ABSTRACT
Background
Toxic Epidermal Necrolysis (TEN), also known as Lyell’s syndrome, is a rare but severe connective tissue condition that is a common cause of substantial skin damage as well as mucous membrane breakdown. This condition is distinguished by the detachment of the epidermis presenting as blisters and denuded skin areas along with erythema. Supportive care is typically the mainstay in the primary treatment of TEN. The effectiveness of systemic steroids remains uncertain due to the limited availability of studies that directly compare the utility of these therapeutic approaches. Here, we report a case where TEN was successfully treated with dexamethasone.
Case Presentation
We present a case of an 18-year-old female patient who presented with complaints of itchy skin lesions, multiple purpuras and petechiae following ingestion of a dose of a cefadroxil tablet. A diagnosis of cefadroxil-induced Toxic Epidermal Necrolysis was made. Hydration therapy along with systemic steroids and topical treatments were administered to the patient. The lesions healed gradually and the condition of the patient improved.
INTRODUCTION
Toxic Epidermal Necrolysis (TEN), also referred to as Lyell’s syndrome, is a rare yet severe mucocutaneous adverse drug reaction with a significant mortality risk. Alan Lyell first characterised it in 1956 as “an eruption resembling scalding of the skin.” Authors and experts typically regard Stevens-Johnson syndrome (SJS) and TEN as separate manifestations of the same disease.1 The dermal detachment between 10% and 29% is used to characterise the overlap between SJS and TEN. TEN is characterised by epidermal detachment of around 30% or more, whereas SJS is characterised by less than 10%. Nikolsky’s sign is caused by a separation of the dermal-epidermal junction, which gives skin the characteristic “wet dressing” appearance.2
TEN is primarily caused by drugs and rarely by infections.3 SJS and TEN have an estimated incidence of 1-6 and 0.4-1.2 cases per million person-years, respectively.4 Anticonvulsants, nonsteroidal anti-inflammatory medicines, antibiotics and sulphonamides are some of the medications that may result in TEN.5 While severe cutaneous reactions caused by cephalosporins are infrequent, there have been documented cases, often arising during concurrent administration with another antibiotic.6
In all instances, the provision of symptomatic and appropriate supportive care holds paramount importance, preferably within a burn unit setting.7,8 However, the utilization of targeted treatments such as systemic corticosteroids or Intravenous Immunoglobulin (IVIG) remains a subject of debate.9 Here, we describe a probable case of cefadroxil-induced TEN treated with dexamethasone.
CASE PRESENTATION
An 18-years-old female presented to the casualty department with complaints of itchy skin lesions over the body for 2 days. 20 days ago, she was presented at the hospital following a road traffic accident where she fell from a bike and sustained an injury to the head, necessitating immediate medical attention. She was diagnosed with diffuse cerebral edema and managed conservatively with intravenous fluids, antiepileptics, analgesics, mannitol and antiemetics. She was discharged with a prescription for tablet phenytoin and nortriptyline with appropriate medical advice. The patient had stopped taking phenytoin and nortriptyline 4 days ago.
The patient was apparently all right until 2 days ago when she developed a fever along with throat pain and abdominal pain for which she was prescribed paracetamol and cefadroxil tablets. She reported having taken one tablet of cefadroxil after which she started developing itchy skin lesions which started on her upper limbs and then progressed to involve the face, lower limbs, trunk and back. The patient denied any history of topical application or photosensitivity, smoking, consumption of alcohol or illicit drug use. She had no history of any comorbidity.
