ABSTRACT
ABSTRACT
The objective of the present was to formulate a novel topical P-herbo dosage form by incorporating the extracts of Azadirachta indica, Centella asiatica, and Ocimum gratissum and compare the efficacy. There is a strong clinical need to establish an appropriate formulation with the possibility to treat Ance by once-a-day application. Topical P-herbo dosage forms were prepared using various concentrations of extracts, with other excipients, and were evaluated for physicochemical parameters such as Viscosity, pH, spreadability, and extrudability to determine the suitability of the topical formulation. Anti-oxidant activity by DPPH assay, anti-microbial assay against Cutibacterium acne, and in vitro skin irritation studies were also carried out. Studies revealed, in vitro skin irritation showed the least irritation score for formulations (Gels-0.2; cream and ointment- 0.3) compared to 1% clindamycin and positive control. On analyzing the results, the OF-2 formulation was found to be optimized, due to its good anti-microbial, antioxidant activity with high skin deposition of herbs extract. Further, the optimized formulation was subjected to stability studies in compliance with ICH guidelines for a period of six months and all evaluated parameters remained intact even after six months, thus confirming the stability of the formulation. Hence, the present research concludes that the ointment formulation containing P-herbo extract showed prolonged action and was an excellent substitute for synthetic antiacne formulations with once-daily application.
INTRODUCTION
Acnes are the most prevalent skin condition that 85% of youngsters experience today, making it the world’s eighth most prevalent disease. Even though acne is not life-threatening it can affect an individual’s psychological, social, and emotional consequences.1,2 Acne can last throughout adulthood and typically affect the most prominent oil glands, such as the face and neck. Seborrhea, inflammatory lesions, comedones, extreme sebum production, and the presence of bacteria for example Cutibacterium acnes, Staphylococcus epidermidis, and Malassezia furfur in the follicles are all characteristics of acne hence, these bacteria could be targeted for acne therapy. The severity of the condition decides the mode of treatment to be followed, accordingly mild to moderate infections, is treated topically by application of benzoyl peroxide, Clindamycin, and retinoid.3 Despite their efficacy in treating mild to moderate acne vulgaris, these topical therapies are known to be irritating and have a history of poor tolerability and patient adherence. Systemic treatment is recommended for moderate to severe acne, i.e., acne resistance to topical therapy, and acne covers a comprehensive body area. Systemic treatment mainly includes oral antibiotics, retinoids, and hormonal treatment. Antibiotics used for long periods of time cause organisms to become unresponsive to the drugs.4 This multifaceted adaptation is impacted by the organism’s susceptibility to the therapy as well as host factors including hormone levels and stress levels. Treatment of acne with these anti-microbials has been linked to the development of resistance to these drugs by P. acne, leading to treatment failure.5 Discovering effective, safe, low-cost antiacne treatments is critical to overcoming these constraints. To address this problem, herbal therapeutic alternatives have been investigated.
