Contents
ABSTRACT
ABSTRACT
Background
As many pharmaceuticals are removed via the kidneys, a dose adjustment for some medications is necessary in individuals with decreased renal function to prevent toxicity. This study aimed to modify the dose in individuals with renal failure using creatinine clearance as a screening tool.
Materials and Methods
From November 2021 to April 2022, prospective observation research was carried out in a government hospital’s Department of general medicine. The study includes all patients diagnosed with renal illness above the age of 30, patients with elevated serum creatinine levels, and renal patients with concomitant diseases. From the patients’ medical records, information on the serum creatinine level, age, sex, and prescribed medications and their dosages were gathered. The Cockcroft-Gault (CG) equation was used to get the predicted creatinine clearance. The benchmark for dose adjustment was the American College of Physicians Guideline for Drug Prescribing in Renal Failure.
Results
In this study, 100 patients were screened, and among them, 60 were found to be CKD patients. 512 drugs were prescribed in 60 patients, of which 505 were appropriately adjusted and 2 drugs were inappropriately adjusted in 7 (11.6%) patients and 53 (83.3) patients were appropriately adjusted. Patients with renal impairment have a median age of 50-60 years (56), a serum creatinine value of 2.4, and an estimated CCR value of 126.8. Comorbidities are present in 45 (or 75%) 60 patients.
Conclusion
According to the findings, patients with renal impairment admitted to the hospital frequently made dosing errors. In patients with renal impairment, improving the quality of drug prescriptions may enhance the quality of care overall.
INTRODUCTION
The two reddish-brown organs, known as kidneys, are surrounded by fatty tissue and are located posteriorly behind the peritoneum on either side of the spinal column. Each kidney is about 4-5 inches long, and Nephrons are the kidney’s functional units. Each kidney contains average 900000-1 million nephrons. The kidney’s primary function is excretion; it filters blood and other waste materials from the blood, maintaining body fluids balance by keeping the right level of electrolytes.1
Renal failure
Acute Renal Failure (ARF)
Glomerular filtration reduces suddenly (over hours to days) and is typically reversible in ARF syndrome. KDIGO criteria from 2012 state that any of the following can identify AKI:
Creatinine increases of 0.3 mg/dL in 48 hr,
Creatinine increases to 1.5 times baseline within last seven days,
Urine volume less than 0.5 mL/kg per hour for 6 hr.
Chronic Renal Failure (CRF)
Persistent impairment of kidney function, or CRF or Chronic Kidney Disease (CKD), is defined as abnormally elevated serum creatinine for more than three months or a calculated Glomerular Filtration Rate (GFR) of less than 60 mL per minute/1.73 m2. Renal replacement therapy, such as dialysis or transplantation, is frequently required because of the frequent and progressive loss of kidney function. At the point when a patient requires renal substitution treatment, the condition is called End-Stage Renal sickness (ESRD).3
Dosage adjustment in renal failure patients
Numerous medications’ metabolism, excretion, and pharmacological activity and metabolites depend on normal renal function. Patients with kidney disease will have poor renal excretion of the parent medication and its metabolites, causing an excessive buildup of these substances in the body. Additionally, the pharmacokinetic distribution and elimination processes may be affected by a significant decrease in the medicines’ plasma protein binding. It has been exhibited that persistent renal disappointment lessens the action of various medications utilizing catalysts and carriers.
The most significant drug-related issues in patients with renal impairment involve medication dose errors. Inappropriate dosing in kidney disease patients might result in toxicity or unsuccessful treatment. In particular, age-related renal function decline and the use of several drugs to address concomitant illnesses put older patients at a higher risk of acquiring severe disease and associated side effects. In individuals with compromised renal function, doses should be reduced to prevent drug buildup and toxicity from developing quickly. The Glomerular Filtration Rate (GFR), which is measured by the kidneys, and drug elimination are related. Therefore, using eGFR or Crcl to alter dosages in individuals with renal insufficiency makes sense.
