Contents
ABSTRACT
Objectives
To develop and validate a sensitive, accurate, simple, precise and cost-effective UHPLC method for the simultaneous determination of Teneligliptin and Pioglitazone in pure and its tablet formulation form and validating this developed method as per Guidelines of ICH (Figure 1).
Materials and Methods
The chromatographic separation was done by using column Agilent C18 (2.5 μm; 4.6×100 mm ID), isocratic mobile phase consists of Methanol: 0.1% TEA (PH-6 WITH OPA) 60: 40%v/v. The flow rate of mobile phase is 0.9 mL/min. The separation was carried out at 241 nm wavelength. The current method for accuracy, precision, linearity, specificity, robustness and ruggedness was validated as per ICH guidelines.
Results
Teneligliptin and Pioglitazone retention time observed at 2.382 and 3.315 min respectively. The graphs showing peak area against concentration demonstrated linear between 2-10 μg/mL for Teneligliptin and 1.5-7.5 μg/mL for Pioglitazone. This relationship exhibited a high level of linearity with a Regression coefficient (R2) of 0.999. The determined limit of detection is 0.0843 and 0.0084 μg/mL while the limit of quantification was found to be 0.255 and 0.025 μg/mL for Teneligliptin and Pioglitazone respectively. The assay percentage of the available formulation was found to be 99.72 and 100.51 for Teneligliptin and Pioglitazone.
Conclusion
The Validation parameters indicate the effective separation of the drug substance from their degradants effectively. This developed method shows the` suitability for the routine quantitative analysis of Teneligliptin and Pioglitazone in its pure and their available pharmaceutical formulations for quality control purpose.
INTRODUCTION
Throughout the world, the prevalence of common endocrinological disorders, such as Diabetes Mellitus Type 2 (DMT2), is already high and is rising at an alarming rate. According to estimates, there will be more than 500 million diabetes patients by 2030 and over 700 million by 2045. A diverse metabolic disease, diabetes mellitus is characterized by changes in the metabolism of fat, protein, and carbohydrates.1 A persistent metabolic illness marked by high blood glucose is called diabetes. The common type of diabetes, which affects adults, arises from the body’s inadequate response to insulin called as Type 2 DM.2 Type I, type II, and gestational diabetes are the three main categories of diabetes. The majority of people with diabetes (90%) have type II diabetes. When monotherapy for type II diabetes does not work, combined treatment is often recommended. The FDA has authorized the use of Teneligliptin (TEN) and Pioglitazone (PIO) together to treat type II diabetes.3
Figure 1:
UHPLC System.
Dipeptidyl peptidase-4 inhibition done by the gliptins, include the antidiabetic medication teneligliptin. It is {(2S,4S)-4-[4- (3-methyl-1-phenyl-1H-pyrazol-5-yl)-1-piperazinyl]2-pyrroli dinyl(1,3-thiazolidin-3-yl) Methanone} Figure 2. Teneligliptin suppresses postprandial hyperglycemia after meals, which elevates activated Glucagonlike Peptide-1 (GLP-1) levels and acts for 24 hr.4,5 Teneligliptin’s distinct structure, which is made up of five successive rings, gives it a lasting and powerful impact. Teneligliptin is now used as a therapy for conditions in which diet, exercise, and medications from the thiazolidine or sulfonylurea classes do not sufficiently improve glucose control. The oral dose of teneligliptin should start with a dose of 20 mg once day and can be increased up to 40 mg. Patients with renal impairment do not require a particular dosage modification because the drug’s metabolites are excreted through the liver and kidneys.6
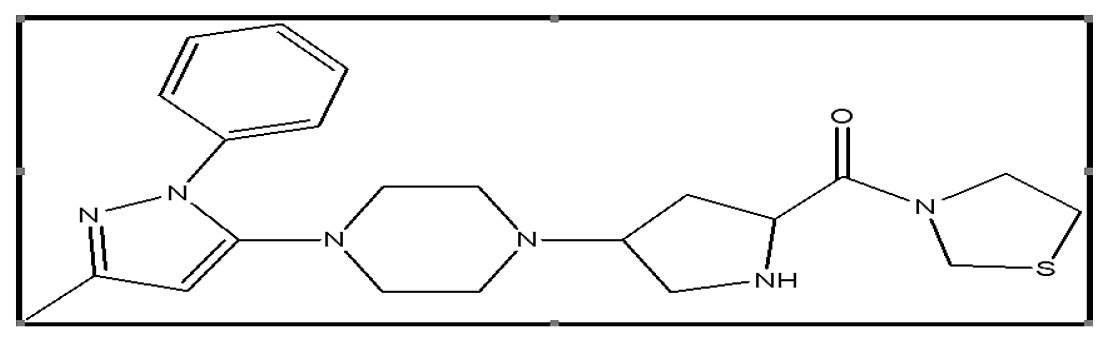
Figure 2:
Structure of Teneligliptin.