Traditional Thai medicine has used the herbal ball to address a number of ailments, including acne. Herbs have such a long history in ancient India that they are even referenced in the Vedas, an ancient Hindu sacred text. Ayurveda and Unani, two ancient herbal medicine systems, use natural remedies and plants to address diseased conditions.6 Although herbal medicines may seem unfamiliar to western healers and physicians, the bulk of currently prescription pharmaceuticals still contain plant extracts. The need for Indian herbal remedies, which are valued by nations worldwide, has led to this traditional kind of therapy’s rapid expansion—an annual growth rate of about 30%.7,8 Even though herbal therapies might seem unfamiliar to western healers and medical professionals, the bulk of today’s prescription drugs still contain plant extracts.9 Many herbs mentioned in traditional literature have been widely used to treat several skin- related problems, such as Centella asiatica, Ocimum gratissimum, and Azadirachta indica.10–12 These herbs exhibit antiacne activity due to their anti-inflammatory, antioxidant, and antibacterial properties. Traditional eastern medicine known as the herbal ball or herbal compress massage ball is widely used in Thailand.13 To prepare the herbal ball, various herbs with anti-oxidant, anti-inflammatory, and anti-microbial properties are combined and firmly wrapped in a square piece of fabric to make a herbal compress ball.14 The herbal massage ball is heated with the use of steam. Multiple illnesses, including acne, have been treated with the help of the herbal ball compress.
The herbal extracts were modified and created as polyherbal formulations because they cannot be utilised directly for the treatment.10 However, the herbal ball needs to be applied repetitively for treating chronic conditions like acne, which is not practical as it is a tedious and time-consuming process. Hence there is a need to incorporate the herbal ball extract into a suitable topical formulation. The current work attempts to develop topical formulations using herbal ball extract of Centella asiatica, Ocimum gratissimum and Azadirachta indica that could be utilized as an alternative of the traditional herbal ball for acne treatment.
MATERIALS AND METHODS
Plant Materials
Centella asiatica, Ocimum gratissimum and Azadirachta indica were collected as fresh herbs from Mandya district, Karnataka, India and authenticated by Dr. H M Mahesh, Head and Coordinator, Department of Botany, Bharathi College – PG and RC, Bharathinagara, Mandya district 571422, Karnataka India. Carbopol 940 was purchased from Loba Chemie Pvt. Ltd., India. Glycerin and triethanolamine were obtained from SD fine chemicals Mumbai, India.15 Isopropyl Myristate (IPM) was ordered from National Chemicals (Vadodara, India). Hard paraffin obtained from Indian research products, Chennai. Emulsifying wax, White soft paraffin and liquid paraffin were procured from Nice Chemicals, Kochi.
Methods
Preparation of P-Herbo Extract
In the microwave method, the same quantity of leave samples (100gm), and distilled water (1000mL) was used. The botanical sample was placed in a Clevenger – type apparatus and was placed within a to completely remove the oil, run a home microwave oven (LG MS2021CW, LG Electronics, India) for 1 min at 80 W of electricity. The mother extract was centrifuged for 10 min at 894 x g (Spinwin MCO1 Micro Centrifuge, Tarsons, India) to produce stick paste form, which was then placed in the desicator till the dried form was achieved.16
Evaluation of P-Herbo extract
Antioxidant activity