According to studies, hospitalized CKD patients everywhere are not having their medicine doses for really excreted medications correctly adjusted. During the hospitalization of CKD patients, the percentage of prescriptions with inappropriately altered drugs may range from 25-77%. Furthermore, it has been noted that almost 63% of these prescriptions may have unfavorable effects, and nearly 3% may have severe or fatal effects. Nevertheless, despite these data and the seriousness of chronic kidney disease, standard clinical practices, particularly in developing nations, continue to assess renal function solely by serum creatinine levels, which raises the risk of medication dosing errors in hospitalized CKD patients.
Drug doses in renal insufficiency should be personalized, when possible, to maximize therapeutic results and reduce toxicity. The two main strategies are to either increase the time between doses or decrease the dose. Sometimes it’s necessary to alter both the interval and the dose. Although there are no specific guidelines for adjusting drug dosage in cases of acute renal injury, it is normal practice for patients with acute or chronic kidney disease. The difficulty lies in accurately estimating a patient’s kidney function in acute and chronic kidney illness, including renal replacement treatment, which is very different. Patients with acute kidney damage and end-stage kidney disease cannot use Scr-based calculations.
The Cockcroft-Gault (CG) equation, one of many ways to estimate renal function, estimates Creatinine Clearance (CrCl). Therefore, in the current study, we will screen for patients with renal failure, and then, using the Cockcroft-Gault equation and creatinine clearance as a tool, we will adjust the dose for patients with renal failure.4–7
Creatinine Clearance (CCR)
Precisely deciding a patient’s kidney capability in instances of intense and ongoing kidney illness, including renal substitution treatment, which is exceptionally unique, is testing. Patients with end-stage renal disease or acute kidney injury cannot use Scr-based estimates.
The Cockcroft-Gault (CG) equation, one of numerous methods for determining renal function, is used to estimate Creatinine Clearance (CrCl). The Cockcroft-Gault equation and creatinine clearance will be used in the current trial to alter the dose for patients with renal failure after screening for these patients.4–7
Estimated Creatinine Clearance Rate (eCCR)-Cockcroft-Gault formula
Serum creatinine levels can be used to calculate creatinine clearance. The Cockcroft- Gault (C-G) formula predicts CCR (mg/dL) based on a patient’s weight (kg) and gender. If the patient is a female, the resulting CCR is increased by 0.85 to account for the lower CCR in females. Age is a key factor in the C-G formula’s prediction of CCR.8
MATERIALS AND METHODS
The study’s main aim is to screen and adjust the dosage of drugs among really impaired patients using creatinine clearance.
Objectives of the study
Primary objective
- Screening of appropriate dosage adjustments to be made in hospitalized patients with Renal impairment.
Secondary objective
- To categorize patients based on demographic parameters.
- To estimate Glomerular Filtration Rate (GFR).
- Categorize the stage of renal failure based on GFR.
- To adjust the dose of drugs based on Creatinine Clearance (CCR) if required/needed.
Methods
Study design and study period
- A prospective observational study will be conducted in the Inpatient department of General Medicine in Government General Hospital (RIMS) Kadapa.
- The present study will be conducted for 6 months, from Dec 2021 to May 2022.
Source of data
Data would be collected from Inpatient (IP), ICU patients admitted in GMC and GH- Kadapa.
Sample size
It is planned to conduct study on 60 patients presented with renal impairment.
Inclusion criteria
- All patients above 40yrs of age diagnosed with CKD.
- Patients with increased serum creatinine clearance level.
- Renal patients with comorbid conditions.
- Subjects who are willing to give their written informed consent form.
Exclusion criteria
- Patients without CKD.
- Patients are not receiving any medications and are exclusively on dialysis.
- Patients with decreased serum creatinine clearance.
Method of collection of data
It is a prospective observational study conducted in RIMS hospital in the Department of General Medicine—informed consent obtained from the study participants after explaining the study protocol.
Statistical analysis
The demographic details of recruited subjects were calculated using descriptive statistics mean, simple percentage, and standard deviation.