Pioglitazone functions as an insulin sensitizer, chemically described as (RS)-5-(4-[2-(5-ethylpyridin-2-yl) ethoxy] benzyl) thiazolidin-2,4-dione, an oral type II diabetes medication Figure 3. This type of diabetes arises from insufficient insulin production in the body. Pioglitazone triggers the ligand-activated transcription factor PPAR-gamma stimulating cell differentiation while inhibiting cell growth and angiogenesis. Activation of macrophages and monocytes inhibited by Pioglitazone and regulates insulin transcription responsive genes and stimulate the differentiation of fat cells. Pioglitazone boosts insulin sensitivity by increasing cellular responsiveness. The patients with type II diabetes, pioglitazone primarily enhances peripheral insulin sensitivity for better glycemic control. In hypoglycemic situations, pioglitazone mask signs like high heart rate, dizziness, and sweating. Additionally, when paired with a sulfonylurea or insulin, it may lead to edema and carries potential side effects such as heart failure and respiratory infections.7,8
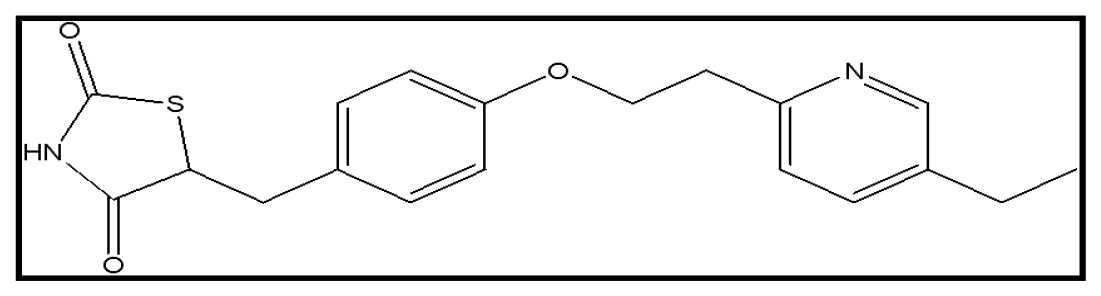
Figure 3:
Structure of Pioglitazone.
In the literature survey, we found that there are several spectroscopic and liquid chromatographic procedures for the determination of Teneligliptin and Pioglitazone by HPLC,9 RP-HPLC,10–14 HPTLC,15–17 UV,18–20 and there is no UHPLC method reported.
Hence, our proposal involved creating an efficient UHPLC technique for concurrently determining Teneligliptin and pioglitazone in both pure and pharmaceutical formulations. This research is focused on developing and validating a rapid, sensitive UHPLC method that offers improved resolution and peak symmetry, adhering to ICH guidelines during validation.
MATERIALS AND METHODS
Reagent and Reference Samples
The standards samples of teneligliptin and pioglitazone obtained as a gift sample from Swapnroop Drugs and Pharmaceuticals, Aurangabad. The chemicals and reagents which are used in analysis are of HPLC grade. A combined fixed dose formulation Zita plus Pio (Glenmark Pharmaceuticals Ltd.,) containing Teneligliptin 20 mg and Pioglitazone 15 mg is available as tablet dosage form. This medication is used in treatment of Type II Diabetes Mellitus. Calibrated glassware’s was used during the study. Calibrated analytical balance is used during all weighing procedures. For UHPLC study, double distilled water and Mili-Q water were utilized.
Instrumentation
During the analysis, we used an Agilent 1100 High-Performance Liquid Chromatography (HPLC) system inbuilt with reciprocating pump (HP-1100) and coupled with UV detector. We utilized the chemstation software to analyse the HPLC reports. The analysis was conducted using an Agilent C18 column (2.5 μm; 4.6×100 mm ID). Additionally, the Digital weighing balance (ME-204) was obtained from Mettler-Toledo (USA) and the digital pH meter by Mettler-Toledo was obtained from (Mumbai-India). The ultra-sonicator Labman was acquired from Ultra chrome Ltd, India. A 50 μ micro-syringe was purchased from Hamilton USA. Nylon membrane filters with pore size 0.20 μ and 0.45 μ were sourced from Phenomenex, Mumbai, India.
