ABSTRACT
Medicinal plants play important roles in the treatment of various diseases. The aim of this review is to compile and document the phytochemical properties and the pharmacological effect in different parts of the various extracts of Hibiscus rosa-sinensis. The information was collected from various electronic databases such as Google Scholar, PubMed, Web of Science, Science Direct, Scifinder and articles published in English. Previous studies have revealed that the leaves of the plant contain important constituents such as tannins, saponins, glycosides, flavonoids, and terpenoids, while the petals contain compounds such as quercetin, kaempferol, and anthocyanins. The chemical composition of the plant may vary depending on the color of the petals, with red petals having more anthocyanins and white petals having slightly higher levels of tannins. The anti-inflammatory, antioxidant, anti-microbial and anti-diabetic activity of Hibiscus rosa-sinensis have been documented. This review will contribute to a more organized finding on the pharmacological effects of Hibiscus rosa sinensis and invigorate further studies.
INTRODUCTION
Nature has been a source for medicine since thousands of years ago and a large number of modern drugs produce are derived from natural sources based on their used traditionally.1 Hibiscus rosa-sinensis is a glabrous shrub cultivated in a tropical climate with varying forms and colors. It is widely grown as an ornamental plant in subtropical or tropical climates since it does not tolerate temperatures below 10°C and it is best grown under glass.2 Red flowered is normally used and preferred as medicinal agents.3 It is originated from India and some claim that Hibiscus rosa-sinensis is not a natural herb, however it is a collection of man-made hybrids.4 Hibiscus rosa-sinensis was previously known as ‘rose mallow’ or ‘Queen of Tropical Flower’ and in Malaysia it is called ‘Bunga Raya’. Hibiscus rosa-sinensis is called differently depends on the country itself such as “Bent El-Kunsil” among Arabic, “Zhu jin” among Chinese, “Haibisukasu” in Japanese and “Rosa della Cina” among Italian.5 Hibiscus rosa-sinensis is one of the medicinal plants that is known to have pharmacological activity and it is traditionally used as a treatment in various diseases. This plant has been used in Chinese and Ayurvedic traditional medicine to treat several conditions such as hair loss, head lice and coughing.6
Based on the previous record in the 19th and early 20th century, Hibiscus rosa-sinensis were believed to have different medicinal effects depended on the parts of the plant. The aerial parts such as flowers were used in traditional Malay healing in Malaya and the Dutch Indies in the treatment of a disease called ‘seriawan’ which is symptomatically similar to thrush, diphtheria, and sprue. It was also worked as an expectorant for bronchitis. The flower petals were also used to stimulate thicker hair growth and to prevent premature graying, hair loss and scalp disorders5. Hibiscus rosa-sinensis leaves were revealed to help in relieving headaches or swellings and also act as laxatives, aperients and emollients.4,7 Moreover, the aqueous and alcoholic extract of the hibiscus leaves also benefits as anti-infective, anti-dandruff, anti-graying action which helps in darken the color of hair, stimulate hair growth and prophylactic action against various skin diseases and allergies.4 The roots’ part was extracted to provide relief in fever, sore eyes, venereal disease, sexual transmitted disease and also served as an antidote for poison especially those from white or red flowered hibiscus.7
Other than medicine, Hibiscus rosa-sinensis has a wide application in the food and cosmetics industries. It is used as a flavoring agent in ketchup, sauces, spices, soup and enhances the flavor and aroma of tea mixtures.4 In cosmetics, Hibiscus rosa-sinensis is formulated as an essential oil, which is useful in preserving elasticity, flexibility and prevents aging of the skin if used regularly daily.4
Phytochemical of Hibiscus rosa-sinensis
Hibiscus rosa-sinensis is made up from a variety of compounds that can be classified based on the different parts of the plant extracted such as leaves, stems, roots and flowers. The compounds present in the plant are responsible for different pharmacological activities and defense systems.