RESULTS
During the study period, a total of 100 patients’ case sheets were reviewed. However, a total of 60 patients were included in the final analysis during the study period from the Department of general medicine in Government General Hospital. All the patients were divided based on gender. Here the Table 1 shows that male predominance was observed among them 32 (53.3%) members were males and 28 (46.6%) members were females. The Figure 1 represents that percentage distribution of subjects based on the gender.
| Male (%) | Female (%) | Total |
|---|---|---|
| 32 (53.3) | 28 (46.6) | 60 |
The Table 2 represents, the renal failure patients based on different age groups, where it Concludes that, the age group of 61 -65 are highly prone to renal failure; The total number of patients with RF were 15(25%) among them males were 9 and females were 6. The Figure 2 shows that percentage distribution of patients based on demographics.
| Sl. No. | Age group | Male | Female | Total (%) |
|---|---|---|---|---|
| 1 | 35-40 | 3 | 3 | 6(10) |
| 2 | 41-45 | 2 | 4 | 6(10) |
| 3 | 46-50 | 3 | 5 | 8(13.3) |
| 4 | 51-55 | 2 | 1 | 3(5) |
| 5 | 56-60 | 4 | 3 | 7(11.6) |
| 6 | 61-65 | 9 | 6 | 15(25) |
| 7 | 66-70 | 4 | 2 | 6(10) |
| 8 | ≥70 | 5 | 4 | 9(15) |
| Total | 60(99.9) | |||
A total of 60 patients were included in the study during the study period from the general medicine government general hospital. All patients were divided based on GFR value and categorized into stages based on GFR. The Table 3 states that Stage 3 and 4 predominances of renal failure were observed The Figue 3 represents that percentage of renal failure based on GFR.
| GFR (ml/n/1.73m2) | Stage of renal | male | Female | Total | Average GFR/ standard deviation |
|---|---|---|---|---|---|
| ≥90 | Stage 1 | 2(3.3 | 0 | 2(3.3) | 97±5.6 |
| 60-89 | Stage 2 | 2(3.3 | 0 | 2(3.3) | 71.5±2.8 |
| 30-59 | Stage 3 | 13(21. | 16(26.6 | 29(48.3) | 40±7.1 |
| 15-29 | Stage 4 | 12(20 | 11(18.3 | 23(38.3) | 22.3±4.5 |
| ≤ 15 | Stage 5 | 3(5) | 1(1.6) | 4(6.6) | 9±2.3 |
The Table 4 represents that the patients were categorized based on comorbidities. Patients with comorbidities of both HTN and DM are more prone to get renal failure.
| Parameter | Duration | Male | Female | Total |
|---|---|---|---|---|
| HTN | <1 year | 2(3.3) | 1(1.6) | 3(5) |
| 1-5 years | 2(3.3) | 10(16.6) | 12(20) | |
| 5-10 years | 0 | 1(1.6) | 1(1.6) | |
| >10 years | 0 | 0 | 0 | |
| DM | <1 year | 1(1.6) | 1(1.6) | 2(3.3) |
| 1-5 years | 2(3.3) | 0 | 2(3.3) | |
| 5-10 years | 0 | 0 | 0 | |
| >10 years | 0 | 0 | 0 | |
| Both | 15(25) | 10(16.6) | 25(41.6) | |
| None | 9(15) | 6(10) | 15(25) |
Figure 4 average GFR and stages of renal failure which states that stage 1 patients have AVG GFR is 97,stage 2 patients have AVG GFR is 71.5,stage 3 patients have AVG GFR is 40,stage 4 have AVG GFR is 22.3 and stage 5 have AVG GFR is 9.
Figure 5 Categorization based on Co-morbidities. Categorization based on Co-morbidities which states that in 60 patients there are 16 patitents with HTN,4 patients with DM, 25 patients with both HTN & DM and 15 patients NONE of the comorbiditites
The Table 5 shows that Male patients were predominant among the total 60 patients (32 [53.3%]). our study reveals that the age group of 61-65 is highly prone to renal failure (15 [25%]) compared to other age groups, all patients were categorized into stages based on GFR values, the stage3 (29 [48.3%]) and stage 4 (23 [38.3%]) predominance of renal failure were observed. Comorbidities are present in most of the patients (45 [75%]), and among them, the patients with both hypertension and diabetics were on the top of the list (25 [41.6]).
