ABSTRACT
Background
An accurate, rapid, inexpensive, and straightforward, reliable simultaneous assay technique was developed and demonstrated to evaluate Levodopa and Benserazide using RP-HPLC.
Materials and Methods
The suggested approach achieved efficient chromatographic separation at a flow rate of 1 mL/min with a wavelength of 228 nm using a Luna phenyl hexyl column (250 mmx4.6 mm, 5 µ), acetonitrile and 0.1% formic acid as the mobile phase.
Results and Discussion
The retention time for Levodopa was 2.457 min, whereas that for Benserazide was 3.887 min. Isocratic chromatography was carried out in around six min at room temperature. Using a mathematical technique, we were able to determine that the system’s suitability parameters fall within reasonable bounds. In a linear analysis, stages with regression coefficients of 0.999 were used. The drug recovery rate was between 98%-102%, which is within acceptable range.
Conclusion
The method was determined to be appropriate for bulk and formulation analysis, and the validation findings were positive. In accordance with ICH recommendations, the suggested action is justified.
INTRODUCTION
A number of plant and animal species, including humans, produce and use L-DOPA (levodopa, L-3,4-dihydroxyphenylalanine) as part of their regular biological processes. Produced via the metabolism of the amino acid L-tyrosine, L-DOPA is used by humans and certain other animals. Dopamine, noradrenaline (Mareev and Cleland, 2015), and adrenaline are all neurotransmitters (Teleanuet al., 2022; Cuevas, 2019) that originate from L-DOPA. This group of chemicals is called a catecholamine (Eisenhoferet al., 2004). In addition, L-DOPA facilitates the release of neurotrophic factors by the central nervous system (Miller and Zachary, 2020; Hiroshimaet al., 2014) and brain. The biochemical route that creates a family of pigments called betalains (Polturaket al., 2016; Gonçalveset al., 2012) begins with L-DOPA in some plant families (of the order Caryophyllales). It is possible to produce L-DOPA and sell it as a psychoactive medicine under many brand names, such as Sinemet, Pharmacopa, Atamet, and Stalevo, when combined with the INN levodopa. It is the pharmacological therapy of choice for dopamine-responsive dystonia (Weissbachet al., 2022; Nygaardet al., 2021) and Parkinson’s disease (Skjærbaeæket al., 2021; Warneckeet al., 2022). A chirality-opposite analogue of L-DOPA is D-DOPA. The human body can only synthesise the L-DOPA version of this chemical, as is the case with many others. While dopamine is unable to traverse the protective blood-brain barrier (Kadryet al., 2020; Harilalet al., 2020), L-DOPA may be tested for enantiomeric purity using optical rotation determination (Plumet al., 2016) or chiral thin-layer chromatography (Singhet al., 2016). Patients with Parkinson’s disease, Parkinsonism, dopamine-responsive dystonia, or Parkinson-plus syndrome may benefit from L-DOPA since it raises blood levels of dopamine.
One such agent that fails to penetrate the blood-brain barrier is benserazide, an inhibitor of aromatic L-amino acid decarboxylase or DOPA decarboxylase that acts peripherally. It goes by the names Madopar and Prolopa, and it is used to control Parkinson’s disease in conjunction with L-DOPA (Levodopa) as co-Beneldopa (BAN). The therapy of restless leg syndrome (Silberet al., 2021; Riccardiet al., 2023) also makes use of these combinations. Administering levodopa, a precursor of dopamine, raises levels of the neurotransmitter in the brain and spinal cord. Nevertheless, the majority of levodopa undergoes decarboxylation to dopamine prior to entering the brain. Since dopamine cannot penetrate the blood-brain barrier, this results in little therapeutic benefit coupled with significant peripheral side effects. Because benserazide blocks the aforementioned decarboxylation, dopamine may accumulate exclusively in the brain without ever crossing the blood-brain barrier. Reduced are peripheral dopamine-induced side effects including nausea, arrhythmia (Kuck, 2020; Batra and Balaji, 2019), and vasoconstriction (Moodley, 2017; Swenson, 2013). The centrally mediated adverse effects of levodopa, especially dyskinesia (Haljeet al., 2012; Fabbriniet al., 2007), cannot be alleviated by benserazide. Levodopa and Benserazide’s chemical structures are shown in Figure 1.
One HPLC (Madhusudan et al., 2020) technique has been documented in the literature so far. As a result, we came up with a way to measure both Levodopa and Benserazide at the same time. The in vitro approach was used to estimate the combination medicines using the devised HPLC method.
MATERIALS AND METHODS
Chemicals
Acetonitrile (HPLC grade), formic acid (purity-99.9%), and water were acquired from Merck (India) Ltd., Mumbai, India (HPLC grade). APIs (purity-99.9%) of Levodopa and Benserazide were given by Glenmark Pharmaceutical Private Ltd., Andheri, Mumbai, India.
Equipment
We used a quaternary pump HPLC of Alliance model and a PDA detector of 2998 in conjunction with chromatographic system. The chromatographic information was analyzed using Empower software version 2.0.
Chromatographic Conditions
Luna Phenyl Hexyl (250 mmx4.6 mm, 5 µ) column was used for the separation, and it was carried out in isocratic mode at room temperature. In this study, the mobile phase consisted of a mixture of acetonitrile and 0.1% formic acid (20:80 v/v) flowing at a rate of 1 mL/min. It was determined that 228 nm was the optimal injection wavelength for both Levodopa and Benserazide, and a volume of 10 µL was used for the injection. Therefore, 228 nm was chosen as the appropriate wavelength.
Diluent
Acetonitrile and 0.1% Formic acid (50+50).
Preparation of standard solution
Working standards of Levodopa (20 mg) and Benserazide (5 mg), were added to a 10 mL flask and diluted with the diluent to the desired amount. Further dilute 1 mL of the above solution to 10 mL using diluents.
Preparation of sample solution
Accurately weigh 33 mg of Levodopa and Benserazide sample (Each tablet contains 200 mg of Levodopa and 50 mg of Benserazide), was added to a 10 mL flask and diluted with the diluent and sonicate to 20 min. Further dilute 1 mL of the above solution to 10 mL using diluents.
RESULTS
Method validation
After adjusting the parameters of the chromatographic run for specificity, plate count, tailing factor, and retention time, we found that the best isocratic condition for eluting Levodopa and Benserazide was with a Luna phenyl hexyl column and a mobile phase of 0.1% formic acid and Acetonitrile in a ratio of 80:20. If the proportion of mobile phase utilised was high, the resultant chromatogram will show background noise or peaks that indicate the tailing phenomenon. The chromatographic settings used in the procedure are shown in Table 1.
Specificity
Prior to the retention of the molecules for the allotted duration, Levodopa and Benserazide were not covered. Figure 2 depicts a blank chromatogram.
System suitability
A 60-min period of stabilisation was carried out to establish a stable baseline. Six injections of Levodopa (200 µg/mL) and Benserazide (50 µg/mL), were administered to test the system’s compatibility. After accounting for tailing, the theoretical plate counts for Levodopa and Benserazide were calculated to be 5634 and 3897 respectively and the tailing factors were 1.15 and 1.02. It was decided that these values were suitable. chromatographic software will be used to collect all of the data (Empower 2.0). Figure 3 shows a standard chromatogram, and Table 2 details the system accuracy achieved.
Linearity
To evaluate the linearity of the strategy, we made a reference solution comprising Levodopa and Benserazide at 200 µg/mL and 50 µg/mL (100% of the targeted level of the assay concentration). This required a series of dilutions of the provided solutions from 25%, 50%, 75%, 100%, 125%, 150% of the desired concentration. Because the peaks were artificially inflated, they may be used to plot calibration curves over the actual data. This pair of analytes was found to have a correlation value of 0.999. Calibration plots for Levodopa and Benserazide are presented in Figure 4 and Table 3 presents the results of the linearity testing. A linearity calculation sheet was used to get the values for slope, intercept, and correlation coefficient.
| Parameter | Proposed method |
|---|---|
| Stationary Phase | Luna phenyl hexyl (150 x 4.6 mm, 3.5 µ) |
| Mobile Phase | 0.1% Formic acid: Acetonitrile (80:20) |
| Injection Volume | 10 µL |
| Flow Rate | 1.0 mL/min |
| Column Temperature | Ambient |
| Wave Length | 228 nm |
| Run Time | 6.0 min |
| Retention time of Levodopa | 2.457 min |
| Retention time of Benserazide | 3.887 min |
Optimized chromatographic conditions.

