ABSTRACT
Studying the immunogenicity of insulin is crucial for managing and treating diabetes and other medical conditions. Although insulin therapy is proven effective for diabetic patients, the presence of insulin antibodies can diminish its effectiveness and lead to adverse immune reactions. Understanding the processes and factors affecting insulin immunogenicity is important to improve treatment outcomes and minimize complications. Research indicates that insulin’s immunogenicity is influenced by various structural characteristics such as size, charge, and hydrophobicity. Additionally, factors like insulin dosage, frequency of administration, patient age, gender, genetic background, and mode of insulin delivery contribute to the likelihood of antibody formation. Therefore, optimizing insulin therapy for diabetic patients requires a comprehensive knowledge of insulin immunogenicity and effective insulin antibody detection assays. The development of advanced point-of-care immunoassays has enabled efficient screening for anti-insulin antibodies and assessment of insulin immunogenicity.
INTRODUCTION
A group of metabolic disorders, collectively referred to as diabetes, is distinguished by chronically elevated levels of blood sugar resulting from insufficient insulin production, ineffective insulin function, or a combination of both factors. Prolonged effects encompass the impairment of multiple body organs, including the eyes, kidneys, nerves, heart, and blood vessels, resulting from persistently raised blood sugar levels linked to diabetes.1,2 Type 1, which depends on insulin for survival, and type 2, brought on by issues with insulin secretion or sensitivity, are the two primary forms of diabetes. Gestational diabetes, which appears during pregnancy and is brought on by hormonal changes, and particular genetic forms of diabetes, which are brought on by gene mutations, are other less frequent types of diabetes.3 Effective diabetes treatments necessitate a multidisciplinary, all-encompassing strategy.4 Type 2 diabetes is identified by increased blood sugar levels resulting from varying levels of pancreatic beta-cell dysfunction and reduced responsiveness to insulin. This worldwide health issue impacts people of all age groups and can lead to harm to both small and significant blood vessels due to elevated blood sugar levels.
This concise evaluation underscores the significant role of insulin immunogenicity in the onset and advancement of diabetes, as it examines various elements contributing to the disease’s pathogenesis. When the immune system attacks and destroys insulin-secreting cells and secretes insulin as a result of antibodies, immune system dysfunction is frequently linked to the development of auto-antibodies against insulin.5,6 One of the many autoantibodies that can be found in people with T1D are antibodies against insulin; additional autoantibodies include those to glutamate acid decarboxylase, islet cell antibody, insulinoma-associated-2 autoantibodies, and zinc transporter-8 autoantibodies, which have also been linked to the progression of the diabetes disease.7
Types of autoantibodies responsible for diabetes
Anti-insulin Antibody
The presence of anti-insulin antibodies may lead to a decrease in the effective amount of insulin in the body. These polyclonal antibodies bind to insulin with varying affinities and capabilities, interfering with the desired effects of administered insulin. This interference can potentially lead to both low blood sugar levels (hypoglycemia) and insulin resistance.8 It has been implicated that the patient experiencing Subcutaneous Insulin Resistance (SIR), where insulin is trapped at the tissue level is either due to the actions of anti-insulin antibodies or issues related to sequestration.9
Anti-Glutamate Acid Decarboxylase (GAD)
Anti-GAD antibodies are autoantibodies that target the enzyme Glutamate Decarboxylase (GAD), involved in producing the inhibitory neurotransmitter GABA. Detecting these antibodies is crucial for diagnosing Type 1 Diabetes (T1D) as they indicate a higher risk of insulin dependence.10
Islet Cell Antibodies (ICA)
In 1974, the traditional Immunofluorescence assay (IF) was introduced to identify islet cell antibodies, providing the initial indication of an immune response against pancreatic islets in people with a diagnosis of type 1 diabetes mellitus.7 Approximately 70-80% of individuals newly diagnosed with type 1 diabetes possess detectable islet cell antibodies. The existence of these antibodies in asymptomatic individuals suggests an elevated risk of developing type 1 diabetes later on.11
Insulinoma-Associated-2 Autoantibodies (IA-2)
Insulinoma-Associated-2 Autoantibodies (IA-2A) are autoantibodies linked to diabetes, particularly type 1 diabetes mellitus. IA-2A specifically targets an enzyme known as insulinoma-associated-2 (IA-2), which is present in the insulin-producing cells of the pancreas. Roughly 60% of individuals who have recently received a diagnosis of type 1 diabetes show the presence of IA-2 antibodies (IA-2A).12,13 The existence of IA-2A in individuals without symptoms implies an elevated likelihood of developing type 1 diabetes in the future.
Zinc Transporter-8 Autoantibodies (ZnT8A)
ZnT8A antibodies are indicative of autoimmune type 1 diabetes and are present in about 60% of individuals recently diagnosed with symptomatic type 1 diabetes. They also serve as a diagnostic marker for Latent Autoimmune Diabetes in Adults (LADA).14 Even without symptoms, the presence of ZnT8A antibodies increases the risk of developing type 1 diabetes. Furthermore, pancreas transplant recipients with ZnT8A antibodies in their blood are at risk of beta-cell failure.15
Patient Population
Diabetes is a pressing global public health concern, impacting developing nations like China, India, and Pakistan, as well as more prosperous countries such as the USA. As per estimates, the prevalence of diabetes in individuals aged 20 to 79 was 10.5% (equivalent to 536.6 million cases) in 2021, and this projected to increase to 12.2% (reaching 783.2 million cases) by the year 2045.16 It’s worth noting that diabetes is more prevalent among individuals aged 75 and above, with similar prevalence rates for both genders. Notably, middle-income countries are expected to experience the most significant proportional increase in diabetes prevalence (21.1%) between 2021 and 2045, surpassing both high-income and low-income nations. According to,17 treating diabetes-related diseases will cost 966 billion USD globally by 2021.
