ABSTRACT
ABSTRACT
Background:
Neuroprotection is a process of recovery and salvage of the nervous system, function, and structure of cells. Several modulators are leading to neuronal damage and cell death, causing many neurodegenerative diseases like Alzheimer’s, Parkinson’s, Huntington’s, etc. Conventional medicinal therapies like Donepezil and Rivastigmine have a wide range of long-term effects. Acacia catechu and Ficus religiosa are two popularly known plants in herbal medicine. Their primary use is in the treatment of various inflammatory diseases. The present study investigates the beneficial effect of these plants in neuroprotection by in vitro and silico methods.
Materials and Methods:
We determined aqueous extracts of both A. catechu and F. religiosa for their antioxidant, anti-inflammatory, and acetylcholinesterase activities. The extracts were subjected to Liquid Chromatography-Mass Spectroscopy (LC-MS) analysis. In addition, molecular docking was performed for the inhibitory activity of the five selected ligand components identified from LC-MS analysis against two well-known neuroreceptors [1b41(Acetylcholinesterase) and 2V5Z (Human monoamine oxidase b1)] by AutoDock tools v 1.5.6. 2.7.3. These results and standards Donepezil and selegiline were compared for their binding energy, inhibition constant, and intermolecular energy.
Results:
Both A. catechu and F. religiosa have shown moderate to high neuroprotective activities in vitro and in silico.
Conclusion:
The A. catechu and F. religiosa extracts possess remarkable antioxidant, anti-inflammatory potential with potent neuroprotective components. Further studies on combining these two plants into a formulation could benefit neuro-restoration and prevent neuroinflammatory processes.
INTRODUCTION
The challenges involved in treating neurological disorders increase substantially as the human population ages. Researchers have recently discovered the function of numerous physiologically active compounds from herbal sources, which hold promise for the development of new medicinal medications.1 Ficus religiosa and Acacia catechu are well-researched plants with vigorous ethnomedicinal and pharmacological activities. They have been identified widely to treat various diseases in folklore medicine. Recent evidence highlights their promising neuroprotective potential, which could be critical to reducing and restoring neurological damages in neuropathological conditions (such as strokes, Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease symptoms).
The bark of Acacia catechu and Ficus religiosa, which is used as a natural cure with decoction or infusions, is principally responsible for the medicinal virtues of these plants documented in ancient literature. Some previous studies claimed that Ficus religiosa possesses anticonvulsant activity,2 acetylcholinesterase inhibitory activity, and anti-anxiety activities.3 The fruits of this plant have been shown to contain a variety of amino acids, with the fig having the highest concentration of serotonin [5-HT]. According to,2,4 serotonin is present in the brain and regulates serotonergic neurotransmission, which is essential for higher cognitive processes. Retention of the anticonvulsant effect in the saponin-rich fraction-treated animals indicated the role of saponins required for the activity.2 Its bark is extensively used in traditional medicine as an analgesic and anti-inflammatory5,6 and has hypoglycaemic properties specifically attributed to β-sitosterol.7
On the other hand, Acacia catechu, a member of the Mimosaceae family and often known as Willd. or Khair, is widely utilised in Indian Ayurvedic herbal medicines. Khair has historically been helpful for treating leprosy and numerous gastrointestinal disorders.8,9 Furthermore, the leaf extract has been shown to possess antioxidant and anti-cholinesterase activity.10 In addition, the Heartwood extracts have been shown to possess antioxidant, anti-inflammatory, and neuroprotective Properties.11,12 Overall, Acacia catechu shows promising neuroprotective properties.
The present study identifies the bioactive components responsible for the various biochemical and neuroprotective properties. It involves testing with different solvent extractions of Acacia catechu (AC) and Ficus religiosa (FR). These extracts were subjected to various biochemical properties such as antioxidant, anti-inflammatory, and acetylcholinesterase inhibition. Based on these assays, the extracts with higher properties were further subjected to LC-MS characterization. After identifying the specific bioactive components in each extract, they were subjected to in silico molecular docking studies to predict potent neuroprotective components and their possible mode of action.
MATERIALS AND METHODS
Plant collection
The bark materials were collected from AC and FR from local gardens around Mangalore. An authorized botanist authenticated the plant material. A summary of the methodology is described as a flowchart in Figure 1.