Figure 1:
Chemical structures of (A) Levodopa and (B) Benserazide.
Limit of detection and quantification
The limit of detection and quantification is the lowest concentration at which an analyte can be identified and measured accurately. The Lowest Detectable doses (LOD) of Levodopa and Benserazide were respectively 0.6 µg/mL and 0.15 µg/mL, with corresponding S/N values of 3, 3 respectively. The LOQ concentrations of Levodopa and Benserazide were 2 µg/mL, 0.5 µg/mL and S/N values of 10 and 10 respectively. Here S/N is the signal-to-noise ratio.
Precision
To gauge the accuracy of the procedure, six replicates of the same batch were made. Maximum responses from each of these six independent standards were injected with a solution, so we can compute their mean and % RSD values. A Relative Standard Deviation (RSD) of less than 2% was obtained using this approach, demonstrating its precision. Sample chromatogram was shown in Figure 5 and method precision results were shown in Table 4.
Accuracy
Accuracy tests in three different concentrations confirmed the treatment’s efficacy (50%, 100% and 150%). The assay was carried out by injecting the solution into three solutions of increasing concentration, as per the prescribed test procedure. Values for recovery ranged from 99.6 to 100.1% for Levodopa and from 99.9 to 100.0% for Benserazide. The results for accuracy are shown in Table 5.
Ruggedness
In order to determine whether there was a difference in the chromatographic patterns, effects of changing the HPLC technique, observer and column. The fact that the RSD rate was less than 2% is a testament to the reliability of the time-tested procedure. Table 6 shows the results of the ruggedness tests.
| Parameter | Levodopa | Benserazide |
|---|---|---|
| Theoretical plate count | 5634 | 3897 |
| Tailing factor | 1.15 | 1.02 |
| Resolution | – | 7.68 |
| Retention time | 2.457 min | 3.887 min |
Results of system suitability.