According to forecasts from the World Health Organization (WHO) and the International Diabetes Federation, the global prevalence of Type 2 Diabetes is expected to reach 700 million people by the year 2045. It is estimated that 134 million people will have diabetes by 2045 in India, the country with the highest number of diabetics.18 Currently, over 72.9 million people in India have the illness.
Around 7 to 10 million individuals with Type 1 Diabetes depend on recombinant Insulin, and there is also a need for insulin therapy among certain Type 2 Diabetes patients. Diagnostic tests such as glucose monitoring and HbA1c tests are commonly used, but there is no routine test available in India for detecting anti-insulin antibodies. Approximately 44% of type 1 diabetes patients have anti-insulin antibodies, but they are not routinely investigated in India when there is an increase in insulin requirements or a lack of metabolic control. In practice, many doctors simply change insulin formulations without conducting systematic follow-up for suspected insulin antibody issues.
Problem and Evidence
The quality, molecular makeup, and storage requirements of the insulin utilized, along with patient-specific elements such as age, HLA (human leukocyte antigen) profile, and the mode of administration. influenced in the development of anti-insulin antibodies.19,20 Postprandial hypoglycemia, caused by anti-insulin antibodies acting as insulin receptor agonists, is a rare but serious condition. When present at high levels, IgG antibodies can interfere with insulin action or counteract its effects, potentially leading to insulin resistance and, therefore, slowing or decreasing the effectiveness of insulin therapy.21,22
Overview of Insulin Immunogenicity
Insulin immunogenicity can be traced back to the 1920s when insulin was discovered and utilised as a diabetes therapy. However, it was quickly recognised that some people developed antibodies to insulin, which could impair its efficiency. Since then, advances in the development of several kinds of insulin with varying levels of immunogenicity have been made.20 These include human insulin mimics and insulin that have been genetically engineered. While these advancements have benefited many patients with insulin therapy, some people continue to suffer difficulties related to insulin immunogenicity. Ongoing research attempts to get a better knowledge of the underlying processes of insulin immunogenicity and to develop novel techniques for dealing with its consequences.23,24
Insulin Resistance and Immunogenicity
Recent research has shown that insulin resistance might diminish the immunological function by increasing the production of inflammatory cytokines, which causes an overaggressive immune response along with metabolic illnesses like obesity, Type 2 Diabetes Mellitus (DM) is linked to cardiovascular disease and can result in chronic health issue, inflammation, and autoimmune disease.25,26 The cells develop insulin immunogenicity when they are less susceptible to the effects of insulin. These findings underline the necessity of treating insulin resistance promptly to stop the emergence of new health issues as stated in.27 In addition to better understanding how metabolic problems and immunological responses are linked, researchers are investigating how insulin regulates immune system function.28
Types of Insulin Immunogenicity
Insulin immunogenicity can be triggered by five types: aggregated insulin, proinsulin, insulin precursors, insulin analogs, and insulin-like molecules.29 Aggregated insulin involves the clustering of insulin molecules, while proinsulin shares similarities with insulin and can also trigger an immune response.30 Understanding these types are crucial for developing effective insulin therapies that balance glycemic control with the risk of adverse immune reactions. Insulin precursors, synthetic versions of insulin, may exhibit different levels of immunogenicity compared to naturally occurring insulin. Insulin-like molecules, although structurally similar to insulin, can still induce an immune response.31
Factors Affecting Insulin Immunogenicity
Insulin Analogues and Immunogenicity
One method to minimize immunogenicity in insulin therapy involves the utilization of insulin analogues. Insulin analogues are modified versions of human insulin that have been structurally altered to closely resemble the pharmacokinetics of insulin, surpassing traditional human insulin formulations.32 These modifications have demonstrated a reduction in the likelihood of an immune response, as the analogues exhibit a lower affinity for insulin antibodies compared to conventional human insulin. However, studies have indicated that certain insulin analogues, such as insulin glargine, can still elicit a neutralizing antibody response, leading to impaired glycemic control over time. In summary, although insulin analogues may offer certain benefits in diminishing immunogenicity, further research is necessary to comprehend their potential impact on glycemic control and the complications of developing autoimmune disorders.32,33
Insulin Delivery
The method of insulin delivery significantly affects insulin immunogenicity. Patients using insulin pumps experience less immune responses compared to those using daily injections. Insulin pumps provide a continuous low dose of insulin, resembling natural pancreatic insulin. In contrast, frequent injections expose the immune system to insulin repeatedly, leading to the production of antibodies.34,35 Factors like amino acid sequence differences, biosimilar product interchangeability, contaminants, genetic factors, dosage frequency, and cold chain integrity also contribute to antibody development. Choosing the right insulin administration route and considering these factors is crucial in reducing the risk of generating anti-insulin antibodies.20 The absorption and duration of action of various insulin analogues are influenced by distinct factors. Degludec, for instance, forms weak hexamers in the vial and stable multi-hexamers post-administration, slowing absorption. Reversible binding to albumin prolongs its action. Insulin glargine U300 precipitates, forming compact aggregates, leading to slow absorption. Insulin glargine U100 also precipitates but is less compact. Insulin detemir forms weak dihexamers, with reversible albumin binding slowing absorption. NPH insulin co-crystalizes with protamine, slowing absorption. Rapid-acting analogues (lispro, aspart, and glulisine) dissociate more rapidly than human insulin, resulting in quicker absorption. Glulisine’s formulation uses polysorbate 20, preventing hexamer formation. Fast-acting insulin aspart’s addition of arginine and nicotinamide accelerates absorption (Table 1).36