The DPPH assay was used to measure the herbal ball extract’s antioxidant activity.17 To get the final concentration of 0.4 mm DPPH, the diphenylpicryl hydrazine solution in methanol was further diluted. Herbal ball extract powder and marketed product was diluted with methanol to produce the concentration of 25-800μg/mL. Gallic acid served as the test substance. To the different concentrations of samples (1 mL) under test, 1 mL of DPPH reagent was added and vortexed, placed in the dark for 30 min. The decrease in absorbance due to the scavenging of DPPH free radical was measured at a wavelength of 554 nm using a UV-visible spectrophotometer.18 The antioxidant activity in terms of percentage inhibition was determined using the equation:
Where, As and Ac are the absorbance of sample solution and control, respectively.
Minimum inhibitory concentration
To measure Minimum Inhibitory Concentration (MIC), the fresh culture of Cutibacterium acne was prepared in the nutrient broth media. Different concentrations of the herbal ball extract, its formulation and marketed standard (25, 50, 100, 200, 400, 800 mg/mL) were prepared in the broth media (1mL). 1 mL of a brand-new bacterium culture was added to each tube as an inoculation. These tubes were then cultivated at 37°C for 24 hr. 200μL of Para iodonitrotetrazolium chloride (6.3 mg/mL) were added to the test tubes to evaluate whether the microorganism was present or not when it grew there. Experiments were conducted in triplicates. The anti-microbial activity of the samples tested was expressed in terms of minimum inhibitory concentration.19
Anti-microbial assay
P-Herbo extracts were tested against the microbial strain Cutibacterium acne produced from microbial-type culture collection (AIMS, B.G Nagara, and Karnataka, India). The microbial cultures served as stock cultures for the duration of the experiment and were kept in their individual agar slants at 4°C. Herbal ball extracts were tested in triplicate for antibacterial efficacy.19 To examine the effectiveness of various samples and formulations against Cutibacterium acne, nutrient agar plates were pre-warmed. Herbal ball extract samples were dissolved in DMSO (1 mg/mL), and the final concentration of DMSO was maintained 20%. The prepared plates were seeded with Cutibacterium acne and 200 μL of various samples under tests were pipette into the cups prepared using sterile borer. For 24 hr, these plates were incubated at 37°C. The anti-microbial potential of the test samples was determined by measuring the diameter of the test substance’s millimeter-representative zone of inhibition.
Preparation of P-Herbo Extract containing gel
Various amounts of carbopol 934 P were used to create gel formulations containing the P-Herbo extract. The herbal ball extract was first dissolved in isopropyl palmitate and glycerol, then preservatives were added to create the herbal ball extract solution. The gel base was prepared in another beaker by dispensing Carbopol 934 P in distilled water under continuous stirring.20 The extract solution was then introduced into the gel base and triethanolamine dropwise until gel formation. The contents of the prepared gels are given in Table 1.
| Ingredients | GF 1 | GF 2 | GF 3 |
|---|---|---|---|
| Herbal Extract (g) | 2gm | 2.5 | 3 |
| Carbopol gel (%) | 1gm | 1.5 | 2 |
| Isopropyl Maleate (mL) | 4 | 4 | 4 |
| Glycerin (mL) | 10 | 10 | 10 |
| Triethanolamine (mL) |
Composition of the P-Herbo extract containing gel.
Preparation of P-Herbo Extract containing ointment
Accurately weighed amounts of emulsifying wax, soft white paraffin, and liquid paraffin were heated to 70-75°C and agitated until a homogenous mass was achieved. The herbal ball extracts were then mixed into the base of the ointment. The composition of the ointment is given in Table 2.
| Ingredients | CF 1 | CF 2 | CF 3 |