At presentation, she was conscious and oriented to time, person, and place. She was cooperative as well. The patient’s Glasgow Coma Scale (GCS) score was 15 out of 15. Her vitals were stable (Pulse rate: 82 beats per minute, blood pressure: 110/80 mmHg, oxygen saturation: 98% at room air and respiratory rate: 18 cycles per minute). Cardiovascular, abdominal, and respiratory system examinations did not reveal any abnormality. Her blood glucose levels were within normal limits. On examination, multiple purpuras and petechiae were noted on bilateral upper limbs, chest, abdomen, trunk, neck, face, and lower limbs. [Figure 1 and Figure 2 A] A few flaccid bullae were seen on bilateral forearm and trunk. Few lesions over the hard palate of the oral cavity were also seen. Nikolsky’s sign (which is the epidermal separation caused by applying slight lateral pressure on the skin’s surface) was easily visible and was noted to be positive [Figure 2 B].
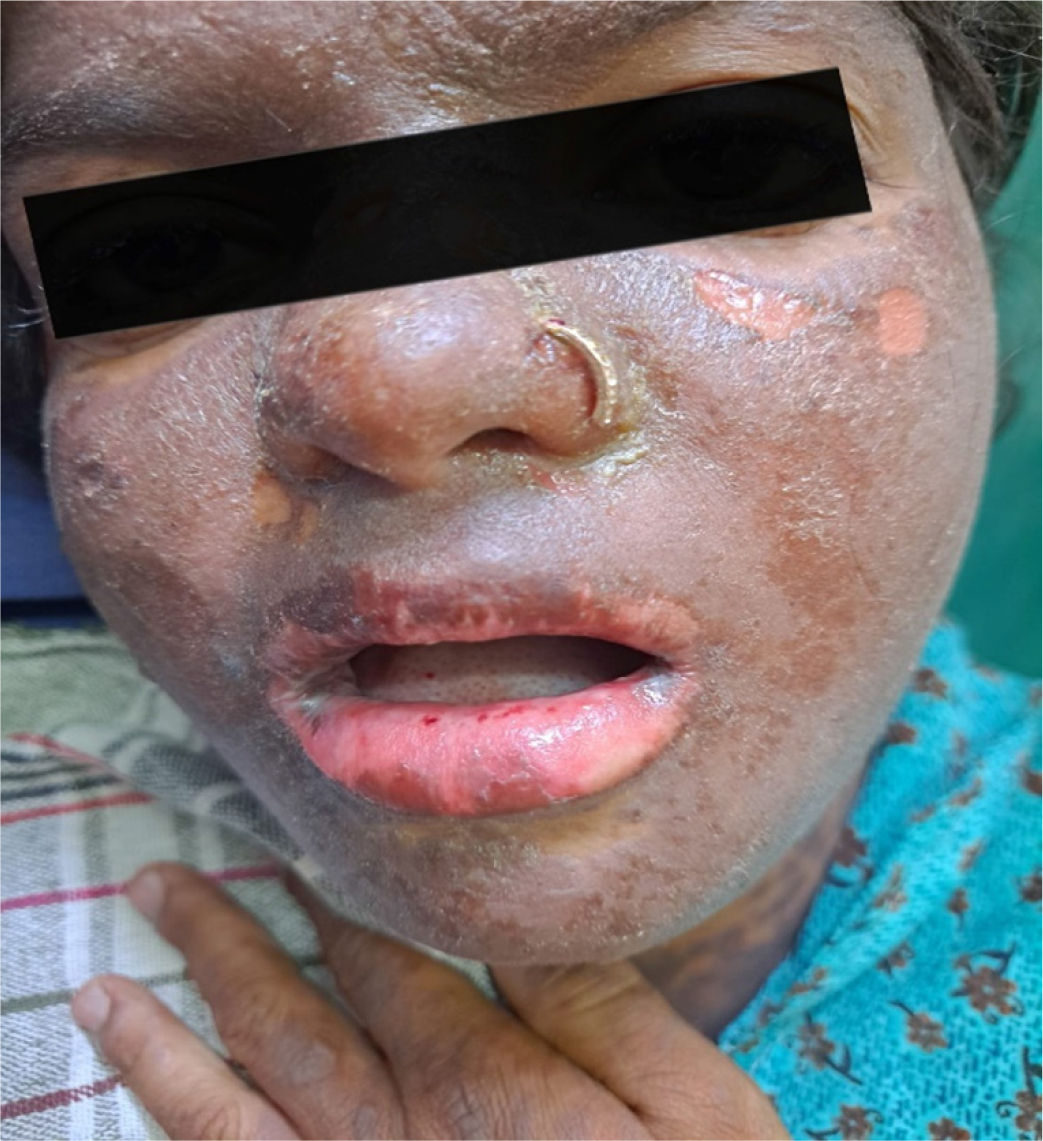
Figure 1.