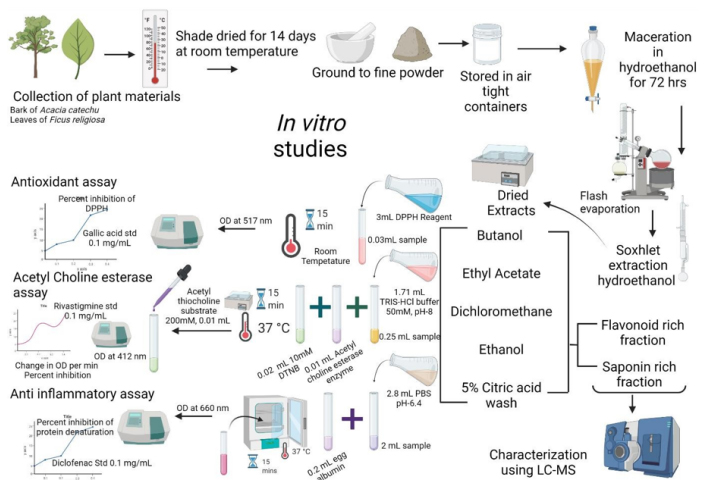
Figure 1:
Shows a summary of the process of extraction, biochemical characterization, and identification of the bioactive components in Acacia catechu and Ficus religiosa.
Preparation of extract
The barks were cut, washed, shade-dried for 30 min, coarsely powdered, and used for solvent extraction. For sample preparation, 500g of dried samples were extracted with 1.5L of a series of solvents in the following order: N-hexane, dichloromethane, chloroform, methanol, and water. Each extract was subjected to lyophilization for 48 hr. These lyophilized materials were stored at -80°C for further use and for studying the biochemical activities.
In vitro studies
To prepare the series concentrations for the in vitro investigations, each sample was dissolved to create a 1 mg/mL concentration, which was then diluted.
DPPH radical scavenging assay
Using the previously established approach, the 2,2′- diphenyl-1- picrylhydrazyl (DPPH) assay was used to assess the free radical scavenging activity of the fractions in vitro.13 A 4 mg/dL stock solution was created. This solution, in aliquots of 3 mL, was combined with 30 ul of the sample at concentrations between 10 and 500 mg/ml. After thoroughly shaking, the reaction mixture was allowed to sit at room temperature for 30 min in the dark. At 517 nm, the absorbance was then measured. As stated previously, the control was created without any sample. The scavenging activity is measured as a percentage of DPPH’s ability to prevent the creation of free radicals.
Protein denaturation assay
The tissue damage can be related to the denaturation of protein constituents within the cells or in the intercellular spaces. Hence, the ability of any substance which can inhibit denaturation can possess anti-inflammatory potential. The assay was performed according to Kumari S et al.14 The results are shown as percentage inhibition of protein denaturation.
Acetylcholinesterase inhibition assay
Based on Ellman’s technique, the acetylcholinesterase inhibitory assay (AChE) activity was carried out.13 The product of the enzyme’s hydrolysis of the substrate acetylcholine is thiocholine, which when combined with Ellman’s reagent (DTNB) produces 2-nitrobenzoate-5-mercapto thiocholine and 5-dinitro benzoate, which can be detected at 412 nm. Results are presented as a percentage reduction in acetylcholine synthesis.
LC-MS analysis15
Both the FR and AC extracts subjected to LC-MS analysis. The samples were examined using an 8050 MS instrument with a quaternary pump in the Shimadzu LC series. An ESI source temperature of 140°C was used for full scan mode from m/z 100 to 1000. For the analysis, a Phenomenex 3 C18 (50 x 4.6 cm) HPLC column was selected. Water was chosen as the diluent. Total flow rate for the solvent delivery was 0.7 mL/min. Gradient elution was used to operate the solvent. Phase A-0.1% formic acid and phase B-100% acetonitrile were the two mobile phase solvents utilised. Both positive and negative acquisition modes for the MS spectra were used. By comparing the compounds’ m/z ratio to the molecular weight in the PubChem database, the chemicals were identified. Based on the identification of precursor m/z ratios and ionisation mode, the components were identified
In silico molecular docking analysis16,18
Preparation of Ligands
Five ligands were chosen from both AC and FR for the docking simulation based on the LC-MS data. The SDF file of each ligand was retrieved from PubChem. From AC (Afzelechin, CID:442154; Emodin, CID:3220; Taxifolin, CID:439533; Quercetin, CID:5280343; Pterocarpin, CID:1715306) and from FR (Ursolic Acid, CID:64945; Beta-sitosterol, CID:86821, Naringenin, CID:932; Procyanidin b1, CID:11250133; Trolamine, CID:7618;) Standards (Selegiline, CID:26757; Donepezil, CID:3152). These files couldn’t be utilised directly for dock simulation; thus, we converted them to PDBQT format using AutoDock tools v1.5.6. Rotatable bonds were specified, Gasteiger partial charges were added, and non-polar hydrogen atoms were combined.