|---|---|---|---|
| Herbal Extract (g) | 2 | 2.5 | 3 |
| Paraffin wax (g) | 25 | 25 | 25 |
| Soft paraffin white (g) | 10 | 10 | 10 |
| Liquid paraffin (mL) | 50 | 50 | 50 |
| Isopropyl maleate (mL) | 3 | 2.5 | 2 |
| Aqueous Phase (mL) | 10 | 10 | 10 |
Composition of the P-Herbo extract containing ointment.
Preparation of P-Herbo Extract containing cream
The beaker was initially filled with the required amount of paraffin wax and set on a hot plate to melt, then weighed amounts of petroleum ether were added to the beaker to make the base. In another beaker, P-Herbo extract was weighed, and 20 mL of liquid paraffin was added to it with steady stirring until the extract dissolved. Then, the herbal ball extract mixture was incorporated into the base with constant stirring, followed by the addition of liquid paraffin to make up the volume. The composition of the cream is shown in Table 3.
| Ingredients | OF 1 | OF 2 | OF 3 |
|---|---|---|---|
| Extract (g) | 2 | 2.5 | 3 |
| Emulsifying wax (g) | 23 | 22.5 | 22 |
| White soft paraffin (g) | 15 | 15 | 15 |
| Liquid paraffin (mL) | 30 | 30 | 30 |
| Aqueous Phase (mL) | 30 | 30 | 30 |
Composition of the P-Herbo extract containing cream.
Evaluation of the topical formulations containing P-Herbo Extract
Physiochemical Properties
Visual inspection was done of the herbal ball extract compositions’ form, colour, homogeneity, consistency, and appearance.
pH Measurement
In advance of obtaining the pH of the formulations determined with the digital pH meter’s probe, formulations were diluted 1:10 with distilled water.
Viscosity
Using a Brookfield viscometer (Brookfield Engineering Laboratories, Inc., USA, DVE) at a speed of 50 rpm and spindle number 21, the viscosity of the optimised formulation was calculated.
Spreadability
On a glass plate, a circle with a radius of 1 cm was drawn. The glass plate’s centre was filled with 1g of the formulation. Glass slide two was taken and placed on the gel. The subsequent glass slide was kept at a weight of 500 g. The weight was taken off after 5 min, and the gel’s diameter was calculated.
Extrudability
In order to carry out the test, a steady weight is applied while the amount of gel extruded from a collapsible tube is measured. With a constant load of 500g, a closed collapsible tube containing 10 g of each gel formulation was squeezed. The gel extruded when the cap was opened, releasing the pressure. After it had been extruded, the gel was gathered and weighed.
In vitro skin irritation study
An in vitro OECD-approved test, the Hen’s embryo test- chorioallantoic membrane, is used to screen for skin irritation (HET-CAM test). In this technique, freshly laid hen eggs were used, and the embryos were fertilized to check for irritation on the growing chick embryo.
Six groups were made as follows:
Group 1: 0.9% NaCl (Negative control).
Group 2: 0.3 mL of 1% SDS+ 0.3 mL of 0.1N NaOH (Positive Control).
Group 3: 1% Clindamycin gel as standard treatment.
Group 4: Optimized gel formulation.
Group 5: Optimized cream formulation.
Group 6: Optimized ointment formulation.
Briefly, the obtained Hen’s eggs were put on a metal tray and placed in incubator at 370.5°C and 58±2% relative humidity, which was necessary for embryo development in the eggs. The eggs were inverted five times a day during incubation, and this technique was repeated for eight days. The incubated eggs were checked for embryo development on the eighth day, and after confirmation, the eggs were returned to the incubator. On the ninth day, the eggs were taken out of the incubator, and without harming the embryo, a hole was made in the air sac of the eggshell on the top surface. After creating the hole, all of the grouped eggs were treated with the appropriate solutions and kept under observation for 5 min to look for symptoms of haemorrhage, coagulation, and lysis of blood vessels. By using the mean irritation score from the formula, the irritation impact was verified. The irritation score value with inference is shown in Table 4 after the Irritation Score (IS) formula is presented below.