| Demographic and clinical data | Number |
|---|---|
| Screening subjects | 100 |
| No. of patients with renal impairment | 60 |
| Male | 32(53.3) |
| Female | 28(46.6) |
| Age(median) | 56(range 50-60) |
| Serum creatinine(mean) | 2.41 |
| Estimated crcl(mean) | 126.8 |
| COMORBIDITY: present | 45(75) |
| Absent | 15(25) |
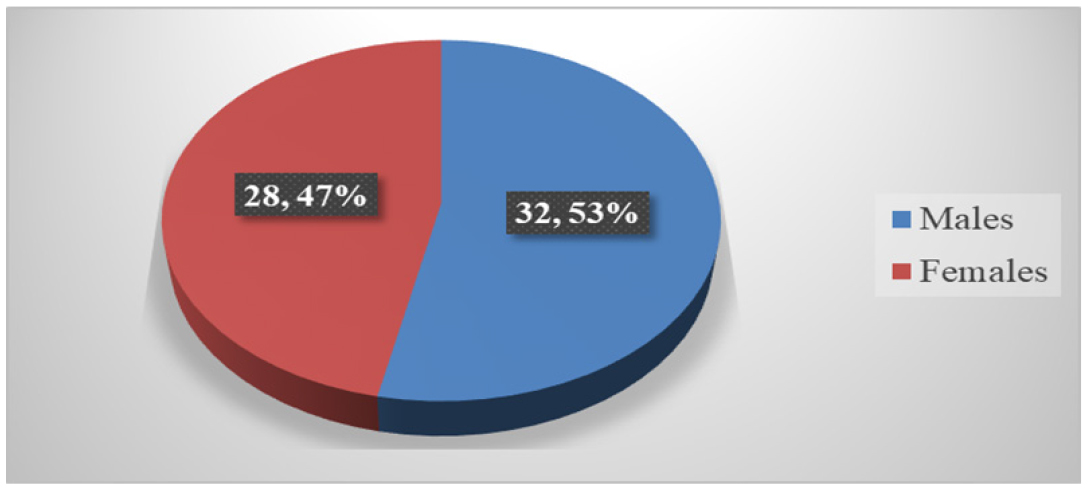
Figure 1:
Distribution of patients based on gender.
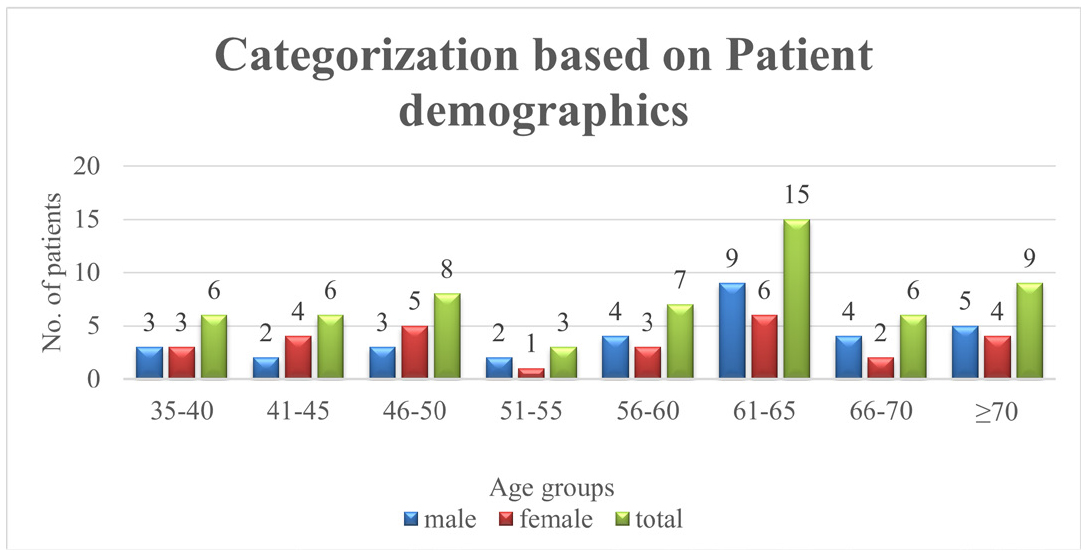
Figure 2:
Categorization based on patient demographics.