Preparation of Receptors
The two receptors’ X-ray crystal structures can be found in the Protein Data Bank (PDB)12 (ACHE, PID:1b41; MAOB, PID:2V5Z). Water, ligands, and heteroatoms (HETATM) were removed from the structure using Discovery Studio Visualizer v 19.1.0 (BIOVIA, San Diego, CA, USA). Changes to atom types, removal of water molecules, addition of polar hydrogen atoms and Gasteiger charges, and conversion into PDBQT format are all required for the preparation of receptor files using AutoDock tools v1.5.6. 2.7.3. Simulations of Docking Using AutoDock tools, molecular docking studies for the chosen compounds were performed at the binding site of the target proteins. Each receptor’s grid box size was selected, and the exhaustiveness was set to 24. For further examination, the results with the best conformation and energy were chosen. The protein-ligand complexes were visualised and analysed using Discovery Studio Visualizer V19.
RESULTS
The results of the biochemical properties of the extracts and their bioactive components are summarised in Figure 2A-D. Figure 2A shows the antioxidant properties of different extracts of AC and FR. The aqueous extracts of both AC and FR show considerably higher antioxidant capacity among the extracts. The results of protein denaturation assay shown in Figure 2B indicated a remarkably higher inhibition displayed by the aqueous extracts of both AC and FR. The Figure 2C shows the acetylcholine esterase inhibition by different extracts of AC and FR. The results show a higher aqueous extract of FR followed by AC when compared to the other solvent based extracts. These results have been summarised in Figure 2D expressed in terms of their respective IC50 values equivalent to the standard used in their assays.
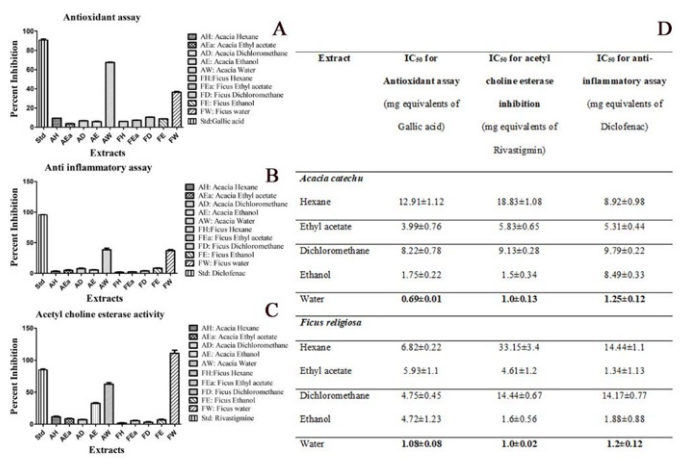
Figure 2:
Shows the biochemical properties of the different types of solvent-based extracts. 2A represents the antioxidant properties, 2B shows the anti-inflammatory activity, 2C shows the acetylcholine esterase activity, and 2D summarises the minimum excitatory doses.
The data from LC-MS analysis and the selected components present in the extracts are summarised in Figure 3A-L. The LC-MS analysis of AC and FR indicate presence of number of phytoconstituents present in the extracts. In the present study, we focused on identifying on the relevant flavonoids and saponins in AC and FR.