Methodology
Statistical Analysis
To ensure the suitability of analytical requirements and the reliability of results, we validate the analytical characteristics of the tested HPLC method.
Preparation of Stock Standard Solutions
20 mg of Teneligliptin and 15 mg of Pioglitazone were weighed carefully and dissolved in 100 mL methanol to get 200 μg/mL and 150 μg/mL respectively. Removal of air bubbles and small particles solubilize by sonicating the both samples for 2-5 min. Then serial of homogeneous mixture consisting Teneligliptin (200 μg/mL) and Pioglitazone (150 μg/mL) were made as needed and used to determine robustness, accuracy, and repeatability and other validation parameters.
Preparation of Sample Solution
Weighing twenty tablets allowed us to determine their average weight. After that, they were grinded into a powder using a mortar and pestle. The resulting powder was precisely weighed and put into a 100 mL volumetric flask, equal to 15 mg of Pioglitazone and 20mg of Teneligliptin. After adding 100 mL of methanol diluent, the small particles dissolved by sonicating the sample for 15 min. The volume was further adjusted with diluent in order to achieve a concentration of 150 μg/mL for Pioglitazone and 200 μg/mL for Teneligliptin.
Preparation of Buffer
To create a 0.1% triethylamine buffer, 0.1 g of triethylamine was diluted to 100 mL using HPLC-grade water and PH is modified to 6 with Ortho-Phosphoric Acid (OPA).
Mobile Phase
The mobile phase consisting of methanol and 0.1% TEA (PH 6 WITH OPA) were added in the ratio of 60:40 v/v.
Method Validation
According to ICH guidelines, method validation was completed.
System Suitability Studies
A uniform mixture of a recently prepared stock solution containing equal concentrations of Teneligliptin (20 ppm) and Pioglitazone (15 ppm) was subjected to five injections in order to assess the consistency of results with respect to the Relative Standard Deviation (RSD), which should consistently remain below 2%. Furthermore, System suitability parameters like capacity factor (k’), theoretical plates (N), Resolution (Rs), tailing factor were evaluated.
Specificity
Specificity refers to a method’s capability to differentiate the target analyte from other components in the sample. The purpose of these studies is to verify whether the optimized approach is free of interferences. To assess the specificity of the procedure both blank and placebo solutions were introduced into the HPLC system under optimal condition. In the chromatogram of the blank and placebo, there should be no interfering peaks observed at the retention time of the selected drugs.
Linearity
The preparation of five distinct concentration calibration standards in five replicates allowed for the determination of the method’s linearity.
Graphs are plotted where the y-axis represents peak areas and the X-axis represents concentrations where the concentration ranges from 1.5-7.5 μg/mL for Pioglitazone and 2-10 μg/mL for Teneligliptin produced the calibration curve plots for PIO and TEN. It is desirable for the correlation coefficient to exceed 0.99.
Accuracy
Recovery experiments involve the addition of measured quantity of pure standard drug to the sample solution and measuring its recovery by assessing the peak areas and were used to evaluate the method’s accuracy. Standard was added to the sample at concentrations of 80%, 100%, and 120% of the test. A duplicate assay was performed on the resulting spiked sample. For every level, the recovery percentage should range from 98% to 102%.
Precision
Precision in a measurement refers to the level of consistency between consecutive measurements conducted under defined conditions from multiple components of the same uniform sample. The Relative Standard Deviation (RSD) or Standard Deviation (SD) are used to express it. A measure of precision could be the analytical method’s reproducibility or degree of repeatability.
Method Precision
Peak areas were measured after sample solutions were injected three times on three separate days, all under optimal conditions. It is not acceptable for the RSD% to exceed 2 for the peak areas of the three standard injection results.
Intermediate Precision
The peak areas of three replicates of the sample solutions were measured the same day they were injected under ideal circumstances. The three replicate injection results’ peak areas’ RSD% shouldn’t be more than 2.
Ruggedness
By conducting the experiment with various instruments, operators, and comparable column types, the robustness of the method was ascertained.
Robustness
The chromatographic methods robustness was evaluated by deliberately introducing minor changes to the chromatographic conditions. Variation in a flow rate, pH, wavelength and mobile phase were specifically evaluated in this. It is possible to track the impacts of each of these variables on variations in the retention pattern, including those on the Tailing factor (Tf), theoretical plates (N), peak area, retention Factor (k’), separation factor and Resolution (Rs).
Effect of variation in Flow
Sample was analysed at 0.9 mL/min flow rate.
Effect of variation in Temperature
In place of 33°C, a temperature of 25°C and 33°C was kept. Following the injection of samples in duplicate, chromatograms were recorded.
