmelos fruits | |
| 25 | 11 | 4 | 7 |
| 50 | 26 | 11 | 16 |
| 75 | 34* | 23* | 25* |
| 100 | 48* | 29* | 32* |
Lactate Dehydrogenase (LDH Assay) of Aegle marmelos leaf and fruit extract mediated AuNPs.

Figure 10a:
Morphological changes in HT-29 cells treated with AuNPs synthesized using Aegle marmelos leaves.
Reducing power assay
The antioxidant activity of the biosynthesized AuNPs was assessed using the reducing power assay, which measures the capability of antioxidants to provide electrons and restore oxidized compounds. The data of the reducing power of the leaves as well as fruits mediated AuNPs elevated with increasing concentration of nanoparticles. The maximum percentage of inhibition was found to be 65.86% and 39.68% for leaves and fruits mediated nanoparticles respectively (Table 4).
ABTS assay
The antioxidant activity of the leaves and fruits mediated AuNPs was examined using the ABTS assay (Table 5), which measures the ability of antioxidants to scavenge the ABTS radical cation and inhibit its formation. As shown in Table 5, the percentage of inhibition was increase with increasing concentrations of the biosynthesized nanoparticles, indicating higher antioxidant activity. Quantitative analysis revealed that leaf-mediated AuNPs exhibited the highest antioxidant activity, with an inhibition percentage of 61% at a concentration of 1000 µg/mL. Fruits-mediated AuNPs showed minimal antioxidant activity, with an inhibition percentage of 19.61%.
Anti-inflammatory activity
Inhibition of albumin denaturation assay
The anti-inflammatory activity of the leaves and fruits mediated AuNPs was examined using the inhibition of albumin denaturation assay, which measures the capability of the biosynthesized AuNPs to prevent albumin denaturation, a protein marker for inflammation. Table 6 shows that both leaves and fruits mediated AuNPs inhibited albumin denaturation to variable degrees at different concentrations.
The data revealed that leaves-mediated AuNPs demonstrated the highest percentage inhibition of albumin denaturation, with values ranging from 2.04-48.78% at concentrations of 25-1000 µg/mL. The fruits-mediated AuNPs showed moderate inhibition, with percentage inhibition values of 0.013-43.10%. The anti-inflammatory activity of both biosynthesized AuNPs was considerable, which displayed their potential to mitigate inflammation.
Heat-induced hemolysis assay
The anti-inflammatory activity of leaves and fruits mediated gold nanoparticles was evaluated using the heat-induced hemolysis assay, which assesses their ability to prevent Red Blood Cell (RBC) membrane damage, a marker of inflammation. The fruits-mediated AuNPs exhibited significant inhibition of hemolysis, with a percentage inhibition of 79.08% at a concentration of 1000 µg/mL. The leaf-mediated AuNPs have a percentage inhibition of 19.54%. Both the biosynthesized AuNPs showed a dose-dependent response, with higher concentrations resulting in increased inhibition of hemolysis (Table 7).

Figure 10b:
Morphological changes in HT-29 cells treated with AuNPs synthesized using Aegle marmelos fruits treated at the IC50 concentrations.
Anti-cancer activity
MTT assay
The anticancer activity of leaves and fruits mediated AuNPs against the HT-29 cell line was evaluated using the MTT assay, which assesses cell viability and proliferation. The morphological changes occurred after incubation with leaves and fruits mediated AuNPs in different concentration were illustrated along with the IC50 concentrations were observed under the phase contrast microscope and the images are presented in in Figure 10a and 10b. There were no changes in the morphology of the cells observed in the control group while the cells exhibited significant morphological changes like roundedness, which is a characteristics of stressed cells, irregular shape, cytoplasmic vacuolation and enlargement of the cells in AuNPs treated group. The leaf-mediated AuNPs demonstrated significant inhibition of cell viability, with a decrease in percentage cell viability to 29% at a concentration of 100 µg/mL. The fruit-mediated AuNPs also exhibited notable inhibition, with a decrease in percentage cell viability to 36%. The leaves, as well as fruits mediated AuNPs, displayed a dose-dependent effect on cell viability, with higher concentrations resulting in greater inhibition of cell proliferation. The anticancer activity of leaves and fruits mediated AuNPs was compared with control groups, indicating a significant reduction in cell viability compared to untreated cells (Table 8a and 8b).
Arrow heads point to the morphological changes induced by AuNPs synthesized using Aegle marmelos leaves.
Arrow heads point to the morphological changes induced by AuNPs synthesized using Aegle marmelos fruits.