Where,
H- Hemorrhage.
L- Lysis of blood vessels.
C- Coagulation.
| Irritation score | Inference |
|---|---|
| 0-0.9 | No irritation |
| 1-4.9 | Weak irritation |
| 5-8.9 | Moderate irritation |
| 9-21 | Severe irritation |
Irritation score value with inference for HET-CAM test irritation.
Determination of the anti-microbial activity of the formulation
The turbidimetric method was used to assess the anti-microbial efficacy. A Petri dish was covered with an aseptically prepared sterile nutritional agar medium. An acne prone face was cleaned with distilled water, and dried for some time. After that, a cotton swab was soaked in 5 mL of distilled water and applied to the ruptured pimple until its complete surface was in contact with the acne. This mixture was evenly applied onto the previously ready surface and cultured for 24 hr at 37°C. On the ninth day, the eggs were taken out of the incubator and a hole was drilled through the top surface of the air sac without harming the embryo.
Stability studies
Using a freeze-thaw technique, the formulations were put through accelerated stability tests for six cycles. The samples for each cycle were put in the plastic tube, kept at 4°C for 24 hr, and then kept at 30°C for 24 hr. Following six cycles, the formulations’ physicochemical characteristics were assessed, and they were contrasted with the freshly made formulation.
RESULTS AND DISCUSSION
The P-herbo extract was obtained by microwave radiation method, containing constituents of Azadirachta indica, Centella asiatica, and Ocimum gratissum. The extract was found to contain Nimbin, Triterpene, flavonoids and polyphenols which possess anti-inflammatory, antioxidant effects, and antifungal activity thereby reducing the damage by reducing and generating reactive oxygen species.21–23
Anti-oxidant activity
The anti-oxidant activity of the P-herbo extract was determined in comparison with gallic acid (positive control). It was observed that there was a decolorization of DPPH, as well intensity of the colour changed from purple to yellow which indicates that P-herbo extract has strong anti-oxidant activity and also the decolorization was concentration dependent as seen in Figure 1. This phenomenon is due to the fact that the P-herbo extract is a crude plant extract contains numerous other secondary metabolites compared to gallic acid (positive control).
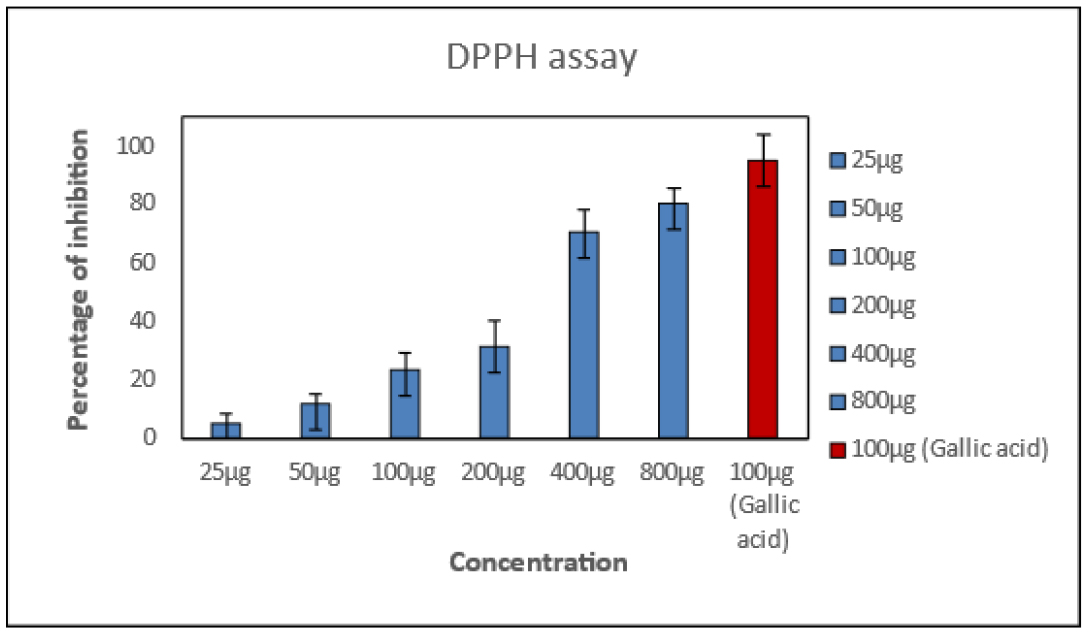
Figure 1:
Anti-oxidant activity of P-herbo extract and gallic acid.
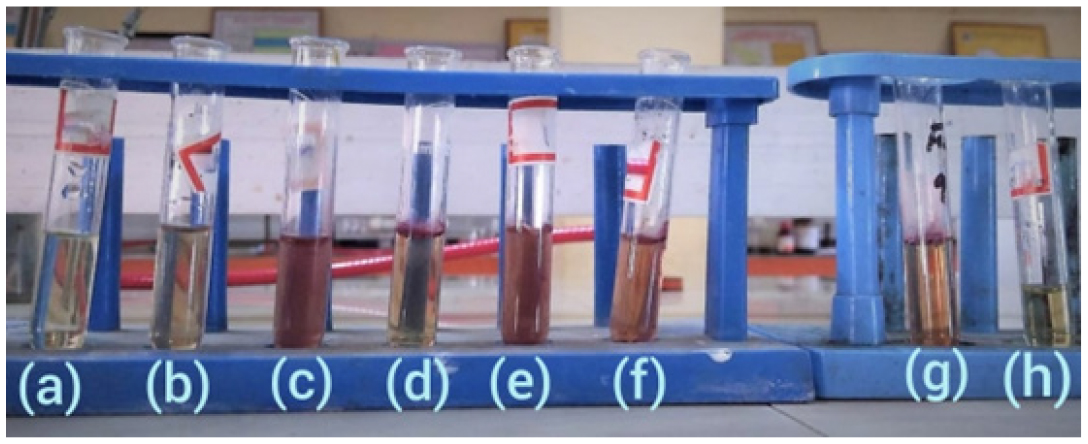
Figure 2:
Determination of MIC of P-herbo extracts by broth dilution method.
Minimum Inhibitory Concentration (MIC)
By calculating the Minimum Inhibition Concentration (MIC) using the broth dilution method, the antiacne activity of the P-herbo extract (25-800mg/mL) was assessed. Results Showed (Figure 2) that 200mg/mL compared to DMSO (vehicle control) did not show any inhibition of the microbial growth.
Zone of Inhibition for P-Herbo Extract
