ABSTRACT
The study’s objective was to evaluate Exenatide for treating Type 2 Diabetic Mellitus (T2DM) in an animal model. The safety profile and dose determination for the in vivo antidiabetic study were established through an acute toxicity study conducted by OECD guideline 425. The plasma levels of exenatide were studied using a pharmacokinetic model in rats. After Exenatide were given to the rats, their behavior, biochemical, and hematological characteristics were tested. Animals were administered Streptozotocin (STZ, 60 mg/mL, i/p) to induce diabetes. Blood glucose levels above 250 mg/dL were diagnostic of diabetes in animals. The drug was non-toxic in toxicity investigation. Results showed that administration of drug did not cause any significant change in behavioral, biochemical, or hematological markers. It was revealed that Exenatide significantly lowered blood glucose levels in diabetic animals. The results of the study indicated that Exenatide has the potential to be utilized as an alternative antidiabetic medicine against Type 2 Diabetes.
INTRODUCTION
India has the second-highest diabetes population worldwide, and its citizens are major contributors to the worldwide epidemic.1In 2017, this condition was anticipated that 425 million people would be affected. It is expected to reach 629 million by 2045.2Prevalence of both obesity and prediabetes are forecast to increase in India over the next several years. Sugar and sweets play an integral role in Indian culture and daily life.3 In India, the rate of diabetes cases is constantly increasing, which may be attributable to the interplay of heritable susceptibility and the insulin resistance brought on by rapid urbanization and changes in dietary and physical activity patterns.4
Recent clinical practice guidelines for Type 2 Diabetes from the American Diabetes Association (ADA), the Indian Council of Medical Research (ICMR), and the Research Society for the Study of Diabetes in India (RSSDI) all recommend medical nutrition therapy as first-line treatment and provide consistent dietary guidelines for daily intake.5–7 One of the most ubiquitous issues in modern public health is the rise of Type 2 Diabetes Mellitus (T2DM). Insulin resistance is the underlying cause of metabolic dysfunction and persistent hyperglycemia.
Traditional medications such as thiazolidinediones, metformin, sulfonylureas, glucagon-like peptide 1 and dipeptidyl peptidase 4 inhibitors are readily available, nonetheless, they each have their own set of drawbacks.8
In recent years, the incretin system has emerged as a promising therapeutic avenue for treating Type 2 Diabetes.9 Blood sugar levels are lowered by incretins, hormones secreted by the intestinal mucosa in response to dietary intake that promote insulin production in reaction to glucose.10 When glucose levels are around normal, incretins also decrease insulin secretion. “The incretin effect” refers to the observation that insulin secretion is higher in response to oral glucose eating than to an isoglycemic intravenous glucose infusion. Glucagon-Like Peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide are two examples of incretin hormones that have been identified.11 The effects of GLP-1 on glucose levels are of particular importance, as are its effects on stomach emptying and glucagon secretion.
T2DM and, in some situations, obesity is treated using a class of drugs called Glucagon-Like Peptide-1 (GLP-1) agonists.12Exenatide (EXE) is a GLP-1 homologue originally extracted from the salivary glands of the lizard Heloderma suspectum and used to treat Type 2 Diabetes. After binding to the GLP-1 receptor on pancreatic endocrine β- and α-cells, GLP-1 and EXE boost glucose-dependent insulin production and decrease glucagon release.13 In order to find efficient methods of meeting the needs of diabetes patients at a reasonable cost, many innovative synthetic pathways were also investigated. Therefore, this study’s methodology was developed to assess the antidiabetic impact of Exenatide in a diabetes experimental model.
MATERIALS AND METHODS
Drugs and chemicals
Samples of exenatide were generously purchase from the local market.