Based on the previous study, phytochemical investigation of Hibiscus rosa-sinensis extracts revealed that there was the presence of compounds such as tannins, saponins, glycosides, anthocyanins, flavonoids, and several other compounds in different parts of the plant as shown in Table 1. The leaves part of the plant consists of important constituents such as tannins, saponins, glycosides, flavonoids and terpenoids (Figure 1).8 There was an absence of phlobatannins, terpenoids and cardiac glycosides in the extract of the root and in the stem, there was no presence of terpenoids.8 Past studies also revealed that the most abundant compounds in the leave and stem of Hibiscus rosa-sinensis that in aqueous and methanolic extracts are phenolics, carbohydrates, flavonoids, and tannins that are responsible for several biological activities9. Polyphenols and flavonoids like kaempferol and quercetinhavebeenshowntoexhibitantioxidantactioninthisplant (Figure 1).9 Structure of some major compounds found in Hibiscus rosa sinensis are shown in Figure 2.
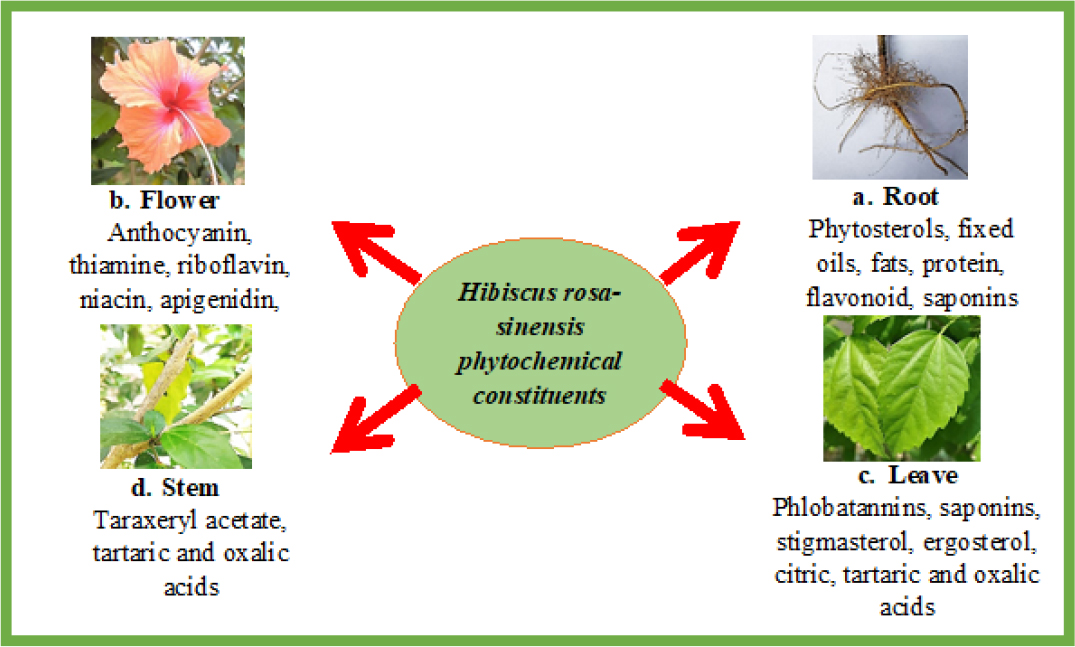
Figure 1:
Hibiscus rosa-sinensis phytochemical constituents.