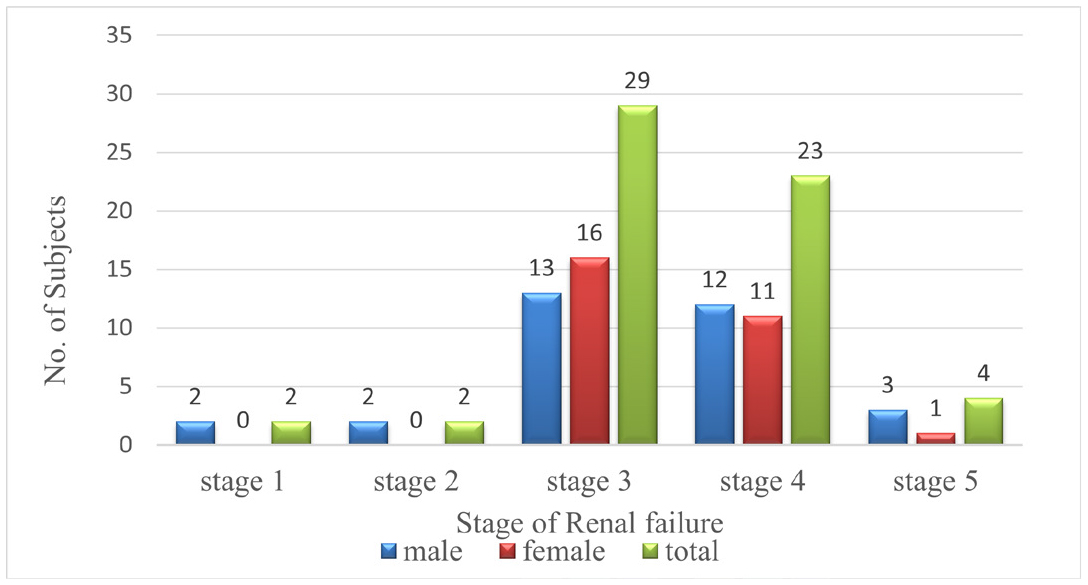
Figure 3:
Stage of Renal failure.
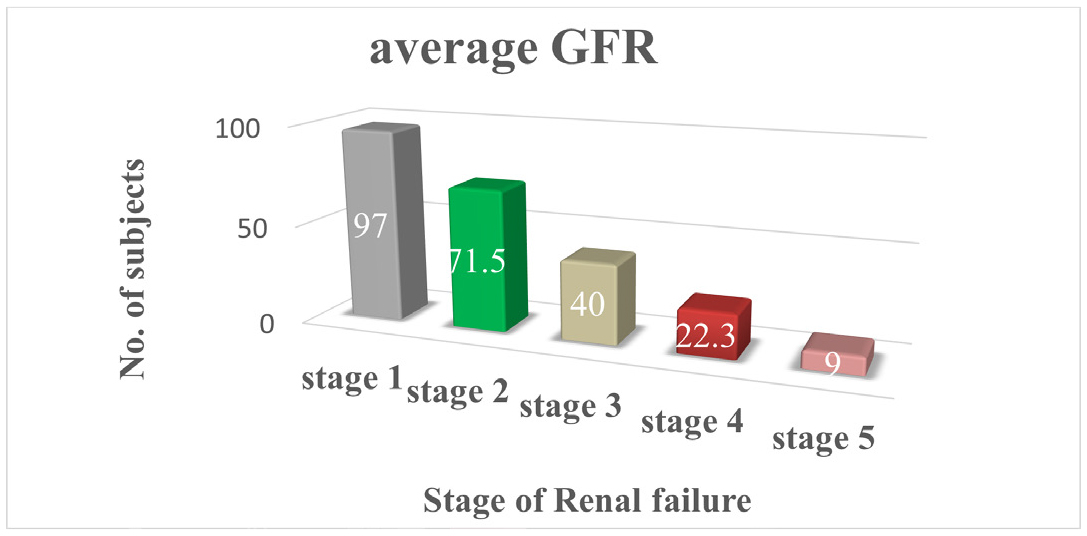
Figure 4:
Average GFR and stages of renal failure.