Pharmacokinetic study
Female Wistar rats weighing 220±20 g was used in a pharmacokinetic investigation. The institute’s animal facility was raided for critters. The animals were housed in a controlled environment with constant access to food and water (25±2°C temperature, 55% humidity, 12 hr dark-light cycle). The animals in the tests were given free access to water, however they were fasted for 8 hr prior to the start of the experiments. The Institutional Animal Care and Use Committee deemed this study to be ethical.
Drug administration and sample collection
Wistar rats were used for the pharmacokinetic investigations and were randomly split into two groups (each group consisting of six replicates). Exenatide was administered just once to those in Group A, while those in Group B were given it over seven days. A solution of sodium carboxymethyl cellulose (CMC-Na) at a concentration of 0.5% was developed. Rats are often given a dose of exenatide equal to 50 μg/kg subcutaneously. Blood samples (0.2 mL) were obtained from the orbital vein and centrifuged at 3,500 rpm for 10 min at 0 hr, 1 hr, 1.5 hr, 2 hr, 3 hr, 4 hr, 5 hr, 6 hr, 8 hr, 10 hr, 12 hr, 24 hr, and 48 hr after medication administration. The supernatant was removed and placed in a fresh tube before being frozen.
Apparatus and conditions
The UPLC-MS/MS apparatus included a Shimadzu Nexera LC-30A UPLC machine (Sciex Co., Ltd., Framingham, MA, USA), a Waters BEH C18 column (2.1 mm 100 mm, 1.7 m), and an API 5500 triple-quadrupole mass spectrometer. The best method called for using acetonitrile in the B mobile phase and formic acid in the A mobile phase. At the beginning of Stage B, the gradient was 40%; after 4 min, it had grown linearly to 75%; and finally, after only 0.8 min, it had increased to 85%. This took 1 min at a flow rate of 0.25 mL/min. The prepared sample (3 L) was loaded into the UPLC-MS/MS. The temperature in each column and Autosampler was maintained at a pleasant 40°C. Within the mass spectrometer itself is an ESI source. Using a declustering potential of 66.5 and 66 V and a collision energy of 35.1 eV, we tracked ion transitions from m/z 462.2 to 191.0 and m/z 451.4 to 71.0 for exenatide.
Preparation of standard and quality control samples
Prepared 1 mg/mL exenatide methanol stock. Adding methanol to stock solutions made an exenatide solution. Spiking an exenatide working solution with plasma/tissue homogenates and extracting the sample produced calibration standards at different concentrations. Lower limit of quantification (5 ng/mL), medium quality control (10,800 ng/mL), and high-quality control (2,400 ng/mL) were utilised as standards.
Preparation of sample
Exenatide was taken out of the blood stream via Liquid-Liquid Extraction (LLE). Separating 50 μL of plasma, 5 μL of IS, and 200 μL of MTBE required 1 min of vortexing and 10 min of centrifugation at 12,000 rpm. Drying the supernatant in a stream of nitrogen. The reconstituted, residual solution measured 50 μL and was aliquoted into sample vials after being centrifuged for 2 min at 12,000 rpm to remove surplus water.
In vivo studies of antidiabetic activity
Acute toxicity studies
OECD 425 criteria tested the acute toxicity of Exenatide. The animals were checked on for the first 4 hr every 30 min up to 24 hr. The animals were observed for 14 days for any change in behavioral, biochemical, and hematological parameters. In an acute toxicity study, a single dose of exenatide was administered subcutaneously in a dose range of 200 to 800 mg/kg.
Behavioral, Biochemical, and Hematological Parameters
Skin color, salivation, weight, respiration, Muscle Spasm, motor activity, and lacrimation were compared to the control group animals. Blood was collected from animals and mixed with anticoagulant for further study. Suggested centrifugation conditions for blood samples (SIGMA 1-15K, Germany): 4°C, 2000 rpm, for 5 min. The samples were analyzed for hematological and biochemical parameters. The hematological tests were performed for collected blood including a Complete Blood Count (CBC), a hematocrit, a clotting time, a Monocyte Count, a Neutrophil Count, and a Granulocyte Count.