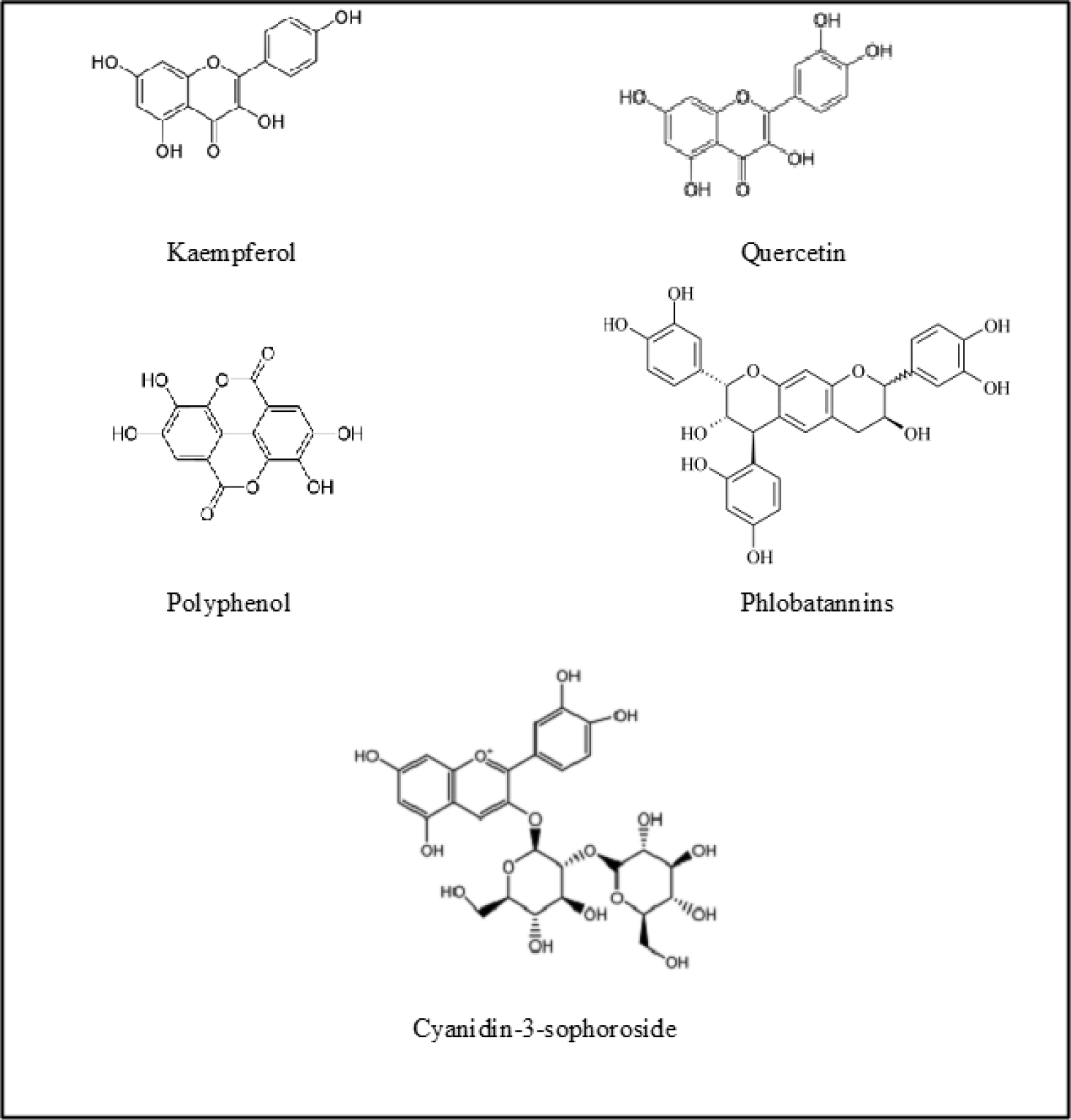
Figure 2:
Some of chemical constituents present in different part of Hibiscus rosa-sinensis.