LOD AND LOQ
LOD means limit of detection represents the minimum detectable concentration, although it may not be precisely quantified. It is determined by following formula,
Where, σ=Standard Deviation; S=Slope.
LOQ stands for limit of quantification refers to minimum quantity of an analyte within the sample that can be precisely and accurately determined.
Where, σ=Standard Deviation; S=Slope.
RESULTS
The optimized chromatographic conditions yielded results as presented in Table 1. All the parameters of system suitability, like theoretical plates, tailing factor, retention time meeting the predefined acceptance criteria. Table 2 summarize of these system suitability parameters. Importantly, no additional peaks were observed at the retention times of Pioglitazone and Teneligliptin, as demonstrated in Figure 4, confirming the method’s specificity. The quantification exhibited linearity within the concentration range of 1.5-7.5 μg/mL for Pioglitazone and achieving a 0.999 correlation coefficient as illustrated in Figure 5. Similarly for Teneligliptin, the linearity was observed within the concentration range of 2-10 μg/mL, with 0.9993 correlation coefficient as depicted in Figure 6. The linearity results have been summarized in Table 3. Pioglitazone and Teneligliptin recoveries fell within the range of 101.11-101.42% and 101.55-99.50% respectively. In accordance with established guidelines, the results have been expressed in percentages as illustrated in Table 4. Notably, the method demonstrates satisfactory precision, with a Relative Standard Deviation (RSD) not exceeding 2%. Ruggedness was assessed by multiple analysts on various days, and the findings are documented in Table 5. These ruggedness studies revealed no significant variations in the results, affirming the method’s robustness, as detailed in Tables 6 and 7. The results of the assay were juxtaposed with the claim by the label of commercially available formulations, and the outcomes are presented in Table 8. To establish the Limits of Detection (LOD) and Limit of Quantification (LOQ), calculations are done depends on the Slope and Standard Deviation (SD) values, and these limits have been documented in Tables 9–10.
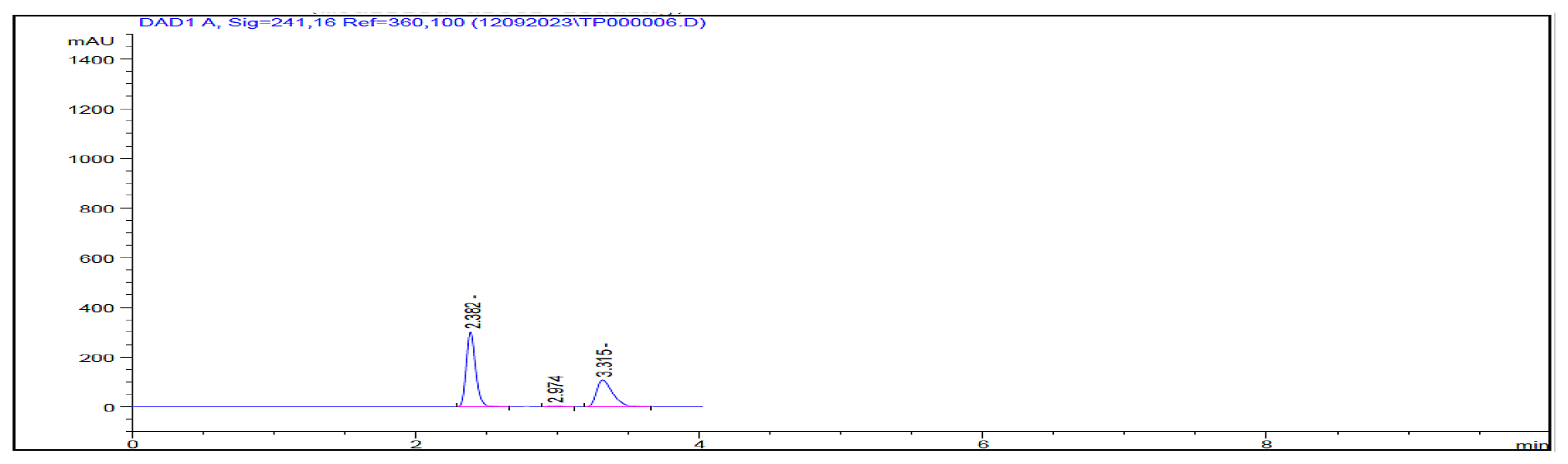
Figure 4:
Chromatogram showing resolved peaks of Teneligliptin and Pioglitazone.