P-Herbo extract exhibited optimum antifungal activity against cutibacterium acne.12 There was no significant difference between zone of inhibition of marketed cream (Figure 3) and P-Herbo extract. But among the test formulation 1000mg/mL exhibited larger zone may be because of antifungal activity of extract (Table 5). According to an in vitro antifungal investigation, the optimised ointment formulation has greater anti-fungal action since it displays a broader zone of inhibition against the acnecausing cutibacterium than the control and commercial creams.
| Formulation | Zone of inhibition |
|---|---|
| Marketed product (1%) | 24 mm |
| 1000 mg/mL | 33 mm |
| 800 mg/mL | 31 mm |
| 600 mg/mL | 35 mm |
| Control (20% DMSO) | No zone of inhibition |
Zone of Inhibition of different concentration of P-Herbo extract.
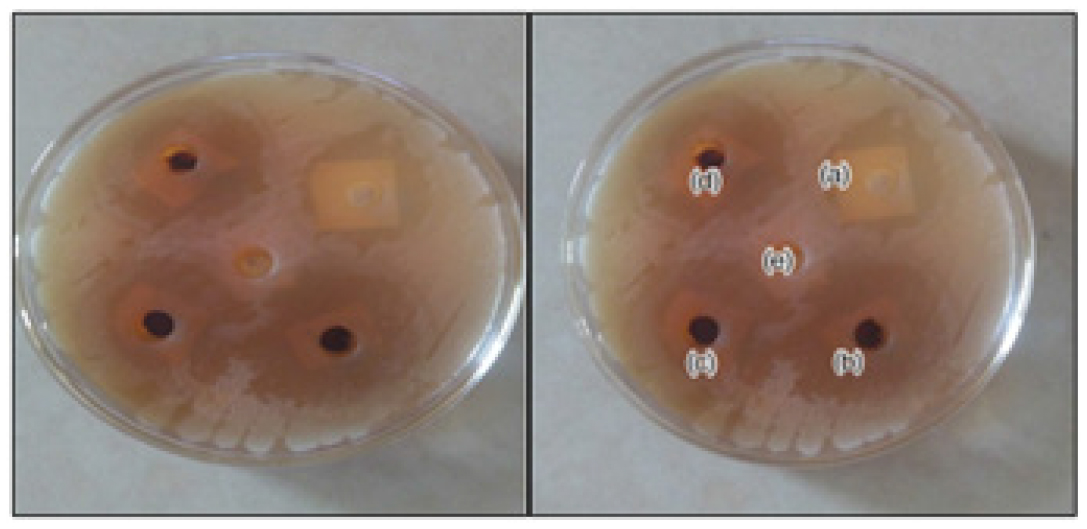
Figure 3:
Zone of inhibition of P-herbo extract and the marketed formulation a) Marketed product `(1%) b) 1000 mg c) 800 mg d) 600 mg e) Control (20% DMSO).
Characterization of topical formulations containing P-herbo extract
Physicochemical properties of P-herbo extract incorporated topical formulation (ointment, cream and gel)
Topical formulations like cream, gel and ointments are prepared containing p-herbo extract and further assessed for their physical and chemical characteristics, such as pH, odour, and colour. spreadability, extrudability, and homogeneity. The results are depicted in the Table 6.
| Properties | Gel Formulations | Cream Formulations | Ointment Formulations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GF 1 | GF 2 | GF 3 | CF 1 | CF 2 | CF 3 | OF 1 | OF 2 | OF 3 | |
| Colour | Dark brown | Brown | Yellow brown | Mid tone brown | Mid tone brown | Mid tone brown | Off white | Off white | Off white |
| Odour | Mild odour | Mild odour | Mild odour | Pungent | Pungent | Pungent | Fragrant | Fragrant | Fragrant |
| pH | 7.2 | 5.9 | 6.2 | 6.1 | 5.9 | 6.2 | 6.3 | 6.5 | 6.4 |
| Homogeneity | +++ | ++ | + | +++ | +++ | +++ | +++ | +++ | +++ |
| Viscosity(cps) | 3800 | 3700 | 4000 | 4600 | 4400 | 7000 | 6,400 | 12,800 | 19,200 |
| Spreadability (gm*cm/sec) | 10.1 | 23 | 23.8 | 9.3 | 9.1 | 9.8 | 6.1 | 6.5 | 6.8 |
| Extrudability (g/cm?) | 0.7 | 0.9 | 1 | 0.5 | 0.4 | 0.3 | 1.3 | 0.6 | 0.7 |
Physico-chemical properties of topical formulations containing the P-herbo extract.
Among the topical formulation containing P-herbo extract, it has been noticed that only the gel formulation was non-homogenous which can be attributed to the fact that on increasing the concentration of herbal extract the quantity of water and oil phase in the formulation was insufficient.
Viscosity is one of the essential parameters for evaluation of topical dosage forms. It was observed that increasing concentration of water phase in gel formulation decreases the viscosity whereas in case of creams and ointment increase in concentration of oil phase makes the formulation more viscous which might help the herbal extract to penetrate through the lipophilic layer of the skin.
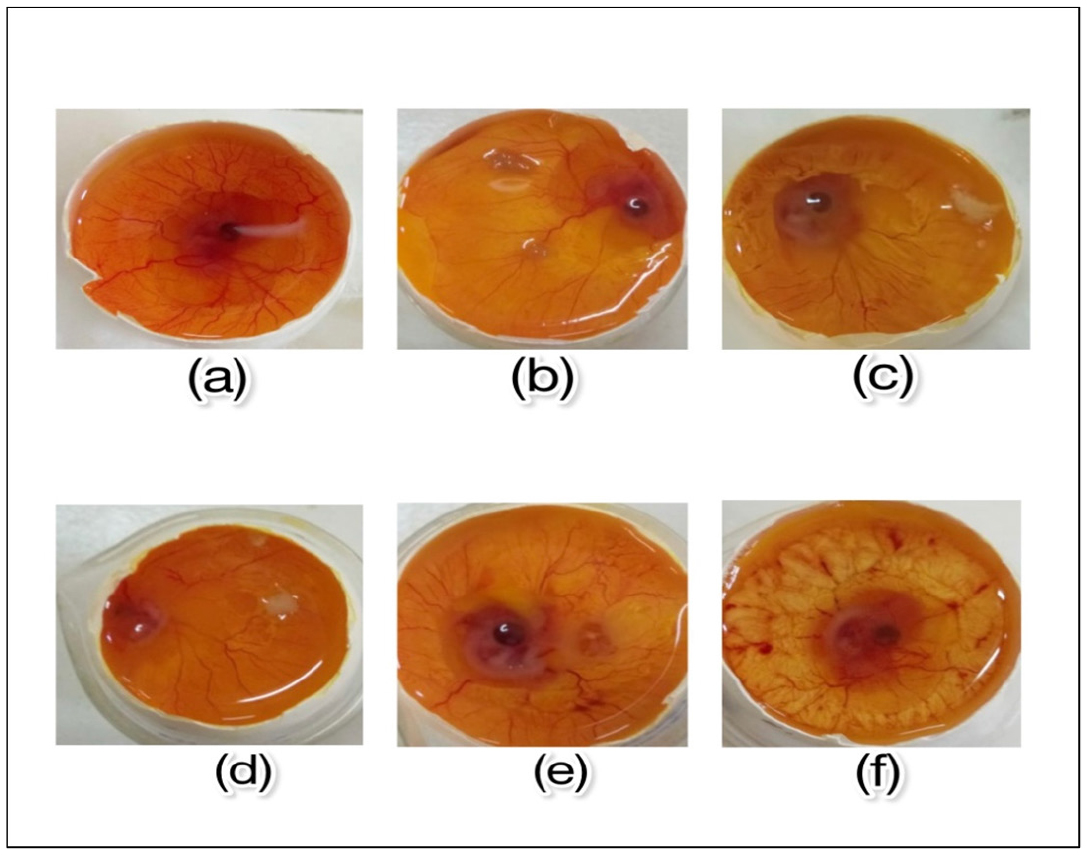
Figure 4:
HET-CAM test for in vitro skin irritation study a) Embryo treated with 0.9% NaCl. b) Embryo treated with gel containing herbal ball extract. c)Embryo treated with cream containing herbal ball extract. d)Embryo treated with ointment containing herbal ball extract. e) Embryo treated with 1% Clindamycin phosphate topical solution (standard). f) Embryo treated with 1% SDS causing lysis of blood vessels and haemorrhage.
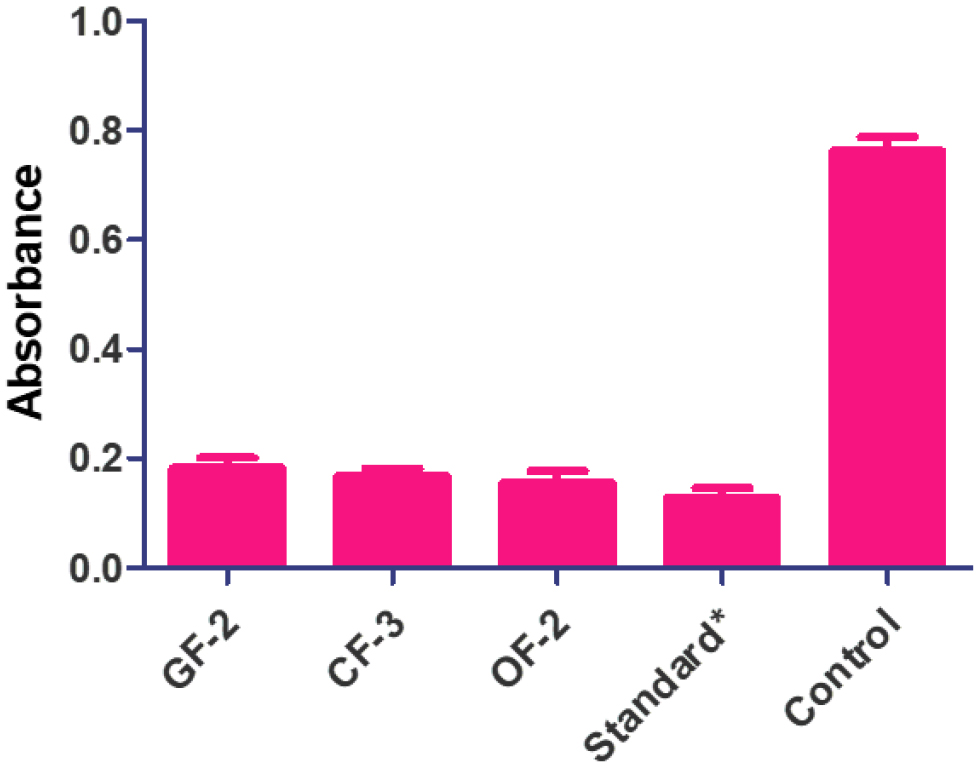
Figure 5:
Antiacne efficacy of the herbal topical formulations in comparison to standard and control. (*Concentration of clindamycin-100 mg/mL).
From Table 6, it was depicted that the formulation OF2 has highest viscosity of 12,800 cps whereas, GF2 has minimum viscosity 3700cps. Maximum viscosity of the optimized formulation OF2 is due the higher amount of oil phase i.e., 30 mg.