| Part Used | Chemical Constituents | References |
|---|---|---|
| Leave | Tannins, phlobatannins, saponins, cardiac glycosides, flavonoids, terpenoids. | 8 |
| Leave | Taraxeryl acetate, ?-sitosterol, campesterol, stigmasterol, ergosterol, citric, tartaric and oxalic acids. | 9 |
| Leave | Phenolic, ascorbic acid. | 10 |
| Leave | Reducing sugar, fatty materials, malvalic acid, sterculic. | 11 |
| Leave | Alkaloids, tannins, flavonoids, phenols, saponins, glycosides, terpenoids. | 12 |
| Leave | Alkaloids, reducing sugars, fatty acid, fatty alcohol, glycosides, resins, and hydrocarbons. | 13 |
| Stem | Tannins, phlobatannins, saponins, cardiac glycosides, flavonoids. | 8 |
| Stem | Taraxeryl acetate, ?-sitosterol, campesterol, stigmasterol, ergosterol, citric, tartaric and oxalic acids. | 9 |
| Stem | Malvalic acid, cyclic acid sterculic. | 11 |
| Flower | Phenolic, ascorbic acid, anthocyanin, flavonoids, tannins, carbohydrate, thiamine, niacin, riboflavin. | 10 |
| Flower | Anthocyanin, thiamine, riboflavin, niacin, apigenidin, citric acid, fructose, glucose, fructose, glucose, oxalic acid, pelargonidin, quercetin. | 11 |
| Flower | Ascorbic acid, thiamine, citric acid, glucose, fructose, oxalic acid and riboflavin. | 13 |
| Flower | Alkaloids, carbohydrates, glycosides, phytosterols, fixed oils, phenolic compounds, proteins, free amino acids, gums, mucilage, flavonoids, terpenoids, lignins, saponins. | 14 |
| Flower | Tannins, saponins, alkaloids, steroids, flavonoids. | 15 |
| Root | Tannins, saponins, flavonoids. | 8 |
| Root | Glycosides, tannins, phytosterols, fixed oils, fats, protein, amino acids, flavonoid, saponins, gums, mucilage. | 11 |
The petal of Hibiscus rosa-sinensis contains quercetin-3-di-O-β-D-glucoside, quercetin-3-7-di-O-β-D-glucoside, quercetin- 3-O-β-D-sophorotrioside, kaempferol and kaempferol-3-O-β- xylosylglucoside.11 The quantitative distribution of the chemical constituents available in Hibiscus rosa-sinensis flower may vary according to the color of the petals. For example, in red petals, the major anthocyanins present were cyanidin-3-sophoroside and they were reported to have a higher number of anthocyanins bands which was different from the yellow or yellow-orange petal of Hibiscus rosa-sinensis.11 Furthermore, a study by Shahi Agarwal et al., revealed that there was a slight difference in the quantity of tannins, phenols, and alkaloids in different colors of Hibiscus rosa-sinensis flower. The quantity of phenols is 0.678±0.14% in red, 0.680±0.11% in white, and 0.678±0.16% in yellow flower while the quantity of tannins was 7.5±0.20% in red, 8.9±0.21% in white and 8.5±0.20% in yellow petals. The quantity of alkaloids in red hibiscus is 0.51±0.16%, 0.50±0.18% in white and 0.48±0.16% in yellow Hibiscus rosa-sinensis.17 This shows that the chemical constituents present in different colors of Hibiscus rosa-sinensis are almost similar with a slightly different percentage amount of three major groups of chemical constituents. The number of tannins was slightly higher in white Hibiscus rosa-sinensis when compared with red flower petals.