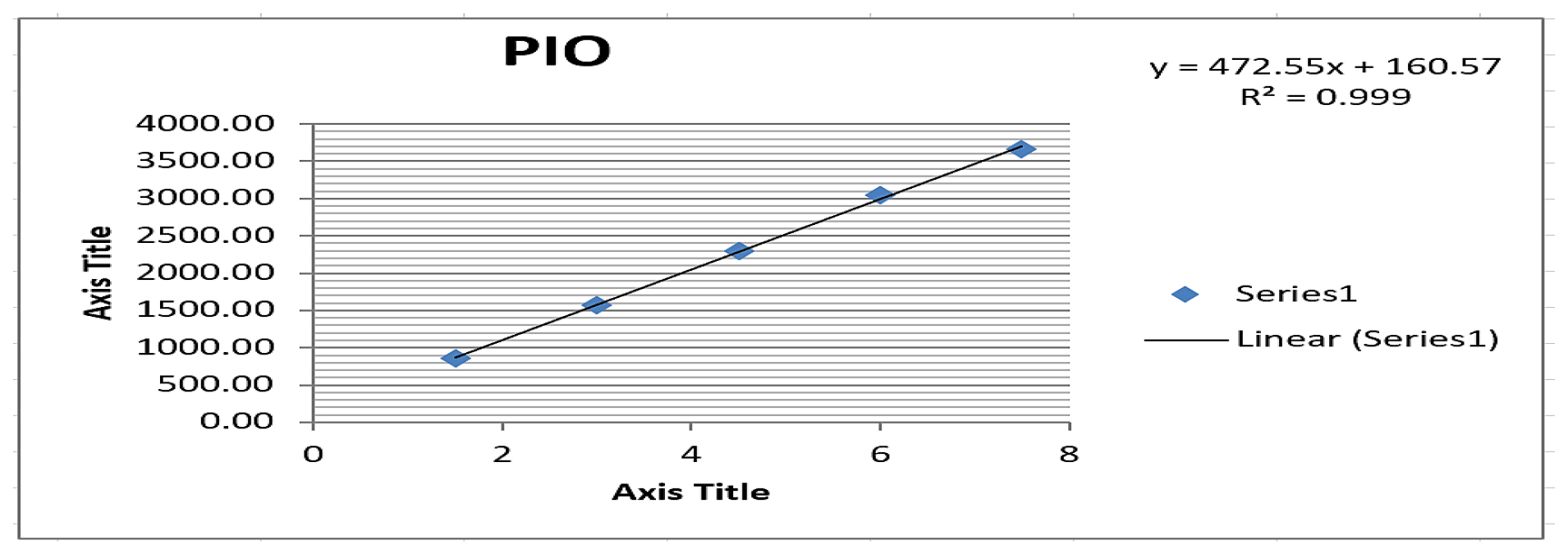
Figure 5:
Linearity plot of Pioglitazone.
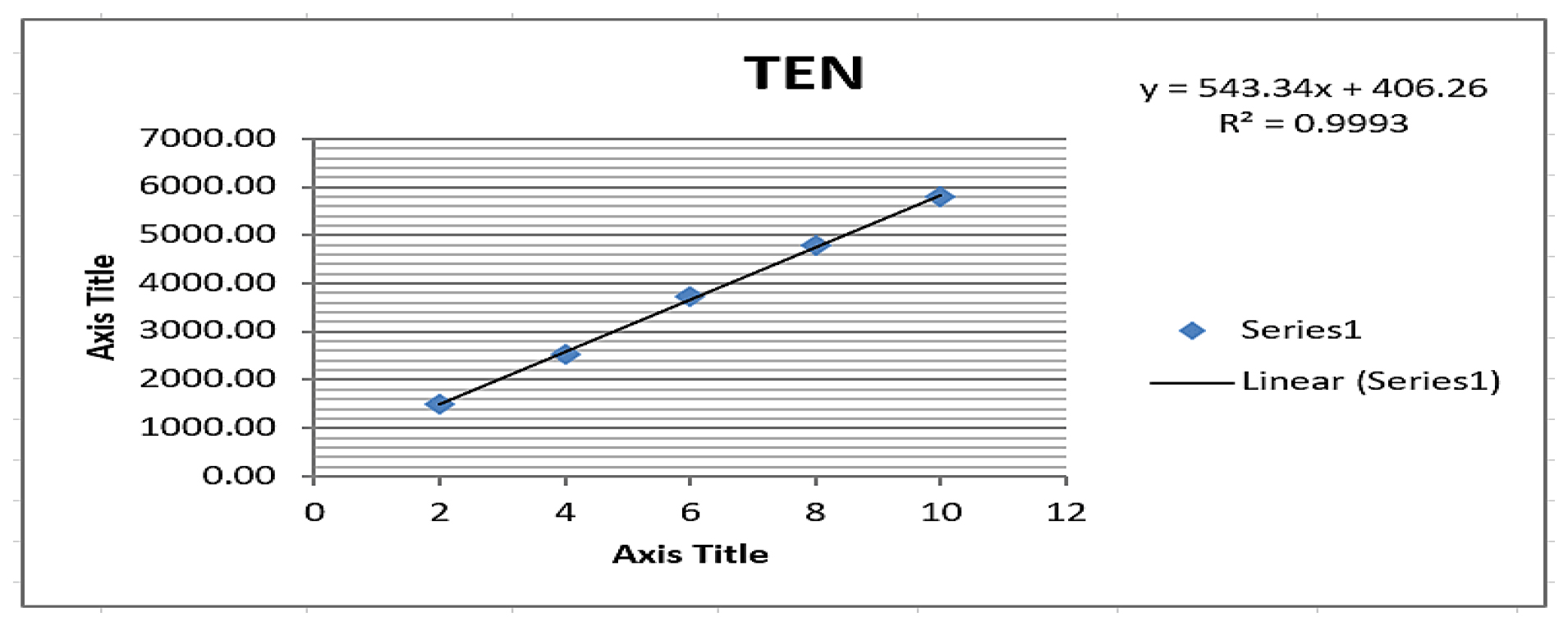
Figure 6:
Linearity plot of Teneligliptin.
| Parameters | Chromatographic Condition |
|---|---|
| Mode of elution | Isocratic |
| Mobile Phase | Methanol: 0.1% TEA (PH 6 WITH OPA); 60:40 |
| Column | Agilent C18 (ID 2.5 μm; 4.6×100 mm Length) |
| Flow Rate | 0.9 mL/min |
| Runtime | 10 min |
| Injection Volume | 20 μL |
| Detection Wavelength | 241 |
| Temperature | 33°C |
| Parameters | Teneligliptin | Pioglitazone | Acceptance criteria |
|---|---|---|---|
| Tailing factor | 0.78 | 0.57 | ≥2 |
| Retention time | 2.382 | 3.315 | ≤2 |
| Theoretical plates | 6503 | 4323 | ≤2000 |
| Parameters | Teneligliptin | Pioglitazone |
|---|---|---|
| Linearity range (μg/ mL) | 2-10 | 1.5-7.5 |
| Regression coefficient±SD | 0.999±13.89 | 0.999±1.20 |
| Slope±SD | 543.34±13.89 | 472.55±1.20 |
| Intercept±SD | 406.2±13.89 | 160.5±1.20 |
| Drug | Level | Analyte amount (mg) | Recovery amount (mg) | Mean% recovery | RSD% |
|---|---|---|---|---|---|
| Teneligliptin | 80% | 1.6 | 1.62 | 101.55 | 0.08 |
| 100% | 2 | 2.057 | 102.87 | 0.04 | |
| 120% | 2.4 | 2.38 | 99.46 | 0.03 | |
| Pioglitazone | 80% | 1.2 | 1.21 | 101.11 | 0.16 |
| 100% | 1.5 | 1.52 | 101.61 | 0.09 | |
| 120% | 1.8 | 1.82 | 101.54 | 0.08 |
| Drug | Analyst-1 (Peak area) | Analyst-2 (Peak area) | SD | RSD% |
|---|---|---|---|---|
| Teneligliptin | 2526.79 | 2528.5166 | 1.882 | 0.048 |
| Pioglitazone | 1568.39 | 1569.16 | 1.098 | 0.070 |
| Chromatographic Condition | Changes | Theoretical plate | Tailing Factor | Resolution |
|---|---|---|---|---|
| Flow (-0.1 mL/min) | 0.8 | 7269 | 0.87 | – |
| Flow (+0.1 mL/min) | 1.0 | 6226 | 0.87 | – |
| M.P (-0.1 mL/min) | 59+41 | 6751 | 0.86 | – |
| M.P (+0.1 mL/min) | 61+39 | 6736 | 0.87 | – |
| Wavelength (-1) | 240 | 6980 | 0.88 | – |
| Wavelength (+1) | 242 | 6980 | 0.88 | – |
| Chromatographic Condition | Changes | Theoretical plate | Tailing Factor | Resolution |
|---|---|---|---|---|
| Flow (-0.1 mL/min) | 0.8 | 3310 | 0.41 | 5.55 |
| Flow (+0.1 mL/min) | 1.0 | 3166 | 0.44 | 5.34 |
| M.P (-0.1 mL/min) | 59+41 | 3004 | 0.42 | 1.44 |
| M.P (+0.1 mL/min) | 61+39 | 3232 | 0.44 | 1.37 |
| Wavelength (-1) | 240 | 3233 | 0.42 | 1.41 |
| Wavelength (+1) | 242 | 3233 | 0.43 | 1.41 |
| Drug | Label claim | Amount found | % Assay |
|---|---|---|---|
| Teneligliptin | 20 mg | 19.94 | 99.72 |
| Pioglitazone | 15 mg | 15.076 | 100.51 |