In vitro skin irritation studies
With a mean irritation score of 14.5, the chick embryo treated with 1% SDS (Positive Control) caused blood vessel lysis and haemorrhage, indicating severe irritation. In contrast, 0.9% NaCl (Negative Control) and the optimised formulations, which had mean irritation scores of (0.2-0.3) respectively, showed no signs of irritation.
The marketed formulation, i.e., Clindamycin, showed a similar mean irritation score as that of the optimized formulations. Thus, the acquired results confirmed that the formulations incorporating herbal ball extract of gel, cream, and ointment were non-irritant and non-toxic in nature. The images of the HET-CAM test are depicted in Figure 4 and mean irritation score metioned in Table 4 and Table 7 suggested that formulations does not have irritation potential.
| Formulations | Irritation score |
|---|---|
| Gel (GF-2) | 0.3 |
| Cream (CF-3) | 0.2 |
| Ointment (OF-2) | 0.2 |
| 1% Clindamycin | 2.3 |
| 0.9% NaCl | 0.2 |
| 1% SDS | 14.5 |
Mean Irritation score of HET-CAM test formulations containing P-Herbo extract.
The efficacy of the optimized topical formulations was studied and represented in Figure 5. The acne-causing bacteria’ ability to multiply could be hampered by anti-acne treatments. The efficacy of all the formulations was higher than that of the usual medication, but the ointment form of the OF-2 formulation demonstrated the best efficacy, showing strong microbe growth even though the extract content was the same. Hence, the gel type of formulation is considered best for preparing antiacne formulations.
Stability Studies
The physicochemical features of the formulation (OF-2) in terms of appearance, homogeneity, pH, viscosity, and spreadability have remained intact even after six freeze thaw cycles as seen in Table 8. As a result, under accelerated conditions, the prepared formulations were physically stable.
| Freeze-Thaw cycle | Appearance | Homogeneity | pH | Viscosity (cps) | Spreadability (gm*cm/sec) |
|---|---|---|---|---|---|
| 1 | Brown | +++ | 5.9 | 9700 | 23 |
| 2 | Brown | +++ | 5.9 | 4680 | 23 |
| 3 | Brown | +++ | 5.8 | 4650 | 22 |
| 4 | Brown | +++ | 5.7 | 4643 | 22 |
| 5 | Brown | +++ | 5.7 | 4637 | 21 |
| 6 | Brown | +++ | 5.5 | 4625 | 21 |
Stability studies of GF-2 formulation on consecutive freeze-thaw cycles.
CONCLUSION
The authors conclude that the P-Herbo extract containing constituents of three different herbs exhibited good antioxidant and anti-acne activity similar to the standard drug. However, for the convenience of application the extract was incorporated into different dosage forms and checked. From the physiochemical parameters evaluated on the same as well the anti-oxidant and anti-acne activity revealed ointment as excellent formulation. The OF-2 showed the highest efficacy in terms of physiochemical parameters and the formulation exhibited least irritation potential. This served the purpose of the research for treating acne in once daily application.
Cite this article
Naveen NR, Raj K, Gaganashree, Biju P, Annegowda HV, Sindhoor SM. Formulation and Comparison of Topical Dosage Forms Containing P-Herbo Extract for Acne Therapy. Pharmacog Res. 2023;13(4):747-53.
ACKNOWLEDGEMENT
The authors are thankful to Sri Adichunchanagiri College of Pharmacy, Adichunchanagiri University for providing the necessary facilities to carry out the project work.
References
- Vora J, Srivastava A, Modi H. Antibacterial and antioxidant strategies for acne treatment through plant extracts. Inform Med Unlocked. 2018;13:128-32. [CrossRef] | [Google Scholar]