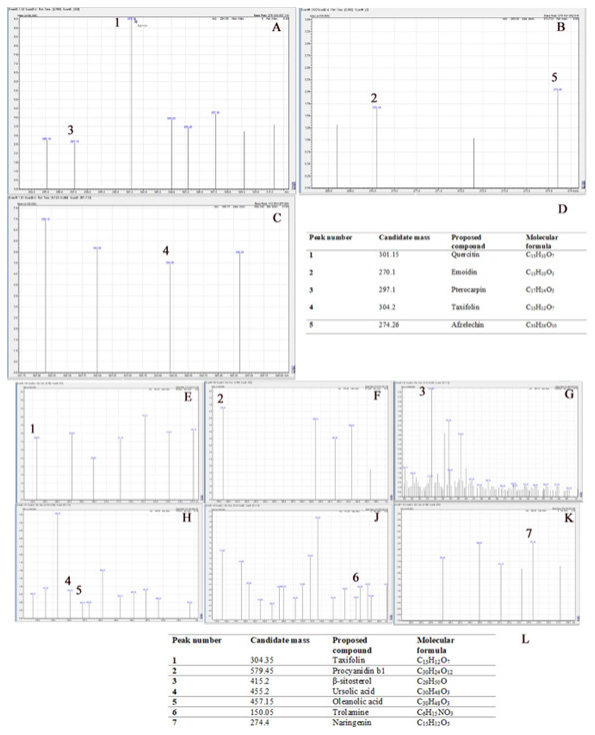
Figure 3:
Shows the LC-MS analysis, with Figures 3A-C showing the LC-MS chromatogram of AC and 3D showing the summary table of the compounds identified. Figures 3E-K represent the chromatogram, and 3 summarizes the compounds identified from the LC-MS data.
The results of molecular docking studies are summarised in Figures 4–5. The docking studies reveal a number of molecules with extensive neuromodulatory potential. These molecules could play a critical role in regulating multiple pathways in mediating the anti-inflammatory activities,
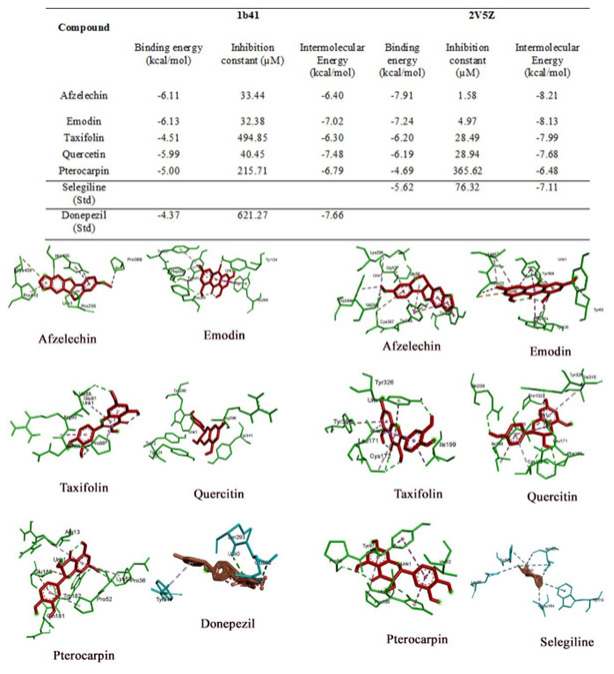
Figure 4:
Summarizes the Binding energies, Inhibition constants, and intermolecular energies of different ligands identified from AC with the receptors. Additionally, the 3D view of ligand-receptor interactions with their best poses is presented in the figure.
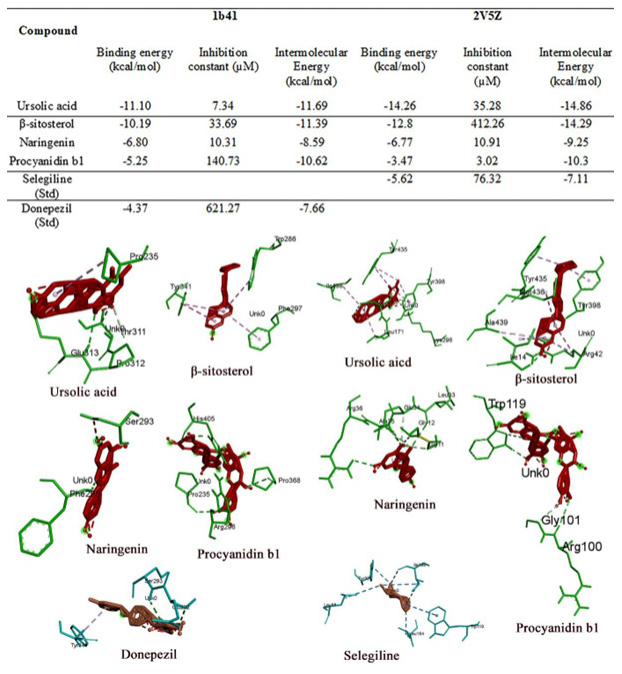
Figure 5:
Summarizes the Binding energies, Inhibition constants, and intermolecular energies of different ligands identified from FR with the receptors. In addition, the 3D view of ligand-receptor interactions with their best poses is presented in the figure.