Facial lesion.
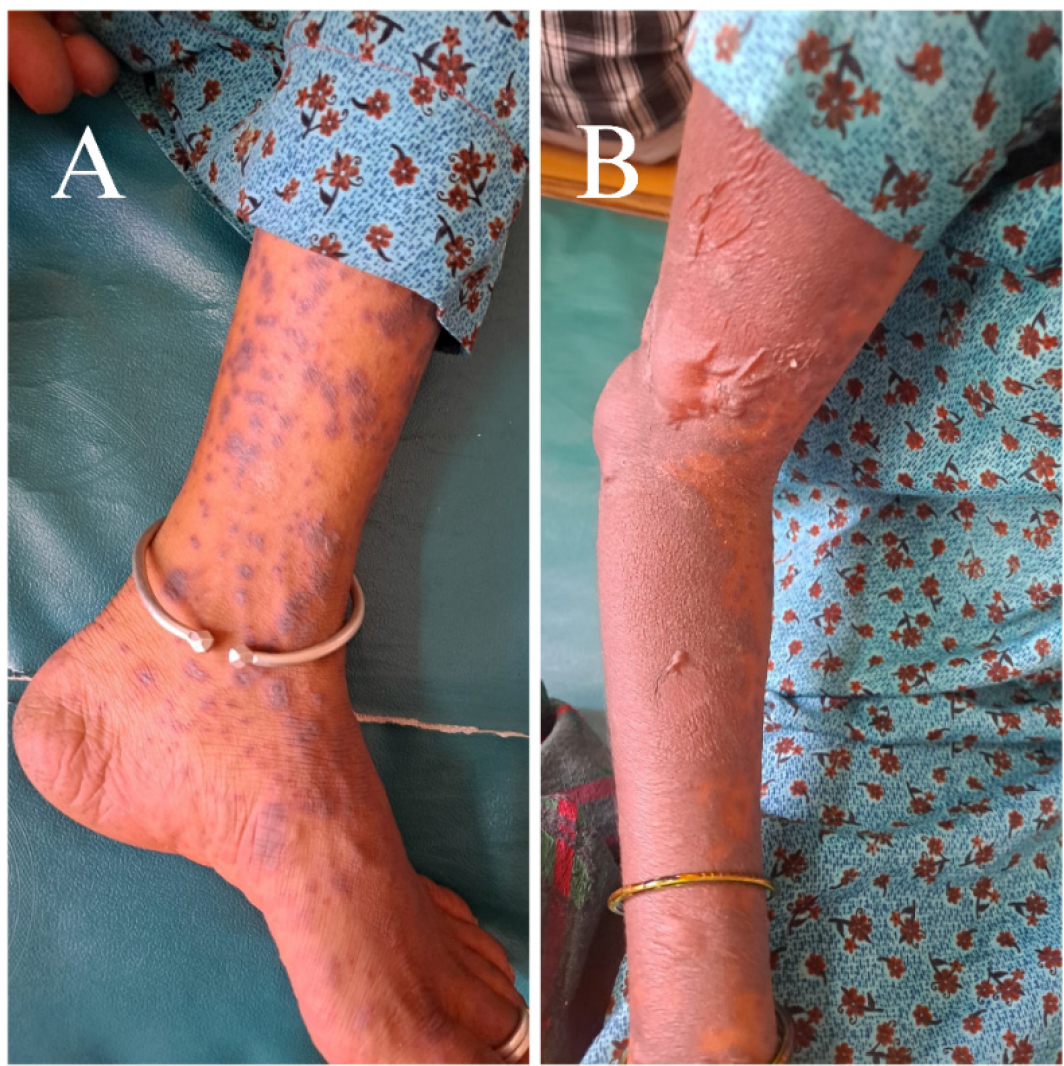
Figure 2.