| Teneligliptin | Pioglitazone |
|---|---|
| LOD=3.3 X Avd.SD/ Slope =45.8321782 =0.084358878 | LOD= 3.3 X Avd.SD/ Slope =3.974476097 =0.00841159 |
| Teneligliptin | Pioglitazone |
|---|---|
| LOQ=10X Avd SD/Slope = 138.8853885 =0.25563296 | LOQ=10X Avd SD/Slope = 12.04386696 =0.025489666 |
DISCUSSION
This linearity of this UHPLC method demonstrated between the range of 2-10 μg/mL and 1.5-7.5 μg/mL for teneligliptin and pioglitazone respectively. The method validation is carried out successfully in the optimized conditions and validation results for various parameters were within the acceptable limits. Sample preparation done with the dilution and filtration of sample. The determination was achieved by using an Agilent C18 column, 4.6×100 mm; 2.5 μm consisting an isocratic mobile phase of Methanol: 0.1% TEA (PH-6 WITH OPA) 60: 40v/v at a flow rate of 0.9 mL/min. Retention times for teneligliptin is 2.382 min and for pioglitazone is 3.315 min. The lower Limit of Quantification (LOQ) was found to be 0.255 μg/mL, 0.025 μg/mL for teneligliptin and pioglitazone respectively and lower Limit of Detection (LOD) were found to be 0.084 μg/mL, 0.0084 μg/mL for both teneligliptin and pioglitazone respectively. Filter compatibility was assessed and variation between % Assay of unfiltered sample and filtered sample did not exceed 2.0%. Validated was conducted by following the ICH guidelines. Determination of teneligliptin and pioglitazone has been successfully done by this method for pure drug and tablet formulations.
CONCLUSION
The UHPLC method was developed for the simultaneous estimation of teneligliptin and pioglitazone in pure form and pharmaceutical formulations offering the speed, convenience, precision and accuracy. This method considered more cost effective as compared to other reported method. It is suitable for the tablet analysis, making it a viable option routine quality control of teneligliptin and pioglitazone in these pharmaceutical formulations.
Cite this article
Kolhe MH, Godase SN, Vikhe KB, Bhor RJ. Method Development and Validation for Simultaneous Estimation of Teneligliptin and Pioglitazone by UHPLC Method. Int. J. Pharm. Investigation. 2024;14(2):577-84.
ACKNOWLEDGEMENT
The author thanks to Swapnroop Drugs and Pharmaceuticals, Aurangabad, Maharashtra, India for providing gift samples of teneligliptin and pioglitazone respectively.
ABBREVIATIONS
| ICH | International Council for Harmonisation |
|---|---|
| UHPLC | Ultra High Performance Liquid Chromatography |