DISCUSSION
The results of antioxidant studies indicate that AC and FR have remarkable free radical scavenging properties. It has been attributed to the high amount of polyphenolic and flavonoid components in AC.2,4,11 In FR, it can be attributed to alkaloid and saponin components in them.12 The redox-potential of phenolic chemicals are crucial for absorbing and neutralising free radicals, scavenging singlet and triplet oxygen, or breaking down peroxidase.17
The results of the protein denaturation assay indicate moderate to high anti-inflammatory potential among the extracts of AC and FR. The phenolic groups present in the flavonoids and the complex groups of saponins tend to stabilize the proteins under extreme conditions of temperature, which in turn results in reduced protein breakdown.12,18,19 Further, under in vivo conditions, these groups stabilize the enzymes involved in the anti-inflammatory pathway and reduce the progression of inflammation.8,9,20 The flavonoid-rich fraction of AC and a saponin-rich fraction of FR show a remarkable inhibition of protein denaturation. This further delineates the prominent role of flavonoid and saponin components responsible for most of the anti-inflammatory properties of AC and FR.
The aqueous extracts of AC and FR also showed higher acetylcholinesterase inhibition than the other solvents. Further, the assay with their flavonoid-rich fraction of AC and the saponin-rich fraction of FR showed a similar range of free radical scavenging potential compared to their parent extracts (Supplementary Figure 1). It indicates that the flavonoid-rich component of AC and the saponin-rich component of FR are the major contributors to most of the observed biochemical properties of their corresponding extracts.
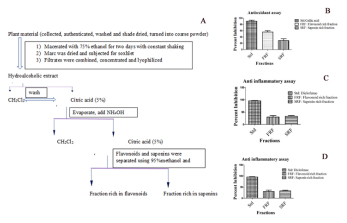
Supplementary Figure 1:
A-D shows the steps in isolating flavonoid and saponin-rich fractions (1sA). Further, Figures 1S A-C show the biochemical activities of these fractions.
For molecular docking, the selected receptors for the study have significantly contributed to developing neurodegenerative drug discovery. Only those ligands with lower binding energy, inhibition constant, and intermolecular energy were compared to a known standard, selected, and analysed. Since both the receptors require an inhibitory action, their values lower than the standard were considered more efficient. The receptors used in the study were 2V5Z and 1B41, with their standard inhibitory compounds selegiline and Donepezil, respectively.
2V5Z (Human Monoamine Oxidase B)
Monoamine oxidase is an enzyme that eliminates the neurotransmitters norepinephrine, serotonin, and dopamine from the brain. MAO inhibitors enable the prolonged availability of these brain chemicals to affect changes in both cells and circuits impacted by depression. In the present study, we selected five compounds from F. religiosa and five compounds from A. catechu against the 2V5Z receptor and compared them with a known standard inhibitor, selegiline. We found that all the selected ligands from both plants are potential compounds as their affinities are either higher or at least equal to the standard. In FR, ursolic acid and β-sitosterol showed twice the effect compared to the standard followed by Naringenin, Procyanidin b1, and Trolamine. Afzelechin in A. catechu has proven to be a potential 2V5Z inhibitor, followed by Emodin, Taxifolin, Quercetin, and Pterocarpin.
1B41 (Human Acetylcholinesterase Complexed with Fasciculin-Ii, Glycosylated Protein)
In the present study, ursolic acid from FR, when docked against the 1b41 receptor, is at least twice as effective as the standard Donepezil with an Inhibition constant of -437kcal/mol. However, in AC, Emodin has proven to be a better inhibitor than the other ligands and the standard.
The present study enables us to identify some new bioactive components present in AC and FR, which are relatively better in their docking scores than their respective standards. We intend to describe these bioactive components and their possible mode of action.