Figure 2:
Chromatogram of blank.

Figure 3:
Chromatogram of standard.
Robustness
Tests conducted by RSD showed that just 2% of RSD were attracted by the strategy despite its apparent durability. Modest adjustments were made to the flow rate (±0.1 mL/min) and the organic concentration of the mobile phase (±10%) before injection. Table 7 shows the findings of the robustness analysis.
Forced degradation
This suggested method is an advancement over existing methods since it permits simultaneous release and stability tests. Forced degradation studies, as specified by the ICH rules, include acid, base, oxidation, reduction and heat degradation. Degraded peaks are depending on the chromatographic technique used, although it seems that the drugs under evaluation were stable. Outcomes of forced degradation were shown in Table 8.
Acid degradation
In a volumetric flask with a 10-mL capacity, 1 mL of the sample stock solution and 1 mL of 1N HCl were added and the mixture was let to stand for 15 min. After 15 min, dilute to the appropriate concentration by adding 1 mL of 1N sodium hydroxide. Inject the solution, after having filtered it with a syringe into a High-Performance Liquid Chromatography (HPLC) apparatus.

Figure 4:
Calibration plot of (A) Levodopa and (B) Benserazide.
| Sl. No. | Levodopa | Benserazide | ||
|---|---|---|---|---|
| Concentration (µg/mL) | Area | Concentration (µg/mL) | Area | |
| 1 | 50.00 | 675471 | 12.50 | 226507 |
| 2 | 100.00 | 1305965 | 25.00 | 449123 |
| 3 | 150.00 | 2054716 | 37.50 | 679481 |
| 4 | 200.00 | 2669587 | 50.00 | 897164 |
| 5 | 250.00 | 3259845 | 62.50 | 1086755 |
| 6 | 300.00 | 4005783 | 75.00 | 1324712 |
| Slope | 13249.80 | 17550.49 | ||
| Intercept | 8439.68 | 8105.32 | ||
| Correl Coeff | 0.99965 | 0.99974 | ||
Results of linearity.
| Sl. No. | Area of Levodopa | Area of Benserazide |
|---|---|---|
| 1 | 2658943 | 896421 |
| 2 | 2625241 | 895705 |
| 3 | 2669583 | 896978 |
| 4 | 2652417 | 890042 |
| 5 | 2678594 | 893130 |
| 6 | 2682396 | 898915 |
Results of method precision.
| Accuracy | Levodopa | Benserazide | ||
|---|---|---|---|---|
| Concentration | % Recovery | Concentration | % Recovery | |
| 50* | 100 | 99.6 | 25 | 99.9 |
| 100* | 200 | 100.1 | 50 | 100.0 |
| 150* | 300 | 99.9 | 75 | 100.0 |
Results of accuracy.
| Sl. No. | Area of Levodopa | % Relative standard Deviation | Area of Benserazide | % Relative standard Deviation |
|---|---|---|---|---|
| 1 | 2665894 | 1.14 | 896582 | 0.33 |
| 2 | 2603256 | 892541 | ||
| 3 | 2645879 | 892257 | ||
| 4 | 2635209 | 897589 | ||
| 5 | 2685481 | 899263 | ||
| 6 | 2674583 | 894526 |
Results of intermediate precision.
| Parameter | % RSD of Levodopa | % RSD of Benserazide |
|---|---|---|
| Flow (0.9 mL/min) | 0.31 | 0.10 |
| Flow (1.1 mL/min) | 0.45 | 0.25 |
| Organic phase (18:82) | 0.46 | 0.26 |
| Organic phase (22:78) | 0.20 | 0.15 |
Results of robustness.
| Stress Parameter | % of Degradation Levodopa | % of Degradation Benserazide |
|---|---|---|
| Acid degradation (1N HCl) | 13.2 | 11.1 |
| Alkali degradation (1N NaOH) | 12.1 | 12.8 |
| Peroxide degradation (10% Peroxide) | 14.7 | 13.4 |
| Reduction degradation (10% sodium bi sulphate) | 1.5 | 2.4 |
| Thermal (sample, 105ºC, 6 Hr) | 9.8 | 3.7 |
| Photo (Photo stability chamber, 6 Hr) | 2.3 | 1.8 |
| Hydrolysis (1 mL HPLC water) | 1.2 | 0.7 |
Results of forced degradation.
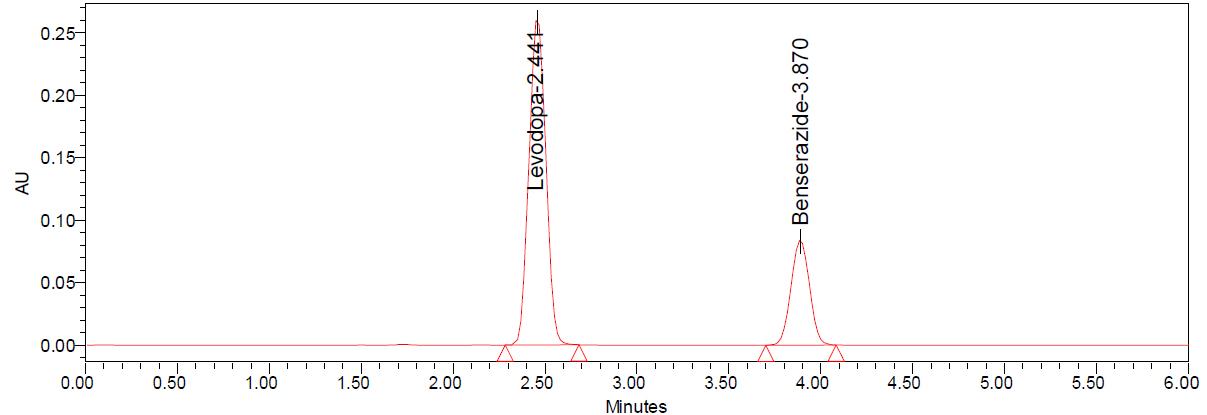
Figure 5:
Chromatogram of sample.
Alkali degradation
In a volumetric flask with a 10-mL capacity, 1 mL of the sample stock solution was transferred, 1 mL of 1N NaOH was added and the combination was allowed to stand for 15 min. Once the 15 min have passed, add 1 mL of 1N HCl to get the concentration up to where it needs to be. This solution will be injected into an HPLC system after being filtered using a syringe filter.
Peroxide degradation