| TEA | Triethylamine |
| OPA | Ortho-Phosphoric Acid |
| DMT2 | Diabetes Mellitus Type 2 |
| FDA | Food and Drug Administration |
| TEN | Teneligliptin |
| PIO | Pioglitazone |
| HPLC | High Performance Liquid Chromatography |
| HPTLC | High-Performance Thin-Layer Chromatography |
| RP-HPLC | Reverse Phase High Performance Chromatography |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| RSD | Relative Standard Deviation |
| S.D | Standard Deviation |
| M.P | Mobile Phase |
References
- David Blessing Rani J, Asha Deepti C. Method development, validation and forced degradation studies of new rp-hplc method for simultaneous estimation of remogliflozin and teneligliptin in pure and tablet dosage form. Int J Pharm Sci Res. 2023;14(7):3452 [CrossRef] | [Google Scholar]
- Biswas B, Kumar M, Sharma JB, Saini V, Bhatt S. Method development and validation for estimation of teneligliptin in tablet dosage form by rp-hplc. Res J Pharm Technol. 2020;13(4):1774-8. [CrossRef] | [Google Scholar]
- Pandit V, Pai RS, Devi K, Singh G, Narayana S, Suresh S, et al. Development and validation of the liquid chromatographic method for simultaneous estimation of metformin, pioglitazone, and glimepiride in pharmaceutical dosage forms. Pharm Methods. 2012;3(1):9-13. [PubMed] | [CrossRef] | [Google Scholar]
- Rao SS, begum KA, Srinivasa Rao S. Glob. Trends Pharmacol Sci. Analytical Method Development and Validation of Teneligliptin And Metformin Hcl By Using Rp-Hplc Method. In J. 2020;11(3) [PubMed] | [CrossRef] | [Google Scholar]
- Lokhande P. Analytical method development and validation of teneligliptin by using RP-HPLC with ICH guidelines. Int J Trend Sci Res Dev. 2019;3(3):2456-6470. [PubMed] | [CrossRef] | [Google Scholar]
- Drug profile for teneligliptin. Available fromhttps://go.drugbank.com/drugs/DB11 950
- Drug profile for pioglitazone. Available fromhttps://go.drugbank.com/drugs/DB01 132
- Maste MM, Gawas NS. RP-HPLC method development and validation for pioglitazone in bulk and marketed formulation. Pharm Chem. 2021;13(6):6-15. [PubMed] | [CrossRef] | [Google Scholar]
- Mirza AZ, Arayne MS, Sultana N. Method development and validation of amlodipine, gliquidone and pioglitazone: application in the analysis of human serum. Anal Chem Lett. 2014;4(5-6):302-12. [CrossRef] | [Google Scholar]
- Patel BD, Dharsandiya NJ, Chaudhary A. Development and validation of RP-HPLC method for estimation of teneligliptin and its impurity in tablet. Int J Pharm Sci Rev Res. 2021;69(2) [CrossRef] | [Google Scholar]