| Details | Degludec | Insulin glargine U300 | Insulin glargine U100 | Insulin detemir | Human NPH Insulin | Human Regular Insulin | Insulin Lazaro Aspart/ Glulisine | Fast Acting insulin Aspart |
|---|---|---|---|---|---|---|---|---|
| In vial | Dihexamer | Hexamers | Hexamers | Dihexamers | Hexamer protamine | Hexamers | Hexamers | Hexamers |
| After Administration under the skin. | Multiple hexamers | Concentrated hexamer aggregates | Hexamer Aggregates | Dihexamers Albumin | Hexamer protamine | Hexamers dimers and monomers | Hexamers dimers and monomers | Hexamers dimers and monomers |
| In the capillary. | Albumin with two insulin monomers. | Insulin monomer | Insulin monomer | Albumin with two insulin monomers. | Insulin monomer | Insulin monomer | Insulin monomer | Insulin monomer |
| Rate of Absorption. | Very slow | Very slow | Very slow | Very slow | Moderately fast | fast | Very fast | Very fast |
| Duration of Action. | Long | Long | Long | Long | Moderately short | Short | Short | Short |
Biological Action Insulin Analogues.
Genetic Susceptibility and Insulin Immunogenicity
Genetic susceptibility is crucial in understanding insulin immunogenicity and its connection to autoimmune diabetes. Certaingenescontributetotheformationofinsulinautoantibodies, while genetic variations in the HLA region increase the risk of autoimmune diabetes. HLA molecules present antigens to the immune system, and their variation influences autoimmune responses.37 This knowledge aids in predicting, preventing, and creating personalized treatments for autoimmune diabetes.
Impact of Insulin Immunogenicity on Diabetes Management
The influence of insulin immunogenicity on the management of diabetes is multifaceted. Conversely, the formation of anti-insulin antibodies can hinder the effectiveness of insulin treatment, resulting in poor glycemic control and the need for higher insulin doses.38 This situation can result in higher expenses and negatively impact patients. However, some research indicates that the existence of anti-insulin antibodies might be linked to a decreased likelihood of experiencing low blood sugar (hypoglycemia), a potentially life-threatening complication of insulin therapy. Hence, physicians should be aware of this phenomenon and keep an eye on it in their patients. Furthermore, knowing the mechanisms driving insulin immunogenicity may lead to novel techniques to increase insulin therapy efficacy and ultimately assist diabetic patients.
Mechanisms of Insulin Immunogenicity
Insulin immunogenicity results from a complex interplay of multiple factors. One primary mechanism involves the immune system’s reaction to the foreign protein introduced during insulin therapy. This immune response is initiated by T-cells, immune cells that activate upon detecting the foreign protein. Subsequently, these T-cells stimulate antibody production, which specifically targets and attacks the foreign insulin molecule.20,39 Consequently, these antibodies can bind to the insulin molecule, rendering it inactive and potentially leading to insulin resistance and issues with glycemic control. Another factor contributing to insulin immunogenicity is the mode of insulin delivery. Insulin administered through subcutaneous injection or insulin pumps is more prone to triggering an immune response compared to other delivery methods such as inhaled insulin or oral insulin.36
Replacement therapy and Immunogenicity
Recombinant insulin therapy commonly employed for Type 1 and certain Type 2 diabetes patients, often results in the formation of anti-insulin antibodies. Even in the absence of exposure to recombinant insulin, insulin autoantibodies are associated with Type 1 Diabetes and Latent Autoimmune Diabetes in Adults (LADA).40 The use of recombinant human insulin and analogs has lowered the incidence of antibody development but hasn’t eliminated it. Notably, Continuous Subcutaneous Insulin Infusion (CSII), Continuous Peritoneal Insulin Infusion (CPII), and inhaled insulin therapy have been found to significantly increase IgG anti-insulin antibody levels in diabetic individuals.15
In addition to analyzing the impact of insulin immunogenicity on therapeutic success, insulin therapy is extensively researched for managing type 2 diabetes, which occurs when the body struggles to regulate glucose levels. When lifestyle changes and oral medications are insufficient, insulin is prescribed. However, some studies indicate that insulin therapy can lead to immunogenicity and the production of anti-insulin antibodies. Clinicians must monitor insulin-treated patients to assess how these antibodies impact treatment efficacy and disease management.
Clinical Consequences of Insulin Immunogenicity
Insulin immunogenicity causes insulin resistance, which makes the body develop antibodies to insulin and requires greater dosages of insulin hormone to meet the desired glycemic index. This will result in poor blood sugar control, which may additionally motivate long-term problems such as retinopathy, neuropathy, and nephropathy.41 Hypoglycemia is probably the fatal facet impact of insulin therapy, and insulin resistance will increase the threat of growing it. Moreover, the immunogenic properties of insulin can lead to the detriment of pancreatic beta cells, potentially aggravating insulin resistance and intensifying the onset of type 1 diabetes.” For efficient diabetic care, it is important to recognize and deal with the medical effects of insulin immunogenicity.42
MATERIALS AND METHODS
Diagnosis of Insulin Immunogenicity
The diagnosis of insulin immunogenicity requires the use of numerous approaches. The insulin-induced lymphocyte proliferation assay examines the proliferative response of T cells, whereas the insulin autoantibody assay quantifies insulin-specific autoantibodies in serum.43 Although they may lack specificity, immunoassays like ELISA and radioimmunoassay can also find insulin antibodies. Even though each technique has drawbacks and the potential to yield false positive or negative results, combining several techniques can lead to an accurate diagnosis.
Anti-insulin antibody measurement Assays
The detection and quantification of antibodies targeting insulin in the bloodstream were accomplished through these assays. Here are a few examples of widely used tests to measure anti-insulin antibodies. E.g.: Various ELISA Techniques, Radio Immunoprecipitation Assay, Affinity Capture and Acid elution ELISA, Surface Plasmon Resonance method, and Charcoal stripping for insulin-neutralizing antibody detection.
ELISA METHODS
Indirect/Sandwich