Experimental animal
Animals were divided into five groups each containing six rats. Feed and water pellets were available to all test animals freely. Exenatide was diluted in acetate buffer (pH 4.0) just before injection. All of the rats fasted for two hours before receiving the medication. A single subcutaneous (SC) injection of the acetate buffer was given to the rats in the control group (Group I), Group II was diabetic group received STZ, Group III was standard group received metformin 200 mg/kg given through oral route, Group IV and Group V received 25 and 50 μg/kg of exenatide, respectively. A blood sample (approximately 0.25 mL) was taken from the retro-orbital sinus at the prescribed time after injection and blood glucose was determined at the same time using a glucometer. After centrifuging the sera (8500 rpm for 5 minutes), they were stored at -70°C until they could be tested for exenatide.
Experimental protocol
The study employed 200-250 g adult female Wistar albino rats. Thirty animals were utilized in the in vivo tests. The rat populations were maintained in polypropylene cages. The study was approved by Institutional Animal Ethical Committee. No animals were injured, and all ethical considerations were taken into account. They were housed in conditions representative of their natural environment, with constant temperatures of 25±2°C, humidity levels of 70±5%, and a light/dark cycle of 12 hr each. Blood sugar levels were checked for all the animals before the experiment. Before inducing diabetes, the experimental rats were fed a standard laboratory rat pellet diet for 15 days.
Diabetes Induction
Blood-sampling
In each research, 0.1 mL of tail blood was taken at 0, 1, 2, 4, 8, 12, and 24 hr. The Glucostrips were used to measure blood glucose levels, and the One Touch®, Blood Glucose monitoring device, was used to analyze the results.
Statistics
Analyst TF 1.7.1 and Analyst software (AB Sciex, Redwood City, CA, USA) were used for the acquisition. Non-compartmental analysis in DAS 2.1.1 was used to process the pharmacokinetic parameters. Statistics were shown using a mean and standard deviation format. Student’s t-test and nonparametric rank-sum test were used to establish statistical significance (*p<0.05).
RESULTS
Pharmacokinetic study
Exenatide plasma concentration was analyzed following dosing in rats using this methodology. Figures 1 and 2 depict the average plasma concentration-time curves of exenatide following a single dosing and following seven days of treatment, respectively. The DAS 2.1.1 software was used to determine the pharmacokinetic parameters, and the findings are reported in Table 1. Single-day dose of exenatide resulted in a 38% increase in AUC0-48 (10678.2 ng/L×h) and a 42% increase in AUC0-∞, (14713.1 ng/L×h) while a decrease in Vz/F (7745.1 L/kg) and a decrease in CLz/F (521.3 L/h/kg) were seen when compared to dosing every seven days
| Parameters | Single-day administration | Seven-days administration |
|---|---|---|
| AUC 0-t (ng/ L×h) | 10678.2±1621.1 | 14713.1±2842.63 |
| AUC 0-∞ (ng/L×h) | 10735±2041.11 | 14837.1±2842.99 |
| MRT (h) | 11.3±0.92 | 12.1±1.1 |
| t1/2z(h) | 8.0±1.17 | 8.4±1.12 |
| Tmax(h) | 2.94±0.91 | 6.58±1.03 |
| Vz/F (L/kg) | 10896.11±202.11 | 7745.1±155.62 |
| CLz/F (L/h/kg) | 873.4±78.22 | 521.3±14.12 |
| Cmax (ng/L) | 712.3±15.35 | 875.8±13.3 |
Effects of exenatide on the pharmacokinetic parameters in rat plasma.
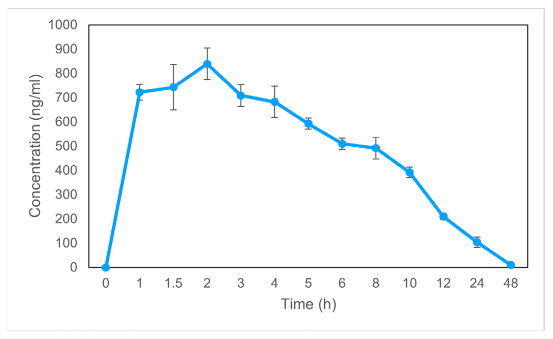
Figure 1:
Dose-response curves for exenatide in a single day’s plasma. Statistics are shown as mean SD for a sample size of 6.
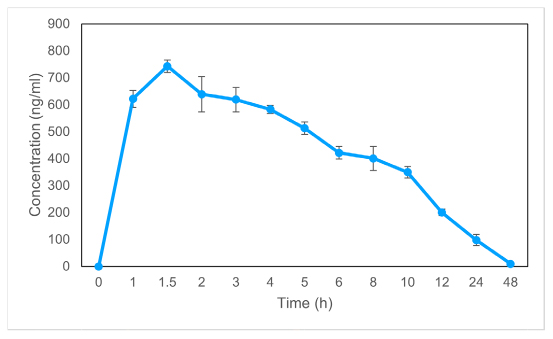
Figure 2:
The average plasma concentration of exenatide vs time after 7 days of treatment. The information is shown as a mean and standard deviation (n=6).
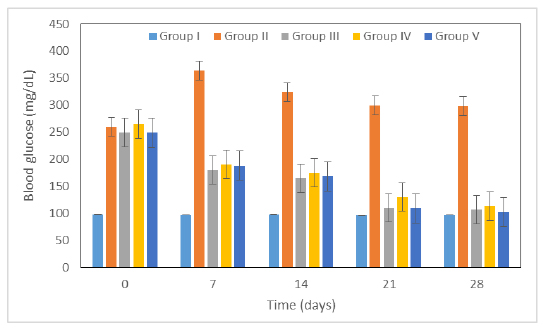
Figure 3:
Figure 3 Antidiabetic activity of exenatide on STZ-induced diabetic rats.
Acute toxicity study