- Chabukswar A, Sakpal P. RP-HPLC and UV-spectrophotometric methods development and validation for simultaneous estimation of teneligliptin and metformin in fixed dose combination Curr. Pharm Res. 2019(3) [CrossRef] | [Google Scholar]
- Prajapati P, Rana B, Pulusu VS, Shah S. Method operable design region for robust RP-HPLC analysis of pioglitazone hydrochloride and teneligliptin hydrobromide hydrate: incorporating hybrid principles of white analytical chemistry and design of experiments. Future J Pharm Sci. 2023;9(1):93 [CrossRef] | [Google Scholar]
- Gaikwad AS, Raut SR, Bargaje GS. Development and Validation of RP-HPLC and UV-spectrophotometric Method for Simultaneous Estimation of Teneligliptin hydrobromide hydrate and metformin hydrochloride in Pharmaceutical dosage form. Int J Creat Res Thoughts. 2021:9 [CrossRef] | [Google Scholar]
- Vetapalem R, Yejella RP, Atmakuri LR. Development and validation of a stability indicating rp-hplc method for simultaneous estimation of teneligliptin and metformin. Turk J Pharm Sci. 2020;17(2):141-7. [PubMed] | [CrossRef] | [Google Scholar]
- Sen AK, Bhimani N. Densitometric simultaneous assessment of teneligliptin hydrobromide and pioglitazone hydrochloride in combined tablet. J Sep Sci. 2023 [CrossRef] | [Google Scholar]
- Patel M, Patel D, Shah U, Kachhiya HM. Simultaneous quantification of teneligliptin hydrobromide and metformin hydrochloride: an improved hptlc method with implementation of plackett-burman design. J Chem Metrol. 2021;15(1):65-75. [CrossRef] | [Google Scholar]
- Patel VB, Patel JS, Shah DA. Estimation of metformin hydrochloride and teneligliptin in pharmaceutical formulation by high performance thin layer chromatography. Ind DRU. 2018;55(10):34-9. [CrossRef] | [Google Scholar]
- Sen DB, Sen AK, Zanwar AS, Pandey H, Maheshwari RA. UV spectrophotometric methods to quantify alogliptin benzoate and pioglitazone hydrochloride. J Pharm Res Int. ;2021:31-41. [CrossRef] | [Google Scholar]
- Ms S, Goyal PK. Simultaneous Estimation of Teneligliptin and Metformin Hydrochloride in Tablet Formulation by UV Spectrophotometric Method. Chemistry Research Journal. 2021;6(6):160-5. [CrossRef] | [Google Scholar]
- Annapurna MM, Naik RR, Pratyusha SM. Simultaneous spectrophotometric determination of compounds: application to an anti-diabetic formulation of Teneligliptin and metformin. Res J Pharm Technol. 2020;13(4) [CrossRef] | [Google Scholar]