As per the articles,44 A mixture of highly purified bovine, porcine, and recombinant human insulin preparations is bound to 96 microcells. Specific antibodies in the patient sample bind to the antigen coated on the reaction wells’ surface. Following that, an enzyme conjugate is added, which binds to the immobilized antibody-antigen complex. Following the addition of TMB substrate, incubate for the reaction to develop before adding acid to stop the reaction, resulting in a yellow end product. The yellow colour intensity correlates with the concentration of the antibody-antigen complex and can be measured photometrically at 450 nm. This ELISA was optimized to be at least as sensitive as the measurement of antibodies by direct 125l-insulin binding. An immunoassay has been developed for the first time to detect and characterize insulin antibodies in sera from a range of patients, including those who had only received highly purified human insulin. The assay has been demonstrated to be most effective in screening for insulin antibodies in resistant patients (Figure 1).
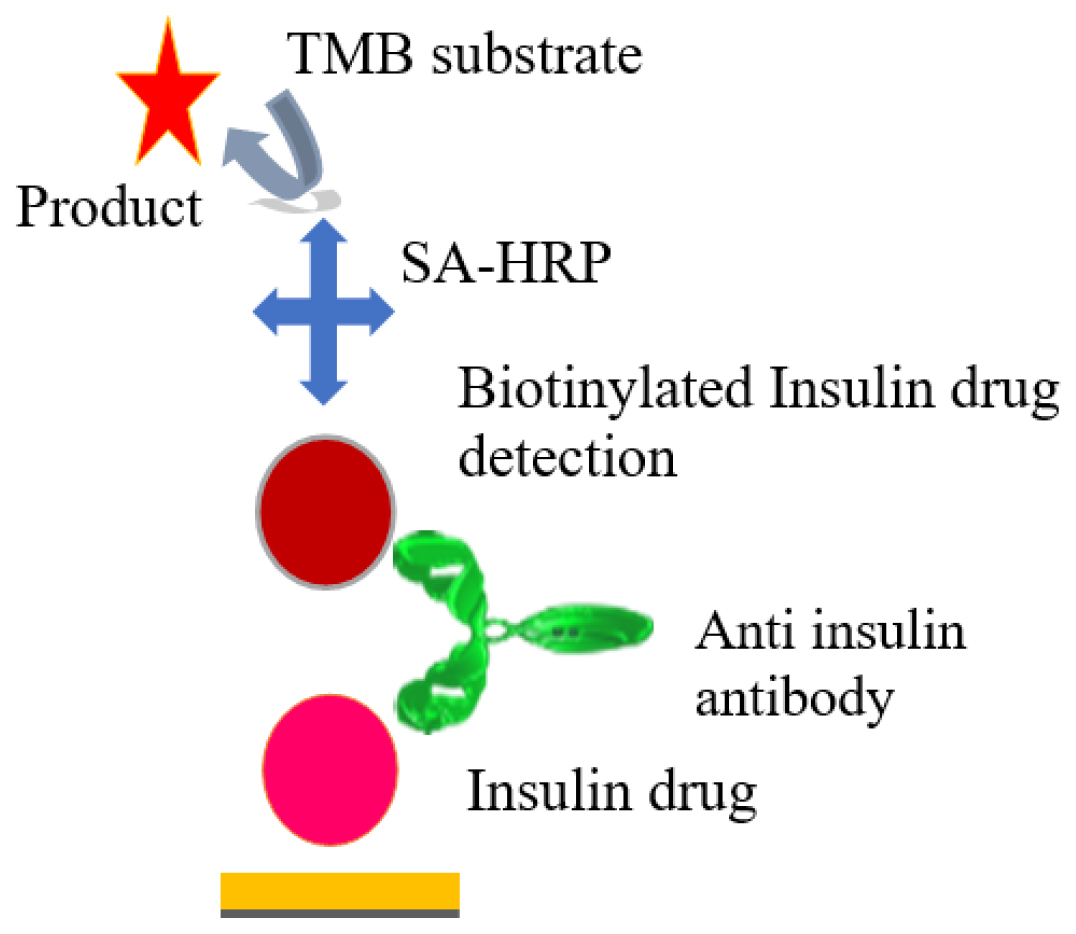
Figure 1:
In the initial step (A) of the sandwich ELISA, microtiter plates were coated with purified insulin antigen, creating a solid foundation for antibody binding. Subsequent (B) blocking prevented non-specific interactions, ensuring specificity between anti-insulin antibodies and immobilized insulin. Patient serum samples suspected of containing anti-insulin antibodies were added in (C) forming primary antibody-antigen complexes. Step (D) introduced a biotinylated anti-human IgG secondary antibody, binding specifically to anti-insulin antibodies. (E) Streptavidin-HRP conjugate formed a tertiary complex at anti-insulin antibody binding sites. (F) Adding the substrate TMB initiated a colorimetric reaction, with color intensity proportional to anti-insulin antibody concentration. Concluding (G) with a stop solution changed color from blue to yellow; absorbance was measured at 450 nm using a microplate reader. Standard curve calibration (H) facilitated anti-insulin antibody quantification in patient samples. Finally, (I) involved analyzing absorbance values to determine anti-insulin antibody presence and concentration, providing a reliable measure of the immune response against insulin.45
ELISA with charcoal stripping followed by acid dissociation assay
This ELISA method follows a series of sequential steps for the detection of anti-insulin antibodies in the presence of Aspart insulin. Initially, in the sample treatment phase, the complex of anti-insulin antibodies and insulin in the sample is dissociated, and endogenous insulin and dissociated insulin are eliminated. In the subsequent affinity capture step, the treated samples are neutralized and introduced to insulin-coated plates, allowing the anti-insulin antibody in the sample to be captured with high affinity. After removing excess free insulin, serum proteins, and non-specific binding agents through washing, the captured anti-insulin antibody is eluted using a mild acid and then attached to a new solid surface upon neutralization.
In the final detection step, the bound anti-insulin antibody is identified by introducing hapten-labeled insulin, followed by the addition of an anti-hapten HRP conjugate. This results in the development of a blue-colored product due to HRP activity upon the addition of the TMB substrate. The color development process is monitored, and the reaction is halted by adding a stop solution, which transforms the blue color to yellow. The ELISA plate is subsequently read in a spectrophotometer at 450 nm.46
Radio Immunoprecipitation Assay
Radio immunoprecipitation assays have proven to be a sensitive method of detecting autoantibodies in the serum of diabetic patients as per the previous articles.47 Antibodies are bound to the radiolabeled antigens in the assays; the antibody-antigen complex is isolated by employing Protein A SepharoseTM. Precipitating the antibody and measuring the labeled antigen. A new “semi-automated” RIP assay uses a 96-well microplate format for convenience. It allows you to reduce the amount of Protein A SepharoseTM you use without losing sensitivity. The results of the radioimmunoprecipitation assay using traditional plastic tubes and the microplate format.
Affinity Capture and Acid Elution ELISA
AIAs (Anti-Insulin Antibodies) are typically identified using bridging ELISAs, which exhibit high sensitivity even to low levels of antigen. We present an ELISA method that involves solid-phase antigen for the affinity capture of AIAs, elimination of excess free insulin, release and transfer of bound AIAs, and the detection of biotinylated insulin. This assay is characterized by its sensitivity and excellent tolerance to free insulin, capable of detecting 500 ng/mL of anti-insulin antibody even in the presence of 500 μg/ mL of insulin. Biopharmaceuticals have the potential to induce an immune response in treated individuals. However, there is a lack of consistent standards regarding the type, quantity, and quality of evidence. Advancements in methods for assessing immune responses have revealed increased immunogenicity rates with modern assays.48
Surface Plasmon Resonance
The paper discusses two bioanalysis methods for detecting biomolecular interactions. The initial technique revolves around harnessing Localized Surface Plasmon Resonance (LSPR) to construct a multiarray optical nanochip (LSPR-based optical nanochip). This innovative method is devoid of labeling and relies on a core-shell-structured nanoparticle layer featuring 300 nanospots on the sensing surface, enabling the anchoring of antibodies. This nanochip exhibits a remarkable detection threshold of 100 picograms per milliliter (pg/mL) and can be readily customized to oversee the interactions involving a diverse range of biomolecules.49 The second method is the Electrochemiluminescent Immunoassay (ECLIA), which employs electrochemical compounds to produce light. ECLIA offers advantages such as low label detection limits and a wide dynamic range for label quantification. It is particularly useful for detecting Insulin Autoantibodies (IAA) associated with autoimmune diabetes. In contrast, the commonly used 125I-insulin Radiobinding Assay (RBA) has limitations in terms of reproducibility, sample processing time, and the need for radiolabeled insulin synthesis.50,51
Electrochemical Nano bio-sensor based Assay
A sensitive electrochemical nano-biosensor has been developed for detecting insulin antibodies related to diabetes antigens. The sensor, created using electron polymerization and gold nanoparticles, accurately determines insulin antibody concentrations in human samples. Its high sensitivity, fast response time, and good reproducibility make it promising for early detection of type 1 diabetes.52 This research marks the first development of a label-free electrochemical immuno sensor for insulin antibody detection.