Quercetin A naturally occurring dietary flavonoid called quercetin has a significant protective effect against neurodegeneration brought on by CNS injury. It has been demonstrated to reduce neuronal apoptosis, which is essential for controlling the mitochondrial apoptotic pathway, preventing the activation of Cytochrome C, activated caspase 3, and cleaved PARP-1 expression, and consequently engaged in preventing neurodegeneration.21
Emodin is a rhubarb anthraquinone derivative (1,3,8-trihydroxy-6-methylanthraquinone). Emodin is essential for shielding cultured cortical neurons from the toxicity caused by A25-35. The ER/PI3K/Akt pathway is activated, the Bcl-2 gene is upregulated, and JNK1/2 phosphorylation caused by A25-35 is inhibited to mediate this neuroprotective action.22
Taxifolin can reduce inflammation by preventing the formation of TREM2-expressing brain cells. Furthermore, it lessens the production of active caspases, which results in apoptotic cell death, reducing glutamate levels and oxidative tissue damage.23
Afzelechin mediates its neuroprotective effect on glutamate-induced neurotoxicity in HT22 cells. Most of its action involves reducing free radical formation in the brain tissue, leading to decreased oxidative stress.24
Pterocarpans are derivatives of isoflavonoids found in the family Fabaceae. There are no previous reports on the potential function of this particular component.
β-Sitosterol is one of the plant sterols that can inhibit platelet A synthesis is, suggesting that it may be effective as a modulator in the prevention of AD. Additionally, Sitosterol has been demonstrated to improve the memory and learning deficits in APP/PS1 mice, possibly by reducing A deposition.25
Naringenin belongs to the class of flavonoids, with naringin and naringenin being extensively effective in regulating redox homeostasis, preventing mitochondrial oxidative damage, and promoting neurogenesis in several CNS disease models in rodents. In addition, its neuroprotective nature is strongly implicated in the activation of Nrf2 signalling, reduced oxidative stress, and inhibits the apoptotic and inflammatory pathways.26
Oleanolic acid Numerous edible and medicinal plants contain the triterpene chemical Oleanolic Acid (OA). It has been shown to lessen the Lipopolysaccharide (LPS)-induced activation of BV2 microglial cells. In AD, neuroinflammation is correlated with chronic microglial overactivation. Inhibiting the release of IL-1, IL-6, TNF-, and NO in cells pre-treated with OA is linked to a downregulation of the genes encoding these cytokines and those activated by Nitric Oxide Synthase (iNOS). These data support a neuroprotective function by reducing oxidative stress and inflammatory reactions in AD-related activated microglia.27
Procyanidin B1 is a dimer of a 4÷8 bond (epicatechm-(4β÷8)- catechin). In glutamate-induced HT22 cell death, it prevented the glutamate-induced chromatin condensation and decreased the number of annexin V-positive cells with an overall reduction in apoptotic cell death. It also has robust antioxidant properties and inhibits glutamate-induced reactive oxygen species accumulation in cells and protein carbonyl formation. Overall, it has a lot of promise in preventing and perpetuating neuropathological conditions.28
Ursolic acid is one of the natural pentacyclic triterpenoid acids, which can be isolated from edible plants of the Oleaceae family; well-known for its antioxidative and anti-inflammatory potential; protection against cerebral ischemic injury by triggering the Nrf2 pathway.29
Overall, the present study focuses on the biochemical properties of AC and FR extracts. The extracts show remarkable antioxidant, anti-inflammatory, and cholinesterase inhibitory activities. These properties can be attributed to their rich flavonoids and saponin components (Supplementary Figure 1). Furthermore, the molecular docking studies also provided evidence for numerous compounds with neuroprotective properties. Hence a dietary supplementation of these extracts could provide substantial benefits of neuroprotection from oxidative and inflammatory damage.
Given the above implications and significance, the application of a combination of a supplement consisting of antioxidants. Anti-inflammatory and a wide range of neuroprotective properties will undoubtedly have a mitigating effect on memory impairment. Furthermore, the flavonoid and saponin-rich FR and AC can be effective and potential cognitive enhancers. Therefore, we predict that these saponins and flavonoid-rich Ficus religiosa and Flavonoid-rich Acacia catechu could be involved in improving cognitive deficits and needs future validation in vivo.