A previous study by Vastrad et al. highlighted the presence of various chemical constituents in Hibiscus rosa-sinensis leaves detected by using different standard qualitative tests, reagents and extraction solvents such as ethanol, methanol and distilled water. For example, the Dragendorff’s test using the Dragendorff reagent detected the presence of alkaloids by the formation of prominent yellow precipitation in the methanol and distilled water extract of the hibiscus. However, in ethanol extraction, a negative result was obtained which indicating the absence of alkaloids.12 For the detection of flavonoids, a sodium hydroxide test was conducted by using 20% Sodium Hydroxide (NaOH) and Hydrochloric Acid (HCl). The presence of flavonoids turned the methanol, ethanol and distilled water extract of the hibiscus into yellow and addition of HCl changed it to colorless.12 Tannins were detected for ethanol, methanol and distilled water extracts of hibiscus using the gelatin and lead acetate tests. However, it was not detected in the distilled water extract using the ferric.12 Other than the major chemical constituents like flavonoids and tannins, previous studies also highlighted the presence of mucilage in the roots and leaves of Hibiscus rosa-sinensis which also contributed to several pharmacological actions such as antiulcer activity.16 A study by Kumari et al. revealed that there was a presence of mucilage in the alcohol and aqueous extract of Hibiscus rosa-sinensis while the opposite results were obtained in the petroleum ether extract of this species. The presence of mucilage was identified in the Hibiscus rosa-sinensis extract by performing Molisch’s and Ruthenium red tests.13
Pharmacological activities of Hibiscus rosa-sinensis
Hibiscus rosa-sinensis is a natural source that contains various compounds exhibiting many pharmacological activities which can contribute to the development of novel therapeutics formulations. Researchers have conducted studies on the pharmacological activity of Hibiscus rosa-sinensis leaves, barks, roots and flower which have been used for the treatment of various diseases such as aphrodisiac, hypertension, wound healing, diabetes mellitus and cancer.18 They have shown that different parts of the plant may have various properties like antioxidant, anti-inflammatory, anti-microbial, anti-ulceration and many more (Figure 3).19 This plant also has been used traditionally as an oral contraceptive and to control dysfunctional uterine bleeding and has been used in many herbal mixes and drinks.18,19 This makes Hibiscus rosa-sinensis a target for pharmacological studies to validate and obtain scientific evidence for the pharmacological activities claim and its efficacy.
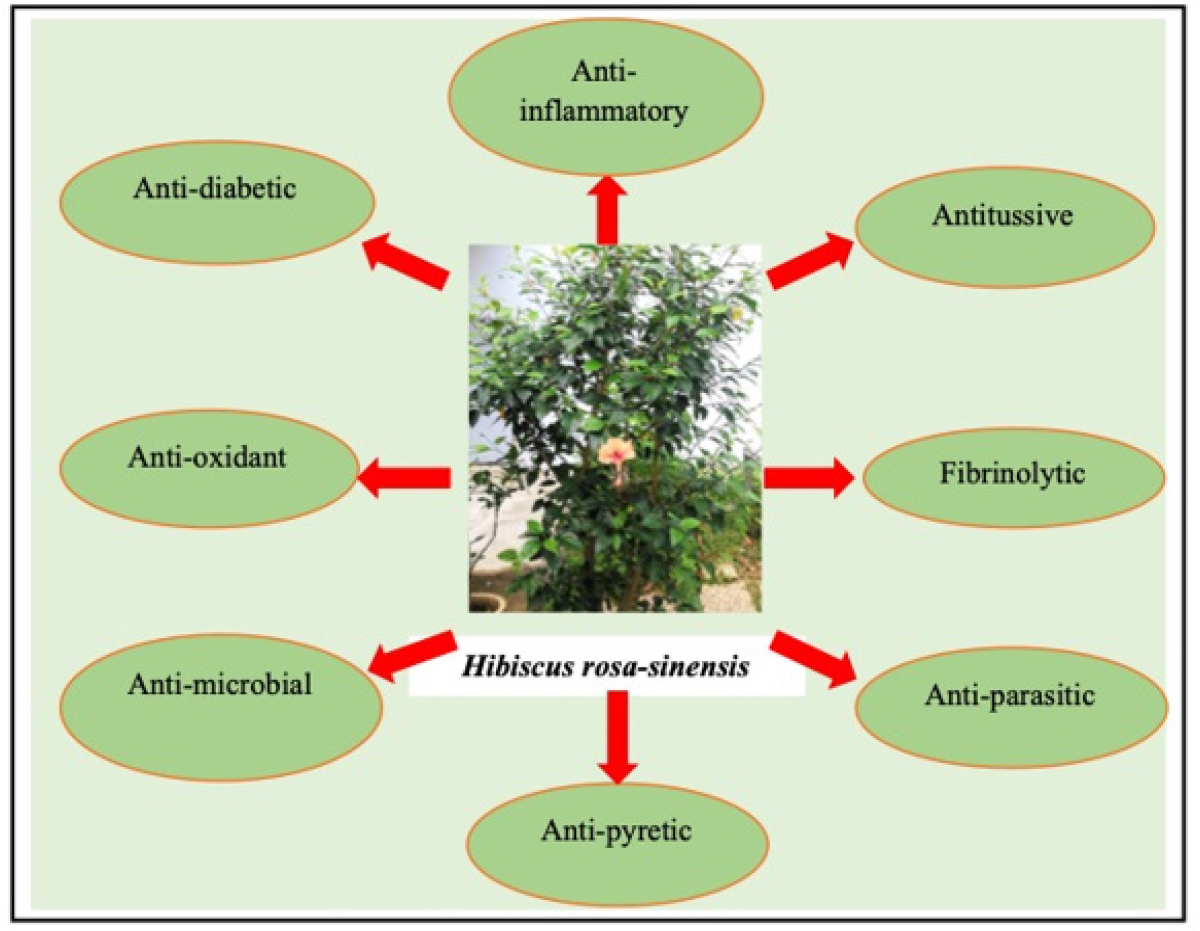
Figure 3:
Hibiscus rosa-sinensis pharmacological activities.