Point of care devices for AIA
The prevalence of anti-insulin antibodies to exogenous recombinant insulin in insulin-dependent diabetics has highlighted the importance of developing point-of-care diagnostics for the early detection of insulin resistance and treatment conditions. Compared with traditional diagnostic methods, simpler diagnostic methods, higher sensitivity and specificity, lower cost, higher efficiency, and the ability to detect on-site are all the benefits of POCT diagnostics. This review article aims to contribute to the significance and simpler method of detecting anti-insulin antibodies and thus pave the way to better understand the impact of insulin immunogenicity by providing new insights and directions for the future development of point-of-care diagnostics for metabolic disease management in general (Table 2 and Figure 2).
| Sl.No. | POCT test | ELISA | Radio Immuno precipitation Assay | Affinity Capture and Acid Elution ELISA | Surface Plasmon Resonance | Electrochemical Nano biosensor based Assay |
|---|---|---|---|---|---|---|
| 1 | POCT methods provide rapid results, often within min. | Time-consuming. | Time-consuming. | Time-consuming. | Rapid but complex procedure. | Rapid but complex procedure. |
| 2 | POCT devices are Compact and portable work well in rural settings. | Laboratory test | Special laboratory test. | Laboratory test. | Laboratory test. | Laboratory test. |
| 3 | POCT methods typically require smaller sample volume. | ELISA requires small volume . | More samples required. | More samples required. | Smaller sample volume required. | Smaller sample volume required. |
| 4 | Radioactive materials not involved. | Radioactive materials not involved. | Dealing with radioactive materials generate radioactive waste. | Radioactive materials not involved. | Radioactive materials not involved. | Radioactive materials not involved. |
| 5 | Cost-effective. | Costly | Too costly | Too costly. | Too costly. | Too costly. |
| 6 | Sensitivity and specificity less compared to ELISA. | Highly sensitive | Highly sensitive | Highly sensitive. | Highly sensitive. | Highly sensitive. |
| 7 | POCT methods are more accessible to healthcare providers and non-technical also. | ELISA requires technical expertise and equipment. | RIPA requires a high level of technical expertise and precision. | Requires technical expertise and equipment. | Requires technical expertise and equipment. | Requires technical expertise and equipment. |
| 8 | Minimized risk of contamination. | High risk of contamination. | High risk of contamination. | High risk of contamination. | High risk of contamination. | High risk of contamination. |
| 9 | Qualitative and quantitative also possible. | Semi-quantitative | Quantitative | Quantitative | Quantitative | Quantitative |
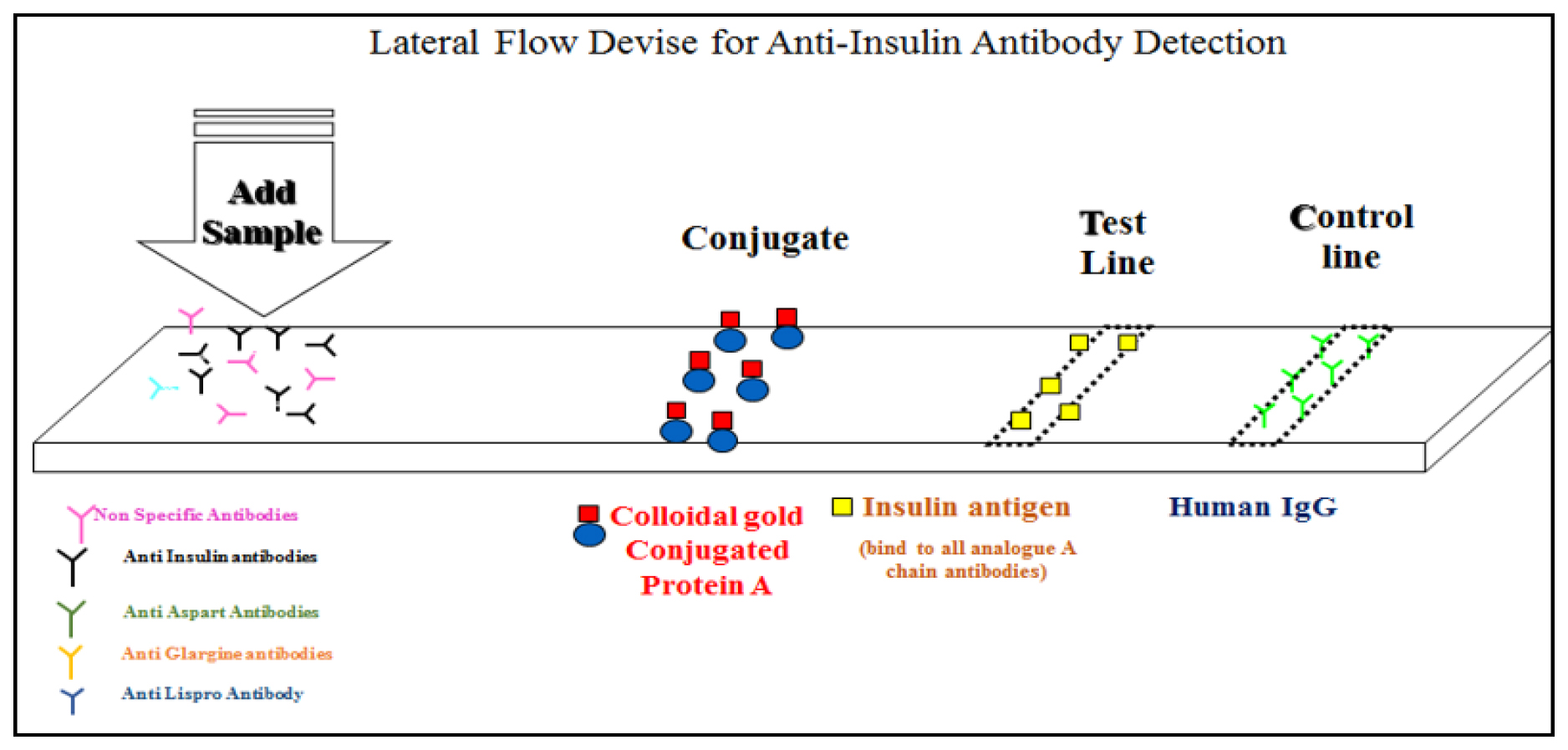
Figure 2:
The lateral flow assay device is composed of essential elements, including a sample pad, conjugate pad, nitrocellulose membrane, and absorbent pad (A). The assay begins by applying patient serum to the sample pad, initiating the process. Gold nanoparticles, equipped with anti-human IgG antibodies and insulin antigen/analogues, serve as detection probes and are fixed on the conjugate pad (B). The sample, potentially containing anti-insulin antibodies, migrates along the lateral flow strip (C). In the presence of anti-insulin antibodies, they bind to immobilized insulin antigens and its analogues on gold nanoparticles, forming an antibody-antigen-gold complex (D). Continuing along the nitrocellulose membrane, a visible line is generated at the capture line, which contains immobilized anti-human IgG antibodies (E). An integral control line, featuring immobilized secondary antibodies, consistently appears as an internal control (F). The presence of anti-insulin antibodies is indicated by the appearance of a test line, and the intensity of the line corresponds to antibody concentration (G). Results are visually examined within a specified timeframe (e.g., 15 min) in the interpretation window after sample application (H).53
POCT kit validation
Method validation for Point-of-Care Testing (POCT) kits is essential to guarantee the accuracy, reliability, and appropriateness of these tests for their intended purpose. These kits are commonly employed for Anti-insulin antibody screening. Below, we outline the crucial steps and factors to consider when validating POCT kit methods.
Accuracy and Precision
Twenty-five positive and twenty-five negative samples were run in duplicates to validate the accuracy and precision. Blank was also run to ensure the background. The obtained results were concordant with known control samples analyzed through indirect ELISA and POCT. The results demonstrated a 100% overall accuracy and precision of the AIA test kit when compared to the known reference serum samples.
Selectivity and Specificity
25 healthy serum samples were spiked with positive samples. The 25 healthy samples were run in parallel with the 25 spiked samples and run in duplicate. The spiked sample has to show positive and healthy samples resulted in negative in both POCT and ELISA which depicts that there is any effect of matrix and interfering compounds.
Sensitivity
An assay was performed to ensure the sensitivity of the kit. The POCT AIA test kit was evaluated with known positive samples (HQC: 4000 ug/mL, MQC 1000 ug/mL, and LQC 50 ug/mL) and the results were verified by a standard commercial ELISA AIA Kit to determine the minimum detection limit of the kit.
Linearity
An assay was performed to ensure the linearity of the kit. The POCT test kit was evaluated with known positive samples (HQC: 4000ug/mL, MQC 1000 ug/mL, and LQC 50 ug/mL) and the results were verified by a standard commercial ELISA AIA Kit to confirm the maximum detection limits of Anti insulin antibody POCT kit.
Specificity
Specificity samples, HPC-Spec, MPC-Spec, and LPC-Spec, will be prepared using nonspecific AIA (Anti insulin Antibody). Specific samples would be analysed in 2 replicates at least one batch by one analyst. Response for specificity samples should be 100% specific to anti-insulin antibody in the POCT kits and results compared with standard commercial ELISA kits.
Cross Reactivity
To determine the cross-reactivity of the method, LPC, MPC, and HPC prepared using Affinity purified Anti-Insulin polyclonal antibody will be incubated with Insulin of 100μg/mL concentration. Cross-reactivity samples would be analysed in 2 replicates at least one batch by one analyst. Response for LPC, MPC, and HPC sample response should be positive, and negative samples should be negative.