Detection of Apoptosis using Fluorescence Dyes
In this study, we examined the induction of apoptosis in HT-29 cells treated with Gold Nanoparticles (AuNPs) synthesized from Aegle marmelos leaves and fruits, at varying concentrations (25 µL, 50 µL, 75 µL and 100 µL), using Acridine Orange (AO) staining to assess cellular fluorescence patterns (Figure 11a and b). The control cells displayed predominantly green fluorescence, indicating intact double-stranded DNA and healthy cellular morphology. In cells treated with increasing concentrations of AuNPs, particularly at 100 µL, minimal green fluorescence was observed, suggesting a potential disruption of DNA integrity or cellular stress. Interestingly, the degree of fluorescence was consistently lower in cells treated with AuNPs derived from Aegle marmelos leaves compared to those treated with AuNPs from fruits of the same plant species. This difference in fluorescence intensity suggests a differential apoptotic response induced by AuNPs depending on their source within the A. marmelos plant.
Arrow heads point to the morphological changes induced by AuNPs synthesized using Aegle marmelos leaves.
Lactate Dehydrogenase (LDH Assay)
The LDH assay results demonstrated the varying effects of Gold Nanoparticles (AuNPs) synthesized from Aegle marmelos fruits and leaves on LDH leakage in HT-29 cells across different concentrations (Table 9). The standard control exhibited increasing LDH leakage percentages with higher concentrations: 11% at 25 µL, 26% at 50 µL, 34% at 75 µL and 48% at 100 µL. In comparison, cells treated with AuNPs derived from A. marmelos fruits displayed lower LDH leakage: 4% at 25 µL, 11% at 50 µL, 23% at 75 µL and 29% at 100 µL. Conversely, HT-29 cells treated with AuNPs using Aegle marmelos leaves showed higher LDH leakage percentages: 7% at 25 µL, 16% at 50 µL, 25% at 75 µL and 32% at 100 µL. These results indicate a concentration-dependent response for both types of AuNPs, with AuNPs from Aegle marmelos fruits exhibiting lower cytotoxicity compared to the standard control, while AuNPs from A. marmelos leaves induced higher LDH leakage at equivalent concentrations.

Figure 11a:
Detection of apoptosis in HT-29 cells treated with AuNPs synthesized using Aegle marmelos leaves with different concentrations.
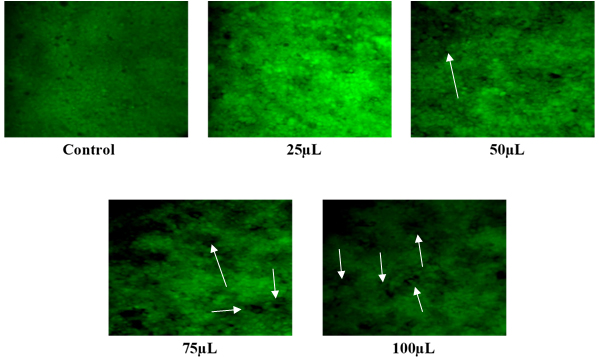
Figure 11b:
Detection of apoptosis in HT-29 cells treated with AuNPs synthesized using Aegle marmelos fruits with different concentrations.
Arrow heads point to the morphological changes induced by AuNPs synthesized using Aegle marmelos fruits.
DISCUSSION
Gold Nanoparticles (AuNPs) have emerged as one of the most extensively investigated nanomaterials due to their distinct physical, chemical and optical properties. These nanoparticles hold a wide range of applications across numerous sectors owing to their extraordinary adaptability and tunable capabilities at the nanoscale (Hammamiet al., 2021). Plants are abundant sources of bioactive substances with intrinsic reducing and capping capabilities. These biomolecules function as both reducing agents, converting metal ions into nanoparticles (Adeyemiet al., 2022). In this study, we aimed to investigate the biological properties of Aegle marmelos AuNPs and elucidate their potential applications in combating oxidative stress, inflammation and cancer. The gold nanoparticles were synthesized in just a few min because of the swift reduction of chloroaurate ions facilitated by the A. marmelos fruit and leaves extracts. These findings highlight that utilizing plant extracts for nanoparticle biosynthesis is rapid, cost-effective, environmentally friendly and safe for potential human therapeutic applications (Samuelet al., 2022). As a result, this eco-friendly technique of nanoparticle synthesis has a substantially lower environmental impact than standard physical, chemical and microbiological techniques. Analysis of research indicates that the biomolecules found in plant extracts play a key role in both reducing and capping the nanoparticles (Bhardwajet al., 2020).