In a 10 mL volumetric flask, 1 mL of sample stock solution was transferred, and then 1 mL of 10% hydrogen peroxide solution was added and the volume was adjusted accordingly. Filter the solution using syringe filter and injected into HPLC system.
Reduction degradation
Place 1 mL of sample stock solution and 1 mL of 10% sodium bi sulphite solution in a 10-mL volumetric flask, mix, and dilute to the desired concentration. This solution will be injected into an HPLC system after being filtered using a syringe filter.
Thermal degradation
The temperature of the sample solution was maintained at 105ºC during the 6 hr heating period. An HPLC system was then used to analyse the solution.
Photo degradation
The sample solution was placed in photo stability chamber for 6 hr. An HPLC system was then used to analyse the solution.
Hydrolysis degradation
Place 1 mL of sample stock solution and 1 mL of HPLC water in a 10 mL volumetric flask, mix, and dilute to the desired concentration. This solution will be injected into an HPLC system after being filtered using a syringe filter.
DISCUSSION
The selected pharmaceutical substances and the degradants they produce were efficiently and rapidly separated using the isocratic elusion-enhanced mobile phase, which consisted of formic acid and acetonitrile. The system adequacy of the method was evaluated by looking at features including resolution, area, retention time, theoretical plates, and tailing factor. At room temperature, the current experiment proved to be more repeatable than before using a Luna phenyl hexyl column (150 mm×4.6 mm, 3.5 μm). According to the specifications set forth by the International Council on Harmonisation (ICH) standard, the system suitability parameters’ results fell within the permitted levels. The active ingredient form of both medications and their associated contaminants was successfully resolved, and a straight baseline was seen in the blank chromatogram of the mobile phase. This method was shown to be sufficiently sensitive for drug identification even when their degradants were present, according to the computed quantification and detection limit values. It was also noted that the percentage of medication recoveries were within acceptable ranges. The specificity of the method was examined by forcing degradation and infusing blank, standard, and sample solutions. Results from robustness studies demonstrate that the developed method can withstand adversity.
CONCLUSION
Levodopa and Benserazide, both in their combined formulation and in their pure forms, may be identified using the high-performance liquid chromatography procedure detailed in the published technique. In this case, high-performance liquid chromatography stands out due to its simplicity, quickness, and low cost. Laboratory quality control is now possible thanks to the suggested method’s satisfactory validity criteria.
Cite this article:
Rayudu S, Manoranjani M. Stability Demonstrating Validated High-Pressure Liquid Chromatographic Method for the Simultaneous Determination of Levodopa and Benserazide. Int. J. Pharm. Investigation. 2025;15(2):10-8.
ACKNOWLEDGEMENT
I thankful to my mentor’s words of wisdom and the help he gave me.
I thankful to my mentor’s words of wisdom and the help he gave me.
ABBREVIATIONS
| RP-HPLC | Reverse phase high performance liquid chromatography |
|---|---|
| CNS | Central nervous system |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| ICH | International Conference on Harmonization |
| PDA | Photo diode array detector |
| RSD | Relative standard deviation |
| HCl | Hydro chloric acid |
| NaOH | Sodium hydroxide |
| DP | Degradation product |
| RS | Related substances |
References
- Batra A. S., Balaji S.. (2019) Fetal arrhythmias: Diagnosis and management. Indian Pacing and Electrophysiology Journal 19: 104-109 Google Scholar