Lesions on the limbs. (A) Multiple purpuras were observed on the lower limb. (B) A positive Nikolsky’s sign was seen on the upper limb.
The patient’s blood work and electrolyte panel were within normal limits. No organisms were isolated during microbiology tests. However, her Liver Function Test (LFT) and Renal Function Test (RFT) were abnormal (Bilirubin unconjugated: 0.2 mg/dl, ALT: 94 U/L, AST: 89 U/L, and Blood urea: 59 mg/dl respectively). Urine analyses were normal, and chest X-ray and electrocardiogram (ECG) readings revealed no abnormalities. A clinical diagnosis of toxic epidermal necrolysis was made and cefadroxil was suspected to have induced the same. The suspected drug was immediately withdrawn.
The patient was hospitalised, and the following treatment was initiated: intravenous fluids (normal saline and dextrose normal saline), 2 cc intramuscular injection of dexamethasone was initiated as a STAT order along with 2 cc intramuscular injection of chlorpheniramine maleate. The patient was moved to an isolated location. Framycetin tulle dressing was carried out twice a day. Framycetin cream and Calamine lotion were prescribed as topical treatments. The patient was advised to do salt water gargles twice a day to treat oral lesions. Nortriptyline and Paracetamol were administered to the patient orally. Supportive care in the form of protein supplements for nutrition and a strict diet was advised by a nutritionist. To prevent her skin from adhering to the cotton bed, the patient was compelled to alter her position on a sterile sheet. Dexamethasone was gradually tapered off and then stopped after ten days. Her vitals were monitored continuously throughout her hospital stay and were within normal limits. The lesions healed gradually, and the patient’s health improved without any sequelae. Due to the early initiation of treatment, the time for arresting disease progression and for re-epithelialization was significantly short.
DISCUSSION
Cutaneous medication reactions are the most often reported adverse drug reactions. TEN, although rare, is considered a severe type of erythema multiform spectrum.10 Because of the high mortality rate, which ranges from 16 to 25%, it is critical to recognise the clinical signs of mucocutaneous eruption at an early stage.11–14 Medication is the most common cause of these disorders. The vast majority of instances of TEN are caused by drug exposure and a subsequent hypersensitivity reaction.15,16 One study revealed that among antibiotics, cephalosporins have been found to trigger TEN.17 Our patient reported itchy skin lesions all over her body after taking a dose of cefadroxil, a cephalosporin. Clinical data obtained on examination supported the diagnosis of TEN. Multiple studies revealed that cephalosporins are the culprit in inducing TEN.6,18,19
The presumption, in this case, was that the patient developed TEN due to the consumption of cefadroxil for the complaints of fever, throat and abdominal pain. The likelihood of the adverse reaction being attributed to cefadroxil was evaluated using the Naranjo probability scale.20 The Naranjo score amounted to 6 out of 13, indicating a probable adverse drug reaction linked to cefadroxil. Points were assigned based on prior documented instances of this adverse reaction (+1), occurrence of the adverse event subsequent to medication administration (+2), absence of any alternative cause solely accountable for the reaction (+2), and corroboration through objective evidence (+1). However, rechallenge with cefadroxil, placebo administration, and measurement of serum cefadroxil concentrations were not carried out.