Administration of exenatide did not show mortality of animal and significant change in animal behavior. There was no change in any of the biochemical (Table 2), hematological or behavioral parameters were seen. No change in body weight was observed, however, skin color, respiration, salivation, lacrimation and motor activity were normal. Blood parameters were in normal range.
| Parameters | Results |
|---|---|
| Body Weight | No significant change |
| Skin Color | Normal |
| Respiration | Normal |
| Salivation | Normal |
| Lacrimation | Normal |
| Muscle spasm | Negative |
| Motor activity | Normal |
Exenatide effect on rat behavioral parameters.
In vivo antidiabetic activity of exenatide
Antihyperglycemic effectiveness of a test drug was assessed over time in a rat model of diabetes. Group I started out with normal blood glucose levels and maintained them throughout the test. STZ caused raised in blood glucose of all groups on week 0. Administration of exenatide in group IV and group V showed significant decrease in raised blood glucose level i.e. 113 and 102 mg/dL (p<0.05), respectively in comparison to the control group. However, metformin showed significant antidiabetic activity and reduced glucose level to 107 mg/dL (p<0.05).
DISCUSSION
Exenatide antidiabetic activity was evaluated in this study using Streptozotocin-induced diabetic rats. STZ is commonly used to induce diabetes in animals by selectively killing pancreatic islet cells. Breaks in pyrimidine nucleotide junctions in pancreatic cells are largely to blame.17 Since STZ has alkylating characteristics, it stimulates poly-ADP-ribose synthetase and causes the degradation of nicotinamide adenine dinucleotide in the islets.18 Therefore, a poly(ADP-ribose) synthetase inhibitor is utilized to safeguard islet functionality and revert insulin secretion suppression, so protecting the pancreas from injury.18
Exenatide pharmacokinetics were effectively analyzed using the UPLC-MS/MS technique. Because of its superior extraction recovery to conventional LLE methods and protein precipitation techniques, this LLE method was chosen to handle the biological materials.19,20 Increased levels of IL-6 and lower levels of circulating adiponectin are hallmarks of this resistance. There is a connection between elevated proinflammatory cytokines and the development of insulin resistance, Type 2 Diabetes, and atherosclerosis. Leptin is known to upregulate adiponectin and IL-6, two cytokines that have been shown to affect insulin sensitivity. An uptick in proinflammatory cytokines is linked to insulin resistance, Type 2 Diabetes, and atherosclerosis.21Exenatide was administered to the rats with streptozocin-induced Type 2 Diabetes (T2D), and their blood sugar levels were measured before and after treatment with exenatide. STZ induced T2D at a dose of 60 mg/kg i.p. Increases in blood sugar and decreases in muscle mass and strength are symptoms of diabetes brought on by STZ treatment.
CONCLUSION
In order to increase Exenatide’s bioavailability and solubility, researchers here used a polyoxy ethylene-polyoxy propylene block copolymer to create stable solid dispersions of the drug. Preparation, characterisation, drug release, and in vivo performance of the formulations were all successful and typical. No chemical reaction was found between the medication and the polymer. The exenatide was superior to the original medicine in lowering blood glucose levels in streptozotocin-induced diabetic rats. In order to extend the duration of the drug’s effects, the proposed formulation provides a workable solution. Tolerable toxicological data were obtained when the drug was administered to healthy rats. As an alternative medicine delivery method, pharmaceutical companies need these innovative formulations to treat common and chronic conditions like Type 2 Diabetes mellitus.
Cite this article
Jamali AN, Soni R. Evaluation of Pharmacokinetic Profile of GLP-1 Receptor agonist Exenatide in Type 2 Diabetes Experimental Model. Int. J. Pharm. Investigation. 2023;13(4):784-8.
ACKNOWLEDGEMENT
We are thankful to the Probecell: Scientific Writing Services for proofreading and editing of this article.
References
- Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10(1):14790 [PubMed] | [CrossRef] | [Google Scholar]

- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-81. [PubMed] | [CrossRef] | [Google Scholar]

- Saboo B, Misra A, Kalra S, Mohan V, Aravind SR, Joshi S, et al. Role and importance of high fiber in diabetes management in India. Diabetes Metab Syndr. 2022;16(5):102480 [PubMed] | [CrossRef] | [Google Scholar]

- Kyrou I, Tsigos C, Mavrogianni C, Cardon G, Van Stappen V, Latomme J, et al. Sociodemographic and lifestyle-related risk factors for identifying vulnerable groups for type 2 diabetes: a narrative review with emphasis on data from Europe. BMC Endocr Disord. 2020;20(S1):1-134. [PubMed] | [CrossRef] | [Google Scholar]

- ICMR, Indian Council of Medical Research. ICMR guidelines for management of type 2 diabetes. 2018 [PubMed] | [CrossRef] | [Google Scholar]

- Chawla R, Madhu SV, Makkar BM, Ghosh S, Saboo B, Kalra S, et al. RSSDI-ESI clinical practice recommendations for the management of type 2 diabetes mellitus 2020. Indian J Endocrinol Metab. 2020;24(1):1-122. [PubMed] | [CrossRef] | [Google Scholar]

- Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod J, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-54. [PubMed] | [CrossRef] | [Google Scholar]

- Lee J, Noh S, Lim S, Kim B. Plant extracts for Type 2 diabetes: from traditional medicine to modern drug discovery. Antioxidants (Basel, Switzerland). 2021;10(1):81 [PubMed] | [CrossRef] | [Google Scholar]

- Hinnen D. Glucagon-like peptide 1 receptor agonists for Type 2 diabetes. Diabetes Spectr. 2017;30(3):202-10. [PubMed] | [CrossRef] | [Google Scholar]

- Holst JJ, Gasbjerg LS, Rosenkilde MM. The role of incretins on insulin function and glucose homeostasis. Endocrinology. 2021;162(7):bqab065 [PubMed] | [CrossRef] | [Google Scholar]

- Boer GA, Holst JJ. Incretin hormones and Type 2 diabetes—mechanistic insights and therapeutic approaches. Biology. 2020;9(12):473 [PubMed] | [CrossRef] | [Google Scholar]

- Collins L, Costello RA. Glucagon-like Peptide-1 receptor agonists. 2023 [PubMed] | [CrossRef] | [Google Scholar]

- Rowlands J, Heng J, Newsholme P, Carlessi R. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol. 2018;9:672 [PubMed] | [CrossRef] | [Google Scholar]

- Devarajan PV, Sonavane GS. Preparation and evaluation of gliclazide loaded Eudragit nanoparticles as a sustained release carriers. Drug Dev Ind Pharm. 2007;33(2):101-11. [PubMed] | [CrossRef] | [Google Scholar]

- Jain S, Saraf S. Influence of processing variables and characterization of glipizide loaded biodegradable nanoparticles. Diabetes Metab Syndr Clin Res Rev. 2009;3(2):113-7. [CrossRef] | [Google Scholar]

- Mohan Kamila M, Mondal N, Kanta Ghosh L, Kumar Gupta B. Multiunit floating drug delivery system of rosiglitazone maleate: development, characterization, statistical optimization of drug release and evaluation. AAPS PharmSciTech. 2009;10(3):887-99. [CrossRef] | [Google Scholar]

- Szkudelski T. Streptozotocin–nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med (Maywood). 2012;237(5):481-90. [PubMed] | [CrossRef] | [Google Scholar]

- Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of Type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;2010:453892 [PubMed] | [CrossRef] | [Google Scholar]

- Kobuchi S, Yano K, Ito Y, Sakaeda T. A validated LC-MS/MS method for the determination of canagliflozin, a Sodium-Glucose Cotransporter 2 (SGLT-2) inhibitor, in a lower volume of rat plasma: application to pharmacokinetic studies in rats. Biomed Chromatogr. 2016;30(10):1549-55. [PubMed] | [CrossRef] | [Google Scholar]

- Iqbal M, Ezzeldin E, Al-Rashood KA, Asiri YA, Rezk NL. Rapid determination of exenatide in rat plasma by UHPLC- MS/MS using negative ionization mode to avoid adduct- ions formation. Talanta. 2015;132:29-36. [PubMed] | [CrossRef] | [Google Scholar]

- López-Jaramillo P, Gómez-Arbeláez D, López-López J, López-López C, Martínez-Ortega J, Gómez-Rodríguez A, et al. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig. 2014;18(1):37-45. [PubMed] | [CrossRef] | [Google Scholar]