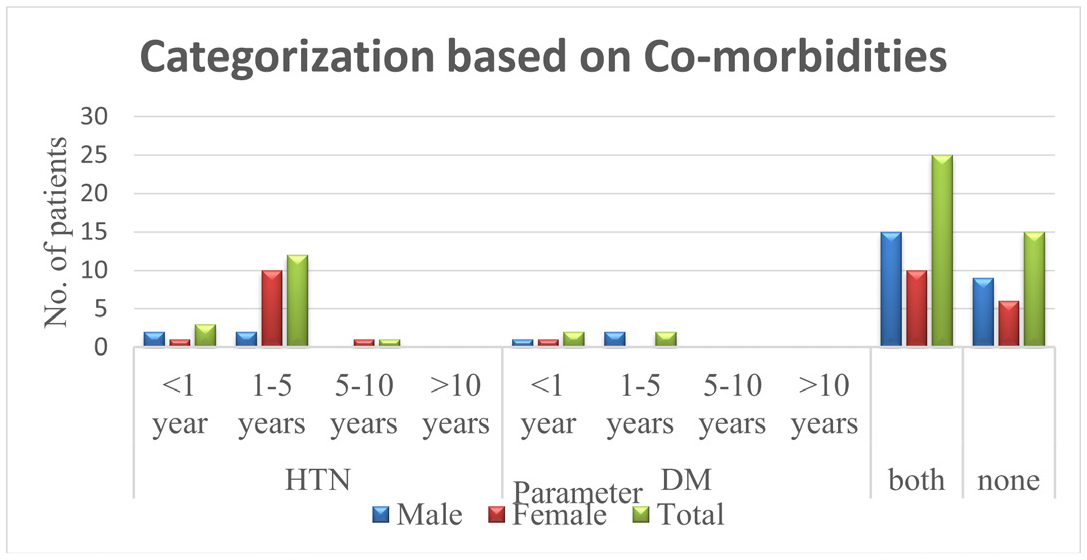
Figure 5:
Categorization based on Co-morbidities.
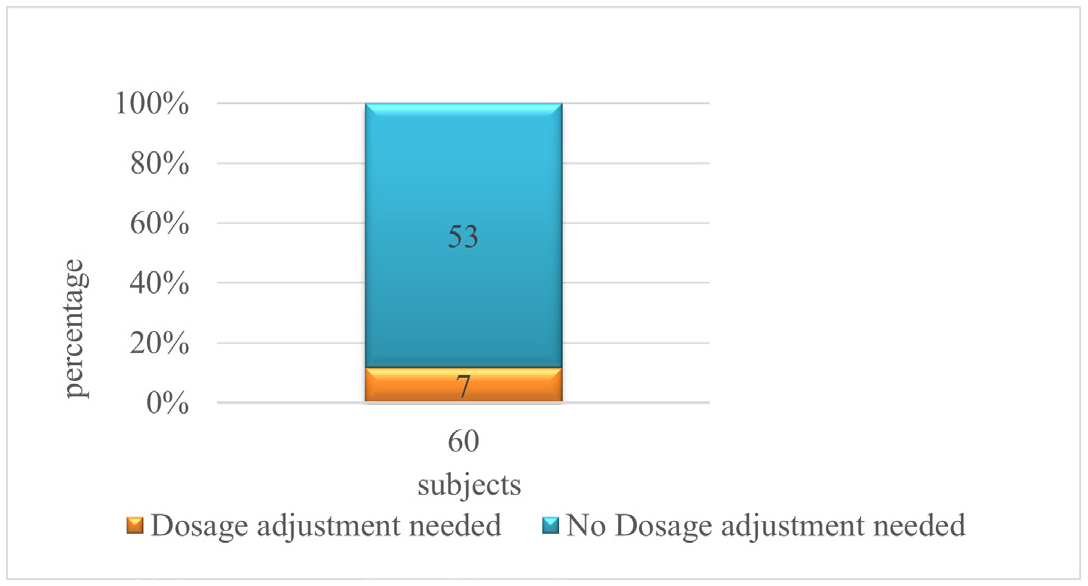
Figure 6:
Distribution of patients based on appropriateness of dosage.