Biosynthesized gold nanoparticles excite surface plasmon vibrations, causing the solution to shift colour from light yellow to dark violet (Bhardwajet al., 2020). UV-vis spectroscopy was utilized to characterize metal nanoparticles based on the surface Plasmon resonance phenomena. The produced Vitis vinifera fruit peel-mediated AuNPs displayed an absorption peak at 540 nm (Nirmalaet al., 2017). The AuNPs synthesized using the leaf extract ofA. muricata exhibited the gold absorbance peak at 530 and 538 nm (Folorunsoet al., 2019). Similarly, the plasmon absorbance peaks were obtained at 525-540 nm for the AuNPs of Ziziphus zizyphus leaf extract (Aljabaliet al., 2018). Our finding also corresponds to the study of Majumdaret al., 2019 UV spectral peak has occurred at 544 nm for AuNPs of Citrus macroptera fruit Extract.
SEM images of biosynthesized AuNPs of Aegle marmelos leaf extract was observed to be triangle-shaped (Arunachalamet al., 2014). However, the SEM and TEM results revealed that the A. marmelos leaf extract-mediated AuNPs doped with copper sulphate were spherically formed (Anuradha and Manimekalai, 2019). Previous studies have reported the role of phytochemicals present in plant extracts in mediating the shape and size of AuNPs (Mubeenet al., 2022). The hexagonal shape observed in this study may be attributed to specific bioactive compounds present in Aegle marmelos extracts, which act as reducing and capping agents, influencing the nucleation and growth kinetics of AuNPs. X-ray Diffraction (XRD) investigation is used to determine the composition, physical characteristics and crystal structure of nanomaterials. The XRD pattern showed five distinct peaks which reflect the face-centered cubic configuration of the AuNPs synthesized using the aqueous extract of Marsilea quadrifoli (Balashanmugamet al., 2018).
Likewise, Devendrapandiet al., 2023 also reported the XRD pattern of AuNPs synthesized from the Aegle marmelos fruit juice. Lakshmi, 2023 also employed EDX studies to characterize the formation of AuNPs from aqueous extract of A. marmelos seeds. The EDX results corroborate the X-ray Diffraction (XRD) findings, further confirming the elemental composition and purity of the synthesized AuNPs. In the present study, FT-IR spectroscopy was used to determine functional groups in Aegle marmelos leaves and fruit extracts that reduce Au3+ ions and serve as a capping agent for AuNPs. Similarly, Arunachalamet al., 2014 FTIR spectra of green synthesized AuNPs with phytochemicals of Aegle marmelos leaf extract showed peaks at 1618, 1452 and 1378 cm-1 corresponding to the ketones, aldehydes and carboxylic acids respectively.
Antioxidant activity refers to the ability of certain compounds to reduce or prevent the adverse effects of free radicals in the body. Medicinal plants include a wide range of secondary metabolites that have a variety of therapeutic uses and are reliable sources of antioxidants (Pancheet al., 2016). Plant extracts with active phytoconstituents synthesize stable AuNPs without hazardous reducing agents and are subsequently utilized for biological purposes (Nirmalaet al., 2017). The antioxidant activity of the Aegle marmelos leaf extract was assessed by DPPH and FRAP assay (Ahmadet al., 2021). Recently, Rahmanet al., 2024 also evaluated the antioxidant potential of A. marmelos fruit pulp using DPPH, FRAP and ABTS assays.
DPPH is a persistent free radical that is commonly employed to test the radical quenching properties of compounds. This approach is based on reducing DPPH utilizing a hydrogen-donating antioxidant, which results in the production of the non-radical DPPH-H (Gulcin and Alwasel, 2023). According to Ohet al., 2018 the AuNPs of aqueous extract of Chaenomeles sinensis fruits exhibited DPPH radical scavenging activity. The DPPH activity of the AuNPs of Rauwolfia serpentine leaf extract was also reported by Alshahraniet al., 2021.
The FRAP (Ferric Reducing Antioxidant Power) assay is a standard method for determining the antioxidant properties of medicinal plants and other natural substances (Munteanu and Apetrei, 2021). This assay evaluated the capacity of the AuNPs of Aegle marmelos fruits and leaves to convert ferric to ferrousions in a redox reaction with a reagent 2, 4, 6-Tripyridyl-s-Triazine (TPTZ). Another study reported the AuNPs synthesized using Pancratium parvum bulb extract exhibited concentration-dependent FRAP activity (Patilet al., 2021). The present study also evaluated the hydrogen peroxide scavenging activity of the AuNPs mediated from A. marmelos leaves and fruits. Because hydrogen peroxide enters the body through inhalation, ocular or skin contact. H2O2 degrades quickly in the body into oxygen and water, leading in the generation of hydroxyl radicals, which can drive lipid peroxidation and damage DNA (Andréset al., 2022).