- Bhoir M., Rao N.. (2020) RP-HPLC Method development, Validation and Forced Degradation for Simultaneous estimation of Benserazide HCl and levodopa in a Marketed Formulation. International Journal of PharmTech Research 13: 206-216 Google Scholar

- Cuevas J.. (2019) Neurotransmitters and their life cycle. Reference module in biomedical sciences Google Scholar

- Swenson E. R.. (2013) Hypoxic pulmonary vasoconstriction. High Altitude Medicine and Biology 14: 101-110 Google Scholar

- Eisenhofer G., Kopin I. J., Goldstein D. S.. (2004) Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacological Reviews 56: 331-349 Google Scholar

- Fabbrini G., Brotchie J. M., Grandas F., Nomoto M., Goetz C. G.. (2007) Levodopa-induced dyskinesias. Movement Disorders 22: 1379-1389 Google Scholar

- Gonçalves L. C. P., Trassi M. A. S., Lopes N. B., Dörr F. A., Santos M. T., Baader W. J., Oliveira V. X., Bastos E. L., et al. (2012) A comparative study of the purification of betanin. Food Chemistry 131: 231-238 Google Scholar

- Halje P., Tamtè M., Richter U., Mohammed M., Cenci M. A., Petersson P., et al. (2012) Levodopa-induced dyskinesia is strongly associated with resonant cortical oscillations. The Journal of Neuroscience 32: 16541-16551 Google Scholar