- Neeraj B. Topical gel: A review. World J Pharm Pharm Sci. 2017:303-13. [CrossRef] | [Google Scholar]

- Hoffman LK, Bhatia N, Zeichner J, Kircik LH. Topical vehicle formulations in the treatment of acne. J Drugs Dermatol. 2018;17(6):s6-s10. [PubMed] | [Google Scholar]

- Rasheed A, Avinash Kumar Reddy G, Mohanalakshmi S, Ashok Kumar CK. Formulation and comparative evaluation of poly herbal anti-acne face wash gels. Pharm Biol. 2011;49(8):771-4. [PubMed] | [CrossRef] | [Google Scholar]

- Vyas A, Sonker AK, Gidwani B. Carrier-based drug delivery system for treatment of acne. Sci World J. 2014:2014 [CrossRef] | [Google Scholar]

- Bakkiyaraj S, Pandiyaraj S. Evaluation of potential antimicrobial activity of some medicinal plants against common food-borne pathogenic microorganism. Int J Pharm Biol Sci. 2011:2 [CrossRef] | [Google Scholar]

- Patil P, Datir S, Saudagar R. A review on topical gels as drug delivery system. J Drug Deliv Ther. 2019:9 [CrossRef] | [Google Scholar]

- Verma A, Singh S, Kaur R, Jain UK. Topical gels as drug delivery systems: a review. Int J Pharm Sci Rev Res. 2013:23 [CrossRef] | [Google Scholar]

- Azimi H, Fallah-Tafti M, Khakshur AA, Abdollahi M. A review of phytotherapy of acne vulgaris: perspective of new pharmacological treatments. Fitoterapia. 2012;83(8):1306-17. [PubMed] | [CrossRef] | [Google Scholar]

- Shen X, Guo M, Yu H, Liu D, Lu Z, Lu Y, et al. Propionibacterium acnes related anti-inflammation and skin hydration activities of madecassoside, a pentacyclic triterpene saponin from Centella asiatica. Biosci Biotechnol Biochem. 2019;83(3):561-8. [PubMed] | [CrossRef] | [Google Scholar]

- Kanlayavattanakul M, Lourith N. Therapeutic agents and herbs in topical application for acne treatment. Int J Cosmet Sci. 2011;33(4):289-97. [PubMed] | [CrossRef] | [Google Scholar]

- Lertlop W. The appropriate temperature of the Thai Herbal Ball compress for relaxing effected. Procedia Soc Behav Sci. 2015;197:1653-60. [CrossRef] | [Google Scholar]

- Cheung LK, Osei-Kuffour D, Drake PJH. Full thickness burns after Thai herbal hot compress massage ball therapy. Open. 2021;5(2):78-80. [CrossRef] | [Google Scholar]

- Arifullah M, Namsa ND, Mandal M, Chiruvella KK, Vikrama P, Gopal GR, et al. Evaluation of anti-bacterial and anti-oxidant potential of andrographolide and echiodinin isolated from callus culture of Andrographis paniculata Nees. Asian Pac J Trop Biomed. 2013;3(8):604-10. discussion 609 [PubMed] | [CrossRef] | [Google Scholar]

- Ibeh SC, Akinlabi OD, Asmau I, Audu J, Muritala AM. Extraction of Ocimum gratissimum using different distillation techniques. Int J Sci Technol Res. 2017;6(5):26-8. [PubMed] | [CrossRef] | [Google Scholar]

- Waghule T, Rapalli VK, Singhvi G, Manchanda P, Hans N, Dubey SK, et al. Voriconazole loaded nanostructured lipid carriers based topical delivery system: QbD based designing, characterization, in vitro and ex vivo evaluation. J Drug Deliv Sci Technol. 2019;52:303-15. [CrossRef] | [Google Scholar]

- Patwekar SL, Pedewad SR, Gattani S. Development and evaluation of nanostructured lipid carriers-based gel of isotretinoin. Part Sci Technol. 2018;36(7):832-43. [CrossRef] | [Google Scholar]

- Budhiraja A, Dhingra G. Development and characterization of a novel antiacne niosomal gel of rosmarinic acid. Drug Deliv. 2015;22(6):723-30. [PubMed] | [CrossRef] | [Google Scholar]

- Hiremath SSP, Dasankoppa FS, Nadaf A, Jamakandi VG, Mulla JS, Sreenivas SA, et al. Formulation and evaluation of a novel in situ gum based ophthalmic drug delivery system of linezolid. Sci Pharm. 2008;76(3):515-32. [CrossRef] | [Google Scholar]

- Baghel S, Nair VS, Pirani A, Sravani AB, Bhemisetty B, Ananthamurthy K, et al. Luliconazole‐loaded nanostructured lipid carriers for topical treatment of superficial tinea infections. Dermatol Ther. 2020;33(6):e13959 [PubMed] | [CrossRef] | [Google Scholar]

- Bylka W, Znajdek-Awiżeń P, Studzińska-Sroka E, Brzezińska M. Centella asiatica in cosmetology. Postepy Dermatol Alergol. 2013;30(1):46-9. [PubMed] | [CrossRef] | [Google Scholar]

- Ugbogu OC, Emmanuel O, Agi GO, Ibe C, Ekweogu CN, Ude VC, et al. A review on the traditional uses, phytochemistry, and pharmacological activities of clove basil (Ocimum gratissimum L.). Heliyon. 2021;7(11):e08404 [PubMed] | [CrossRef] | [Google Scholar]

- Islas JF, Acosta E, G-Buentello Z, Delgado-Gallegos JL, Moreno-Treviño MG, Escalante B, et al. An overview of Neem (Azadirachta indica) and its potential impact on health. J Funct Foods. 2020;74:104171 [CrossRef] | [Google Scholar]