Stability
The robustness of the assay was evaluated with an AIA test kit at various temperatures, accelerations, and different periods of up to 14 days. The kit’s quality was determined by accelerating it over various temperatures and different periods with known positive samples and negative samples. The outcome was consistent with the QC specifications. As a result, we concluded that the assay kit’s stability was satisfactory or not.
Room Temperature Stability
The POCT kit will be subjected to storage at room temperature for 24 hr±2 hr and the quality and performance of the kit.
Acceptance Criteria
The precision of the 3 replicates LPC, MPC, and HPC will be assessed and should be ≤20%.
The percentage difference between the mean of the PCs that have undergone stability and those that have not undergone the indicated storage conditions (Freeze-thaw and Room Temperature) will be calculated. The percentage difference between the responses of the stability samples (PCs) from the baseline PC response (which has not undergone additional storage conditions) should be ±20%.
DISCUSSION
Individuals suffering from type 1 diabetes as well as some cases of type 2 diabetes rely on a daily insulin injection to sustain their lives. The formation of anti-insulin antibodies, which can render the administered insulin ineffective, poses a threat to glucose regulation, potentially leading to complications and even fatality. Given the variations in insulin sequence, expression, manufacturing processes, formulation, and storage shelf life, it is imperative to conduct an immunogenicity assessment of recombinant medicines. Antibodies, with their precision and sensitivity in identifying specific target antigens, serve as valuable diagnostic instruments. The objective is to generate target-specific antibodies in guinea pigs, utilizing insulin as the target antigen, with the potential application as a positive control in the validation of insulin immunogenicity kits.
The existing methods for detecting anti-insulin antibodies encompass Radio Immunoassay, Surface plasmon resonance, and acid elution test. While these methods exhibit high sensitivity and specificity, they come with the drawbacks of using expensive reagents, being time-consuming, involving multiple intricate steps, and necessitating highly skilled individuals along with well-equipped systems for both execution and data analysis. An ideal solution to address these limitations would be the implementation of a straightforward point-of-care device like the lateral flow assay, particularly for the detection of anti-insulin antibodies in serum. This approach would prove especially advantageous in rural areas where access to technical specialists and advanced equipment is limited. The Point-Of- Care Test (POCT) kit empowers healthcare providers to make real-time adjustments to treatment plans, resulting in enhanced management and ongoing monitoring of chronic conditions.
In India and various other parts of the world, there is a notable gap in the medical need for an Insulin immunogenicity kit that is tailored for the detection of Insulin antibodies, particularly Neutralizing NAb. Our objective is to develop a companion diagnostic kit for the management of diabetes that is straightforward and budget-friendly, akin to the glucose and HbA1c level measurement kits. This solution would enable healthcare practitioners to promptly and reliably determine the presence or absence of insulin antibodies, equipping them to make informed decisions and enhancing the efficiency of diabetes patient care. Future developments in insulin immunogenicity research include identifying biomarkers to predict insulin immunogenicity and personalized treatment regimens. Gene therapy is growing to modulate the immune response to insulin and prevent immunogenicity. Alternative insulin formulations, based on human analogs or orally administered insulin, are also being explored. Further investigation into the clinical implications, particularly insulin resistance and complications, is needed. Advancements in understanding insulin immunogenicity can significantly improve diabetes management and patient quality of life.
Diabetes treatment is severely impacted by insulin immunogenicity because the production of antibodies can reduce the efficiency of insulin therapy in regulating blood glucose levels. Healthcare practitioners need to keep an eye on the formation of insulin antibodies in patients and think about other treatment alternatives, such as moving to a new formulation or non-insulin drug. Improving diabetes care and lowering problems related to insulin therapy depend on the development of stable, less immunogenic insulin variants. It is crucial to have a better understanding of insulin immunogenicity to treat chronically ill patients effectively. In conclusion, the immunogenicity of insulin has important effects on the treatment of diabetes.
CONCLUSION
Our point-of-care In Vitro Diagnostic (IVD) kit is based on the antigen-antibody interaction principle that detects the presence or absence of antibodies against insulin and insulin analogues. Our idea has the potential to be developed and marketed as qualitative and quantitative kits that can be used as a single-use stand-alone test or multiplexed with other biomarker detection kits. To the best of our knowledge, there is currently no In Vitro Diagnostic (IVD) kit available in the market for the detection of anti-insulin and insulin analog-specific anti-insulin antibodies. We believe that our simple, user-friendly, and economical kit will become the preferred companion diagnostic kit in diabetes management, alongside the standard glucose test and HbA1c measurement.
Cite this article
Kumar VR, Sathiya V. Harmonised Methods for Detection of Insulin Immunogenicity. Int. J. Pharm. Investigation. 2024;14(2):306-16.
ACKNOWLEDGEMENT
I want to express my heartfelt appreciation to Dr. Arumugam Muruganandam, who serves as the Managing Director of Affigenix Biosolutions Pvt. Ltd., I am thankful to him for granting me the opportunity and permission to carry out research and a thorough evaluation of the manuscript along with its recommendations. Additionally, I wish to convey my thanks to the dedicated technical team at Affigenix for their indispensable guidance and unwavering support during the entire undertaking.
ABBREVIATIONS
| HQC | High-quality control |
|---|---|
| MQC | Middle-quality control, and Low-quality control |
| POCT | Point-of-care test kit |
| PCs | Positive controls |
| AIA | Anti insulin antibody |
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67-74. [PubMed] | [CrossRef] | [Google Scholar]

- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62-9. [PubMed] | [CrossRef] | [Google Scholar]

- Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11):1-21. [PubMed] | [CrossRef] | [Google Scholar]

- Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):1-34. [PubMed] | [CrossRef] | [Google Scholar]

- Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol. 2021;17(3):150-61. [PubMed] | [CrossRef] | [Google Scholar]

- Li M, Song LJ, Qin XY. Advances in the cellular immunological pathogenesis of type 1 diabetes. J Cell Mol Med. 2014;18(5):749-58. [PubMed] | [CrossRef] | [Google Scholar]

- Kawasaki E. Anti-islet autoantibodies in Type 1 diabetes. Int J Mol Sci. 2023;24(12):10012 [PubMed] | [CrossRef] | [Google Scholar]

- Censi S, Mian C, Betterle C. Insulin autoimmune syndrome: from diagnosis to clinical management. Ann Transl Med. 2018;6(17):335 [PubMed] | [CrossRef] | [Google Scholar]

- Jassam N, Amin N, Holland P, Semple RK, Halsall DJ, Wark G, et al. Analytical and clinical challenges in a patient with concurrent type 1 diabetes, subcutaneous insulin resistance and insulin autoimmune syndrome. Endocrinol Diabetes Metab Case Rep. 2014;2014(1):130086 [PubMed] | [CrossRef] | [Google Scholar]

- Belhiba O, Aadam Z, Jeddane L, Saile R, Salih Alj H, Bousfiha AA, et al. Research of anti-gad and anti-ia2 autoantibodies by elisa test in a series of Moroccan pediatric patients with diabetes type 1. Afr Health Sci. 2020;20(3):1337-43. [PubMed] | [CrossRef] | [Google Scholar]

- Mufioz A, Gallart T, Usac EE. Anti-Islet Cell Anti-Insulin Antibody Prod CD;5 + and CD5-B Lymphocytes in IDDM. [PubMed] | [CrossRef] | [Google Scholar]

- Andersson C, Kolmodin M, Ivarsson SA, Carlsson A, Forsander G, Lindblad B, et al. Islet cell antibodies (ICA) identify autoimmunity in children with new onset diabetes mellitus negative for other islet cell antibodies. Pediatr Diabetes. 2014;15(5):336-44. [PubMed] | [CrossRef] | [Google Scholar]

- Singh G, Singh U, Singh SK, Singh S. Immunogenetic study of diabetes mellitus in relation to HLA DQ and DR. Indian J Endocrinol Metab. 2020;24(4):325-32. [PubMed] | [CrossRef] | [Google Scholar]

- Gomes KFB, Semzezem C, Batista R, Fukui RT, Santos AS, Correia MR, et al. Importance of zinc transporter 8 autoantibody in the diagnosis of type 1 diabetes in Latin Americans. Sci Rep. 2017;7(1):1-7. [PubMed] | [CrossRef] | [Google Scholar]

- Radermecker RP, Scheen AJ. Allergy reactions to insulin: effects of continuous subcutaneous insulin infusion and insulin analogues. Diabetes Metab Res Rev. 2007;23(5):348-55. [PubMed] | [CrossRef] | [Google Scholar]

- Bettencourt-Silva R, Aguiar B, Sá-Araújo V, Barreira R, Guedes V, Marques Ribeiro MJ, et al. Diabetes-related symptoms, acute complications and management of diabetes mellitus of patients who are receiving palliative care: A protocol for a systematic review. BMJ Open. 2019;9(6):1-5. [PubMed] | [CrossRef] | [Google Scholar]

- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119 [PubMed] | [CrossRef] | [Google Scholar]

- Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J, et al. Epidemiology of Type 2 diabetes – Global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107-11. [PubMed] | [CrossRef] | [Google Scholar]

- Hu X, Chen F. Exogenous insulin antibody syndrome (EIAS): A clinical syndrome associated with insulin antibodies induced by exogenous insulin in diabetic patients. Endocr Connect. 2018;7(1):R47-55. [PubMed] | [CrossRef] | [Google Scholar]

- Fineberg SE, Kawabata TT, Finco-Kent D, Fountaine RJ, Finch GL, Krasner AS, et al. Immunological responses to exogenous insulin. Endocr Rev. 2007;28(6):625-52. [PubMed] | [CrossRef] | [Google Scholar]

- Takaya M, Nagao M, Takemitsu S, Nakajima Y, Sugihara H, Uchigata Y, et al. Severe insulin-resistant diabetes due to insulin antibodies associated with eosinophilia. Intern Med. 2015;54(18):2367-71. [PubMed] | [CrossRef] | [Google Scholar]

- Sahni P, Trivedi N, Omer A. Insulin Autoimmune Syndrome: a rare cause of postprandial hypoglycemia. Endocrinol Diabetes Metab Case Rep. 2016:2016 [PubMed] | [CrossRef] | [Google Scholar]

- Hirsch IB, Juneja R, Beals JM, Antalis CJ, Wright EE. The evolution of insulin and how it informs therapy and treatment choices. Endocr Rev. 2020;41(5):733-55. [PubMed] | [CrossRef] | [Google Scholar]

- Ilag LL, Deeg MA, Costigan T. original article. Published online. ;2016:159-68. [PubMed] | [CrossRef] | [Google Scholar]

- Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol Rev. 2019;14(1):50-9. [PubMed] | [CrossRef] | [Google Scholar]

- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793-801. [PubMed] | [CrossRef] | [Google Scholar]

- Neubauer HP, Schöne HH. Potentation of insulin immunogenicity by different types of adjuvant. Basic Clin Aspects Immun Insulin. 2019 [CrossRef] | [Google Scholar]

- Putula E, Hannula P, Huhtala H, Metso S. Glycaemic control in insulin deficient patients using different insulin delivery and glucose sensoring devices: cross-sectional real-life study. Diabetes Epidemiol Manag. 2022;7:100072 [CrossRef] | [Google Scholar]

- Weiss M, Steiner DF, Philipson LH. [Published online 2014];Insulin biosynthesis, secretion, structure, and structure-activity relationships – endotext – NCBI bookshelf. Endotext. [CrossRef] | [Google Scholar]

- Brezar V, Carel JC, Boitard C, Mallone R. Beyond the hormone: insulin as an autoimmune target in type 1 diabetes. Endocr Rev. 2011;32(5):623-69. [PubMed] | [CrossRef] | [Google Scholar]