Specific epidemiological research on the prevalence of TEN in developing countries such as India has not been reported. One study revealed that in an Indian population anti-microbials (27.1%), anti-virals (23%), anti-seizure medications (8.4%), and analgesics (8.4%) were the most frequently associated with TEN. Because the main etiologic agent of TEN, drugs such as anti-biotics, anti-convulsants, and nonsteroidal anti-inflammatory drugs (NSAIDs), are easily available without a prescription, the prevalence of TEN may be much higher.21 The pathogenesis of TEN is still not completely understood. TEN was previously linked to Fas-Fas ligand or granulysin-mediated apoptosis. Recent research has revealed that reactive oxygen species (ROS) induce keratinocyte destruction and that they predate the activation of the apoptotic mechanisms outlined above.
There is no mainstay treatment for TEN due to its complexity. The primary treatment for TEN is supportive care until the damaged skin has re-epithelialized. Fluid resuscitation along with management of pain, sterile wound care, and nutritional support are examples of supportive measures. The casualty department must prioritise the following: withdrawal of the offending medicine and early referral to a burn unit or critical care unit with experience in dealing with such situations. These two methods, when done during the initial 24 hr of blister formation, reduce infection rates and hospital stays while also improving overall survival.22,23 To prevent secondary infection until re-epithelialization, aseptic wound care is essential. Good wound care will also lessen the need for analgesics.24 Our patient was regularly provided sterile wound care which acted as the cornerstone in the treatment of TEN.
In the past, systemic steroids were commonly employed as the primary treatment for TEN. Certain research suggests that administering corticosteroids in significant doses during the early stages of the disease can improve the patient’s condition and potentially save lives25 while contrasting studies have indicated an elevation in both mortality rates and hospital stays because of corticosteroid usage.26 However, a recent retrospective case-control study conducted in Europe did not find any instances of mortality associated with steroid treatment. There has been growing interest in the administration of short-term, high-dosage steroid therapy at the initial stages of the disease before substantial loss of the epidermis occurs.9,27 Our patient was successfully treated with dexamethasone along with chlorpheniramine intramuscularly as a part of her initial therapy without any sequelae. According to one Indian study, TEN cases were successfully managed with systemic steroids.28 However, systemic steroids, cyclosporin, plasmapheresis, anti-tumour necrosis factor-α (TNF-α), and Intravenous Immunoglobulin (IVIG) are all inadequate therapies. All of the clinical data published for these drugs is anecdotal and is mostly based on observational studies. Due to the fact that TEN cases are rare, conducting high-quality randomised controlled clinical trials to assess the efficacy of these treatment approaches is extremely difficult.29,30 Systemic steroids have been utilised in the treatment of the condition in India for decades. The majority of instances are attributable to an antibody-dependent cell-mediated cytotoxicity type of hypersensitivity phenomena that is susceptible to corticosteroids. Early steroid treatment was linked to a better outcome. Oral steroids, administered within 24-48 hr of disease start and tapered over the next 7-10 days, produce the best results. Dexamethasone 8-16 mg/day is advised, although the amount can be increased if necessary. If the recovery is insufficient, the corticosteroid dose may be increased by 4 mg dexamethasone the following day, and the evaluation repeated the following day. However, no randomised controlled trials have been conducted to determine the efficacy of steroids.31 Considering the successful treatment of our patient, systemic steroids must be considered as a treatment for TEN/SJS.
CONCLUSION
In the context of a developing country like India, where infectious diseases pose a significant burden due to sanitation challenges, the widespread use of cephalosporin antibiotics as broad-spectrum bactericidal agents is evident. However, the potential risk of drug-induced TEN necessitates careful management and vigilant monitoring to ensure patient safety and treatment effectiveness. Despite ongoing research and a better understanding of its underlying mechanisms, no single treatment has significantly improved the related morbidity and mortality in TEN patients. The use of steroids in TEN treatments remains a topic of debate, with conflicting evidence from various meta-analyses and retrospective studies. In this particular case, administration of a steroid demonstrated a gradual improvement in the patient’s skin lesions, but rigorous randomized controlled trials are needed to establish its true efficacy. Due to the rarity of TEN cases, conducting high-quality trials to assess treatment approaches presents significant challenges.