The Table 6 reveals that a total of 2 drugs were inappropriately adjusted among 7 patients. Those drugs are Ranitidine and Tranexamic acid.
| Drugs | Inappropriately adjusted | Appropriately adjusted |
|---|---|---|
| Ceftriaxone | 0 | 60 |
| Amlodipine | 0 | 54 |
| Atorvastatin | 0 | 52 |
| Piracetam | 0 | 49 |
| Multivitamin | 0 | 43 |
| Pantoprazole | 0 | 56 |
| Ranitidine | 2 | 2 |
| Telmisartan | 0 | 24 |
| Furosemide | 0 | 60 |
| Tranexamic acid | 5 | 21 |
| Paracetamol | 0 | 46 |
| Cefperazone-sulbactam | 0 | 5 |
| Sodium bicarbonate | 0 | 46 |
| Cilnidipine | 0 | 28 |
| Iron folic acid | 0 | 20 |
| Calcium | 0 | 15 |
The below Table 7 shows that Dosage adjustment based on GFR which states if GFR is > 50 the dose should be changed to 75% ,if it is 10-50 dose should be changed 25% and it is < 10 it should be 10% for Tranexamic acid and for ranitidine the dose should changed if GFR is > 50 the dose should be changed to 100% ,if it is 10-50 dose should be changed 75% and it is <10 it should be 25% The below Table 8 shows that a total of 7 patients needed dosage adjustment among them 2 patients were needed dosage adjustment of ranitidine, and 5 patients were needed dosage adjustment of tranexamic acid.
| Drug name | Usual dosage | Dosage adjustment based on glomerular filtration (ml/min/1.73m 2) | ||
|---|---|---|---|---|
| >50 | 10-50 | <10 | ||
| Tranexamic acid | 25mg/kg/6-8hr | 75% | 25% (6.25mg/kg/6-8hr) | 10% |
| ranitidine | 150-300mg bed time | 100% | 75% (112-225mg) | 25% (37-75) |
| Sl. No. | Drug name | Stage | male | female | total |
|---|---|---|---|---|---|
| 1 | Tranexamic acid | Stage 4 | 1 | 4 | 5 |
| 2 | Ranitidine | Stage 3 | 0 | 1 | 1 |
| Stage 5 | 1 | 0 | 1 |
The Table 9 shows that a total of 60 patients were included in the study; among them 7 (11.6) patients were Inappropriately adjusted for dose and 53(83.3) were appropriately adjusted.
| Sl. No. | Inappropriately adjusted | Appropriately adjusted | Total subjects |
|---|---|---|---|
| 1 | 7 (11.6%) | 53 (88.3%) | 60 |
Figure 6 distribution of patients based on appropriateness of dosage adjustment it states that 7 patients needed to adjusted with their dosage and for 53 patients there is no need of adjustment in dosage.
DISCUSSION
The study builds up a representative profile of dosage adjustment screening in renal failure patients in the General medicine department in a tertiary care hospital Kadapa. During the study period, a total 60 patients were included in the study, of which 28 are females and 32 were males; comparing this result to Henok Getachew et al.,4 the results suggest a similarity with previous studies where male preponderance was observed.
Distribution of data based on age group and gender (Table 2) was observed in which the patients were categorized into different age groups. Where it concludes that the age group of 61-65 are highly prone to renal failure, total 15 number of patience in the study are from this group in, which males are 9 females are 6 comparing this result to Ahsan Saleem et al.,9 the results suggest that the age group of 41-60 are highly prone to renal failure.
Distribution of data based on GFR (Table 3) was observed in which all patients are divided based on GFR value and categorized into stages based on GFR. Stage 3 and 4 predominance’s of renal failure were observed. Comparing this result to Mauricio Stefanie et al.,10 the results suggest a similarity with a previous study where stage 3 and stage 4 patients are highly prone to renal failure.