Reducing power is associated with antioxidant activity, so the compounds with reducing power donate electrons and can reduce the oxidized precursors of lipid peroxidation processes (Spiegelet al., 2020). The oxidation of ABTS produces the ABTS radical, which can be used to assess the antioxidant effectiveness of chain-breaking antioxidants. The AuNPs formed Terminalia bellirica Fruit Extract inhibited ABTS radicals effectively (Annavaramet al., 2017).
In vitro anti-inflammatory tests, such as albumin denaturation inhibition and heat-induced hemolysis, are widely used to assess the potential anti-inflammatory characteristics of drugs, extracts and formulations. These tests measure the ability of test compounds to reduce two types of inflammation such as protein denaturation and cell membrane damage (Eshoet al., 2021). Kumaret al., 2021 studied the anti-inflammatory properties of several components of Aegle marmelos.
Methanolic extracts from Aegle marmelos leaves have been investigated. The protein denaturation test showed excellent anti-inflammatory outcomes. According to the findings of Uzma et al., (Nirmalaet al., 2017) the AuNPs mediated from Commiphora wightii also inhibited albumin denaturation in a concentration-related manner. Heat-induced hemolysis assay assessed the capacity of AuNPs of Aegle marmelos leaves and fruits to safeguard red blood cell membranes against heat-induced damage, which replicates the oxidative stress and membrane instability seen in inflammatory instances (Aidooet al., 2021). In this study, the results demonstrated that both leaf and fruit extract-mediated AuNPs exhibit significant cytotoxic effects against HT-29 cells, suggesting their promising role as potential anticancer agents. The observed cytotoxicity of Aegle marmelos AuNPs against HT-29 cells could be attributed to the synergistic effects of biomolecules such as phenols, alkaloids, flavonoids assumed to have anti-proliferative activities capped on the leaves and fruits gold nanoparticles. Our study also aligns with previous studies reporting the anticancer properties of various plant-mediated nanoparticles. Similarly, in the work done by Vijayakumar 2019 the A. marmelos fruits mediated AuNPs exhibited increased cytotoxicity against the MCF-7 cell line upon increasing concentration of nanoparticles. According to the findings of Devendrapandiet al., 2023. AuNPs of A. marmelos fruits inhibited the lung cancer cell line (A-549). Recently we have reported the various anticancer properties of Aegle marmelos fruits and leaf extracts (Sivakumaret al., 2024).
Acridine orange is an important dye that can stain the DNA in the cells with intact membranes (Vairavelet al., 2020). In this study, the apoptosis was produced by AuNPs treatments, as seen by the development of green colour. Lactate Dehydrogenase (LDH) is a common indicator for cytotoxicity assays since it is a key sign of irreversible cell death when released from cells (Al-Zharaniet al., 2021). The consistency of LDH release is a key criterion for establishing apoptosis and cytotoxicity and the amount of intracellular LDH changes the accessibility of cellular membranes. The LDH leakage was observed in the HT-29 cells treated with AuNPs of Terminalia bellirica fruits (Namasivayamet al., 2021). Furthermore, the differential cytotoxicity observed between leaf and fruit extract-mediated AuNPs may be attributed to variations in phytochemical composition and nanoparticle characteristics.
CONCLUSION
Our present article has demonstrated the potential of gold Nanoparticles (AuNPs) synthesized from Aegle marmelos leaves and fruits as versatile therapeutic agents with antioxidant, anti-inflammatory and anti-cancer properties. Through a comprehensive synthesis and characterization process, we successfully developed AuNPs with well-defined physicochemical properties, including size, shape and surface chemistry, which are crucial for their biological activities. The antioxidant assays revealed the potent radical scavenging and reducing abilities of Aegle marmelos AuNPs, indicating their efficacy in mitigating oxidative stress-related damage. Furthermore, the anti-inflammatory assays demonstrated the nanoparticles’ ability to suppress inflammatory processes, offering potential therapeutic applications in inflammatory disorders. Additionally, the cytotoxic effects of Aegle marmelos AuNPs against cancer cell lines, such as HT-29, highlight their promising role as novel anti-cancer agents.
Cite this article:
Mrudhulla S, Narendhirakannan R.T. Green Synthesis of Gold Nanoparticles from Aegle marmelos Extract: Evaluation of Antioxidant, Antiinflammatory and Anticancer Properties. Int. J. Pharm. Investigation. 2025;15(2):10-8.