Cite this article:
Hegde M, Raj S, Ashvil A. Cefadroxil Induced Toxic Epidermal Necrolysis: A Case Report. Int. J. Pharm. Investigation. 2024;14(1):263-7.
ABBREVIATIONS
| TEN | Toxic epidermal necrolysis |
|---|---|
| SJS | Stevens-Johnson syndrome |
| IVIG | Intravenous immunoglobulin |
| GCS | Glasgow coma scale |
| LFT | Liver function test |
| RFT | Renal function test |
| ECG | Electrocardiogram |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| TNF-α | Tumour necrosis factor-α |
References
- Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68(11):355-61. [PubMed] | [CrossRef] | [Google Scholar]
- Wolkenstein P, Revuz J. Toxic epidermal necrolysis. Dermatol Clin. 2000;18(3):485-95. [CrossRef] | [Google Scholar]
- Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333(24):1600-7. [PubMed] | [CrossRef] | [Google Scholar]
- Mockenhaupt M. Severe drug-induced skin reactions: clinical pattern, diagnostics and therapy. J Dtsch Dermatol Ges. 2009;7(2):142-60. quiz 161 [PubMed] | [CrossRef] | [Google Scholar]
- Devi K, George S, Criton S, Suja V, Sridevi PK. Carbamazepine-the commonest cause of toxic epidermal necrolysis and Stevens-Johnson syndrome: a study of 7 years. Indian J Dermatol Venereol Leprol. 2005;71(5):325-8. [PubMed] | [CrossRef] | [Google Scholar]
- Boroda K, Li L, Riina L, Ahmed S. Cephalosporin-induced toxic epidermal necrolysis treated with intravenous immunoglobulin. Cureus. 2015;7(10) [PubMed] | [CrossRef] | [Google Scholar]
- McGee T, Munster A. Toxic epidermal necrolysis syndrome: mortality rate reduced with early referral to regional burn center. Plast Reconstr Surg. 1998;102(4):1018-22. [PubMed] | [CrossRef] | [Google Scholar]
- Palmieri TL, Greenhalgh DG, Saffle JR, Spence RJ, Peck MD, Jeng JC, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehabil. 2002;23(2):87-96. [PubMed] | [CrossRef] | [Google Scholar]
- Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M, et al. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58(1):33-40. [PubMed] | [CrossRef] | [Google Scholar]
- Patel JB, Agrawal P, Soitawala S, Sattigeri BM. Amoxicillin induced toxic epidermal necrolysis (TEN): a case report. Int J Res Med Sci. 2015;3(4):1011-4. [CrossRef] | [Google Scholar]
- Mockenhaupt M. The current understanding of Stevens-Johnson syndrome and toxic epidermal necrolysis. Expert Rev Clin Immunol. 2011;7(6):803-13. quiz 814 [PubMed] | [CrossRef] | [Google Scholar]
- Badia M, Trujillano J, Gascó E, Casanova JM, Alvarez M, León M, et al. Skin lesions in the ICU. Intensive Care Med. 1999;25(11):1271-6. [PubMed] | [CrossRef] | [Google Scholar]
- Neff Ph, Meuli-Simmen C, Kempf W, Gaspert T, Meyer VE, Künzi W, et al. Lyell syndrome revisited: analysis of 18 cases of severe bullous skin disease in a burns unit. Br J Plast Surg. 2005;58(1):73-80. [PubMed] | [CrossRef] | [Google Scholar]
- Rzany B, Mockenhaupt M, Baur S, Schröder W, Stocker U, Mueller J, et al. Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990-1992): structure and results of a population-based registry. J Clin Epidemiol. 1996;49(7):769-73. [PubMed] | [CrossRef] | [Google Scholar]
- Chung WH, Hung SI. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J Dermatol Sci. 2012;66(3):190-6. [PubMed] | [CrossRef] | [Google Scholar]