Distribution of data based on comorbidities (Table 4) was observed in which patients were categorized based on comorbidities in which both hypertensive and diabetic patients are more prone to get renal failure. Comparing this result to Ahan Saleem et al.,9 the results suggest that the patients with comorbidities of hypertension and diabetics are highly prone to renal failure.
Distribution of data based on dose adjustment (Table 8) was observed in which 7 (11.6) patients were inappropriately adjusted for dose and 53 (83.3) were appropriately adjusted comparing this result to Ahan Saleem et al.;9 the results suggest that more than half of the drugs prescribed to CKD patients requiring dose adjustment which was not similar to our study results.
CONCLUSION
In the present study, we conclude that dosage adjustment is made in the majority of the cases in the availability of clinical calculators to estimate the renal function of CKD patients in which GFR and CCR should be assessed. Ranitidine and tranexamic acid were the drugs identified in the present study where dosage adjustment is required. Clinical pharmacists can play an essential role in dosage adjustment among renal-impaired patients.
Cite this article
Kumar MP, Afreen S, Kalpana M, Sailaja T, Naik MV, Reddy MS. Creatinine Clearance as A Screening Tool for Dosage Adjustment among Renal Failure Patients. Int. J. Pharm. Investigation. 2023;13(4):798-804.
ACKNOWLEDGEMENT
We sincerely thank the superintendent Government General Hospital, clinical guide Dr. M. Sureshwar Reddy, Department of General Medicine, and all the study participants for taking part in this study as well duty nurse of general medicine for helping in data acquisition.
ABBREVIATIONS
| CRF | Chronic Renal Failure |
|---|---|
| CKD | Chronic kidney disease |
| GFR | Glomerular filtration rate |
| ESRD | End-stage renal disease |
| Scr | Serum creatinine |
| CG | Cockcroft-Gault |
| CrCl | Creatinine clearance |
| eCCR | Estimated creatinine clearance rate |
References
- Available fromhttps://www.webmd.com/kidney-stones/picture-of-the-kidneys
- Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26(9):1529-33. [PubMed] | [CrossRef] | [Google Scholar]
- Bindroo S, Quintanilla Rodriguez BS, Challa HJ. [Updated 2021];StatPearls [internet]. 2021 [PubMed] | [CrossRef] | [Google Scholar]
- Getachew H, Tadesse Y, Shibeshi W. Drug dosage adjustment in hospitalized patients with renal impairment at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. BMC Nephrol. 2015;16(1):158 [PubMed] | [CrossRef] | [Google Scholar]
- Clara Drenth-van Maanen A, van Marum Rob J, Jansen Paul AF, Zwart Jeannette EF, van Solinge Wouter W, Egberts Toine CG, Reboldi Gianpaolo, et al. Adherence with Dosing Guideline in Patients with Impaired Renal Function at Hospital Discharge. PLOS ONE. 2015;10(6):1-14. [CrossRef] | [Google Scholar]
- Zuber K, Liles AM, Davis J. Medication dosing in patients with chronic kidney disease. J Am Acad Phys Assist. 2013;26(10):19-25. [PubMed] | [CrossRef] | [Google Scholar]
- Karsch-Völk M, Schmid E, Wagenpfeil S, Linde K, Heemann UHA, Schneider A, et al. Kidney function and clinical recommendations of drug dose adjustment in geriatric patients. BMC Geriatr. 2013;13:92 [PubMed] | [CrossRef] | [Google Scholar]
- Shahbaz H, Gupta M. [Updated. 2021];Creatinine clearance. StatPearls [Internet]. [PubMed] | [CrossRef] | [Google Scholar]
- Saleem A, Masood I, ImranSands JM. Pattern and predictors of medication dosing errors in chronic kidney disease patients in Pakistan: A Single Center retrospective analysis. PLOS ONE. 2016;11(7):1-12. [PubMed] | [CrossRef] | [Google Scholar]
- Stefani M, Singer RF, Roberts DM. How to adjust drug doses in chronic kidney disease. Aust Prescr. 2019;42(5):163-7. [PubMed] | [CrossRef] | [Google Scholar]