- Harilal S., Jose J., Parambi D. G. T., Kumar R., Unnikrishnan M. K., Uddin M. S., Mathew G. E., Pratap R., Marathakam A., Mathew B., et al. (2020) Revisiting the blood–brain barrier: A hard nut to crack in the transportation of drug molecules. Brain Research Bulletin 160: 121-140 Google Scholar

- Hiroshima Y., Miyamoto H., Nakamura F., Masukawa D., Yamamoto T., Muraoka H., Kamiya M., Yamashita N., Suzuki T., Matsuzaki S., Endo I., Goshima Y., et al. (2014) The protein Ocular albinism 1 is the orphan GPCR GPR143 and mediates depressor and bradycardic responses to DOPA in the nucleus tractus solitarii. British Journal of Pharmacology 171: 403-414 Google Scholar

- Kadry H., Noorani B., Cucullo L.. (2020) A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids and Barriers of the CNS 17: 69 Google Scholar

- Kuck K.-H.. (2020) Arrhythmias and sudden cardiac death in the COVID-19 pandemic. Herz 45: 325-326 Google Scholar

- Mareev Y., Cleland J. G. F.. (2015) Should β-blockers be used in patients with heart failure and atrial fibrillation?. Clinical Therapeutics 37: 2215-2224 Google Scholar

- Miller A. D., Zachary J. F.. (2020) Nervous system. Pathologic Basis of Veterinary Disease : 805-907.e1 Google Scholar

- Moodley D. S.. (2017) Local anaesthetics in dentistry-Part 3: Vasoconstrictors in local anaesthetics. South African Dental Journal 72: 176-178 Google Scholar

- Nygaard G., Szigetvari P. D., Grindheim A. K., Ruoff P., Martinez A., Haavik J., Kleppe R., Flydal M. I., et al. (2021) Personalized medicine to improve treatment of dopa-responsive dystonia-A focus on tyrosine hydroxylase deficiency. Journal of Personalized Medicine 11: 1186 Google Scholar

- Plum E., Fedotov V. A., Zheludev N. I.. (2016) Specular optical activity of achiral metasurfaces. Applied Physics Letters 108: 141905 Google Scholar

- Polturak G., Breitel D., Grossman N., Sarrion-Perdigones A., Weithorn E., Pliner M., Orzaez D., Granell A., Rogachev I., Aharoni A., et al. (2016) Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants. The New Phytologist 210: 269-283 Google Scholar

- Riccardi S., Ferri R., Garbazza C., Miano S., Manconi M.. (2023) Pharmacological responsiveness of periodic limb movements in patients with restless legs syndrome: A systematic review and meta-analysis. Journal of Clinical Sleep Medicine 19: 811-822 Google Scholar

- Silber M. H., Buchfuhrer M. J., Earley C. J., Koo B. B., Manconi M., Winkelman J. W., et al. (2021) The management of restless legs syndrome: An updated algorithm. Mayo Clinic Proceedings 96: 1921-1937 Google Scholar

- Singh M., Malik P., Bhushan R.. (2016) Resolution of enantiomers of (RS)-baclofen by ligand exchange thin layer chromatography. Journal of Chromatographic Science 54: 842-846 Google Scholar

- Skjærbæk C., Knudsen K., Horsager J., Borghammer P.. (2021) Gastrointestinal dysfunction in Parkinson’s disease. Journal of Clinical Medicine 10: 493 Google Scholar

- Teleanu R. I., Niculescu A.-G., Roza E., Vladâcenco O., Grumezescu A. M., Teleanu D. M., et al. (2022) Neurotransmitters-key factors in neurological and neurodegenerative disorders of the central nervous system. International Journal of Molecular Sciences 23: 5954 Google Scholar

- Warnecke T., Schäfer K. H., Claus I., Del Tredici K., Jost W. H.. (2022) Gastrointestinal involvement in Parkinson’s disease: Pathophysiology, diagnosis, and management. npj Parkinson’s Disease 8: 31 Google Scholar

- Weissbach A., Pauly M. G., Herzog R., Hahn L., Halmans S., Hamami F., Bolte C., Camargos S., Jeon B., Kurian M. A., Opladen T., Brüggemann N., Huppertz H.-J., König I. R., Klein C., Lohmann K., et al. (2022) Relationship of genotype, phenotype, and treatment in dopa-responsive dystonia: MDSGene review. Movement Disorders 37: 237-252 Google Scholar