- Perkins JR, Ayuso P, Cornejo-García JA, Ranea JA. The study of severe cutaneous drug hypersensitivity reactions from a systems biology perspective. Curr Opin Allergy Clin Immunol. 2014;14(4):301-6. [PubMed] | [CrossRef] | [Google Scholar]
- Yamane Y, Aihara M, Ikezawa Z. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan from 2000 to 2006. Allergol Int. 2007;56(4):419-25. [PubMed] | [CrossRef] | [Google Scholar]
- Lam A, Randhawa I, Klaustermeyer W. Cephalosporin induced toxic epidermal necrolysis and subsequent penicillin drug exanthem. Allergol Int. 2008;57(3):281-4. [PubMed] | [CrossRef] | [Google Scholar]
- Hafermann MJ, Barber GR, Dreskin SC, Lindberg GK. Fatal case of cephalexin-induced toxic epidermal necrolysis. SAGE Open Med Case Re. 2014;172 [PubMed] | [CrossRef] | [Google Scholar]
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-45. [PubMed] | [CrossRef] | [Google Scholar]
- Hirapara HN, Patel TK, Barvaliya MJ MJ, Tripathi C. Drug-induced Stevens-Johnson syndrome in Indian population: a multicentric retrospective analysis. Niger J Clin Pract. 2017;20(8):978-83. [CrossRef] | [Google Scholar]
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, Sun J, Markova A, Beachkofsky TM, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82(6):1553-67. [PubMed] | [CrossRef] | [Google Scholar]
- Koh MJ, Tay YK, Koh MJA, Tay YK. An update on Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Curr Opin Pediatr. 2009;21(4):505-10. [PubMed] | [CrossRef] | [Google Scholar]
- Boorboor P, Vogt PM, Bechara FG, Alkandari Q, Aust M, Gohritz A, et al. Toxic epidermal necrolysis: use of biobrane or skin coverage reduces pain, improves mobilisation and decreases infection in elderly patients. Burns. 2008;34(4):487-92. [PubMed] | [CrossRef] | [Google Scholar]
- Herndon DN. Toxic epidermal necrolysis: a systemic and dermatologic disorder best treated with standard treatment protocols in burn intensive care units without the prolonged use of corticosteroids. J Am Coll Surg. 1995;180(3):340-2. [PubMed] | [Google Scholar]
- Heimbach DM, Engrav LH, Marvin JA, Harnar TJ, Grube BJ. Toxic epidermal necrolysis. A step forward in treatment. JAMA. 1987;257(16):2171-5. [PubMed] | [CrossRef] | [Google Scholar]
- HALEBIAN PH, CORDER VJ, MADDEN MR, FINKLESTEIN JL, SHIRES GT. Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann Surg. 1986;204(5):503-12. [PubMed] | [CrossRef] | [Google Scholar]
- Saha K, Gupta AK. Toxic epidermal necrolysis: current concepts in pathogenesis and treatment. Indian J Dermatol Venereol Leprol. 2000;66(1):10-7. [PubMed] | [Google Scholar]
- Ririe MR, Blaylock RC, Morris SE, Jung JY. Intravenous immune globulin therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis complicated by hemolysis leading to pigment nephropathy and hemodialysis. J Am Acad Dermatol. 2013;69(2):221-5. [PubMed] | [CrossRef] | [Google Scholar]
- Chaidemenos GC, Chrysomallis F, Sombolos K, Mourellou O, Ioannides D, Papakonstantinou M, et al. Plasmapheresis in toxic epidermal necrolysis. Int J Dermatol. 1997;36(3):218-21. [PubMed] | [CrossRef] | [Google Scholar]
- Kumar R, Das A, Das S. Management of Stevens-Johnson syndrome-toxic epidermal necrolysis: looking beyond guidelines! Indian J Dermatol. 2018;63(2):117-24. [PubMed] | [CrossRef] | [Google Scholar]