- Weert Mvd, Møller EH. [Immunogenicity Biopharm]. [Published online]. Immunogenicity of Biopharmaceuticals. 2008:97-111. [CrossRef] | [Google Scholar]

- Evans M, Schumm-Draeger PM, Vora J, King AB. A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes Obes Metab. 2011;13(8):677-84. [PubMed] | [CrossRef] | [Google Scholar]

- Abramenko NA, Vnukova PI, Golovina ES, Makarenko IE, Mosikian AA, Nikiforova AG, et al. Development and validation of approach for the detection of neutralizing antibodies against insulin (glargine) in human blood plasma. Drug Dev Regist. 2019;8(3):70-8. [CrossRef] | [Google Scholar]

- Shah RB, Patel M, Maahs DM, Shah VN. Insulin delivery methods: past, present and future. Int J Pharm Investig. 2016;6(1):1-9. [PubMed] | [CrossRef] | [Google Scholar]

- Berget C, Messer LH, Forlenza GP. A clinical overview of insulin pump therapy for the management of diabetes: past, present, and future of intensive therapy. Diabetes Spectr. 2019;32(3):194-204. [PubMed] | [CrossRef] | [Google Scholar]

- Mathieu C, Gillard P, Benhalima K. Insulin analogues in type 1 diabetes mellitus: getting better all the time. Nat Rev Endocrinol. 2017;13(7):385-99. [PubMed] | [CrossRef] | [Google Scholar]

- Piko P, Werissa NA, Adany R. Genetic susceptibility to insulin resistance and its association with estimated longevity in the Hungarian general and Roma populations. Biomedicines. 2022;10(7):1-18. [PubMed] | [CrossRef] | [Google Scholar]

- Hirano M, Arima H, Oiso Y. Immunological insulin resistance due to insulin antibodies developed after cessation of insulin Therapy in a Patient with Type 2 Diabetes. Diabetes Care. 2008;31(11):e84 [PubMed] | [CrossRef] | [Google Scholar]

- Fineberg SE, Kawabata TT, Finco-Kent D, Fountaine RJ, Finch GL, Krasner AS, et al. Immunological responses to exogenous insulin. 2007;35294:6320 [CrossRef] | [Google Scholar]

- Zaharieva ET, Velikova TV, Tsakova AD, Kamenov ZA. Prevalence of positive diabetes-associated autoantibodies among Type 2 diabetes and related metabolic and inflammatory differences in a sample of the Bulgarian population. J Diabetes Res. 2017;2017:9016148 [PubMed] | [CrossRef] | [Google Scholar]

- Genuth S, Lipps J, Lorenzi G. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. J Am Med Assoc. 2002;287(19):2563-9. [CrossRef] | [Google Scholar]

- Koyama R, Nakanishi K, Kato M, Yamashita S, Kuwahara H, Katori H, et al. Hypoglycemia and hyperglycemia due to insulin antibodies against therapeutic human insulin: treatment with double filtration plasmapheresis and prednisolone. Am J Med Sci. 2005;329(5):259-64. [PubMed] | [CrossRef] | [Google Scholar]

- Yi L, Swensen AC, Qian W jun, Division BS. Northwest P. diabetes. Published online. ;2019:13-25. [CrossRef] | [Google Scholar]

- Kikkas I, Mallone R, Tubiana-Rufi N, Chevenne D, Carel JC, Créminon C, et al. A simple and fast non-radioactive bridging immunoassay for insulin autoantibodies. PLOS ONE. 2013;8(7):e69021 [PubMed] | [CrossRef] | [Google Scholar]

- Elisa S. [Published online 2022];applied sciences Efficient Detection of Pre-Proinsulin by Double Antibody. [PubMed] | [CrossRef] | [Google Scholar]

- Kohno T, Ishikawa E, Sugiyama S, Kamano M, Kuzuya H, Imura H, et al. A highly sensitive enzyme immunoassay of anti-insulin antibodies in human serum. Clin Chim Acta. 1987;168(1):97-107. [CrossRef] | [Google Scholar]

- Barth JA, Levels A, Keilacker H, Ziegler M. Measurement of insulin in human sera using a new RIA kit. 1987;90(3):271-7. [CrossRef] | [Google Scholar]

- Bourdage JS, Cook CA, Farrington DL, Chain JS, Konrad RJ. An Affinity Capture Elution (ACE) assay for detection of anti-drug antibody to monoclonal antibody therapeutics in the presence of high levels of drug. J Immunol Methods. 2007;327(1-2):10-7. [PubMed] | [CrossRef] | [Google Scholar]

- Malmqvist MM. Surface plasmon resonance for detection and measurement of antibody-antigen affinity and kinetics. Curr Opin Immunol. 1993;5(2):282-6. [PubMed] | [CrossRef] | [Google Scholar]

- Valdez SN, Iacono RF, Villalba A, Cardoso Landaburu A, Ermácora MR, Poskus E, et al. A radioligand-binding assay for detecting antibodies specific for proinsulin and insulin using 35S-proinsulin. J Immunol Methods. 2003;279(1-2):173-81. [CrossRef] | [Google Scholar]

- Lo B, Swafford ADE, Shafer-Weaver KA, Jerome LF, Rakhlin L, Mathern DR, et al. Antibodies against insulin measured by electrochemiluminescence predicts insulitis severity and disease onset in non-obese diabetic mice and can distinguish human type 1 diabetes status. J Transl Med. 2011;9(1):1-16. [PubMed] | [CrossRef] | [Google Scholar]

- Farrokhnia M, Amoabediny G, Ebrahimi M, Ganjali M, Arjmand M. Ultrasensitive early detection of insulin antibody employing novel electrochemical nano-biosensor based on controllable electro-fabrication process. Talanta. 2022;238(1):122947 [PubMed] | [CrossRef] | [Google Scholar]

- Mirica AC, Stan D, Chelcea IC, Mihailescu CM, Ofiteru A, Bocancia-Mateescu LA, et al. Latest trends in lateral flow immunoassay (LFIA) detection labels and conjugation process. Front Bioeng Biotechnol. 2022;10:1-22. [PubMed] | [CrossRef] | [Google Scholar]

- Nell LJ, Virta VJ, Thomas JW. Application of a rapid enzyme-linked immunosorbent microassay (ELISA) to study human anti-insulin antibody. Diabetes. 1985;34(1):60-6. [PubMed] | [CrossRef] | [Google Scholar]

- Bergström G, Mandenius CF. Orientation and capturing of antibody affinity ligands: applications to surface plasmon resonance biochips. Sens Actuators B. 2011;158(1):265-70. [CrossRef] | [Google Scholar]

- Sodoyez-Goffaux F, Koch M, Dozio N, Brandenburg D, Sodoyez JC. Advantages and pitfalls of radioimmune and enzyme linked immunosorbent assays of insulin antibodies. Diabetologia. 1988;31(9):694-702. [PubMed] | [CrossRef] | [Google Scholar]