Anti-inflammatory activity
Several compounds present in Hibiscus rosa-sinensis such as flavonoids, steroids and saponins possess anti-inflammatory activities. A study by Raduan et al. highlighted that these compounds were present in the ethanol extracts of flower and leaf of two different Hibiscus rosa-sinensis: red Hibiscus rosa-sinensis L. and white Hibiscus rosa-sinensis var alba.20 Flavonoids, saponins and steroids carried out their anti-inflammatory action by different mechanisms. Flavonoids and saponins exert their anti-inflammatory activity by inhibiting the production of Reactive Oxygen Species (ROS) and suppress several inflammatory mediators such as Nuclear Factor-kappa B (NF-kB) and Signal Transducers and Activators of Transcription (STAT) in the signaling pathway.21 According to Raduan et al., carrageenan induced paw edema in rat anti-inflammatory test was conducted for Hibiscus rosa-sinensis L. (red) and Hibiscus rosa-sinensis var alba (white). Flower and the leaf of Hibiscus rosa-sinensis L. (red) at dose 50 mg and 100 mg per kg of rat body weight showed significant effects on the inhibition of paw edema induced by carrageenan while Hibiscus rosa-sinensis var alba (white) exhibited significant inhibition of the paw edema in various ranges of doses which were 5 mg, 50 mg and 100 mg per kg.20 The inhibition activity indicates that both of the hibiscuses could reverse acute inflammation of paw edema induced by carrageenan in a different range of doses. In a study conducted by Singh et al., protein denaturation assay was used to observe the anti-inflammatory action of this plant. Bovine Serum Albumin (BSA) was used as control and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) such as diclofenac sodium and aspirin were used as standard drugs that inhibit the protein denaturation process. Hibiscus rosa-sinensis inhibit 56% of protein denaturation while aspirin and diclofenac sodium inhibit protein denaturation 68% and 88% respectively. The percentage might be not as high as standard anti-inflammatory drugs, but Hibiscus rosa-sinensis inhibited more than half percent protein denaturation process.
Anti-inflammatory effect of methanolic extract of Hibiscus rosa-sinensis L. leaves was reported by Tomar et al. A carrageenan and dextran induced rat paw edema test was conducted to observe the anti-inflammatory action of this plant.23 In this test, Indomethacin, a Non-Steroidal Anti-Inflammatory Drug (NSAIDs) was used as standard which showed significant anti-inflammatory activity.23 According to Tomar et al., there are three different phases of the inflammation process which includes the release of mediators histamine and serotonin for the first phase, followed by the second phase which involved the kinin released and the third phase referred to the released of prostaglandin.23 500mg per kg of methanolic extract of Hibiscus rosa-sinensis L. showed its effect on the third phase of the inflammation process and there was no significant anti-inflammatory activity was observed in the first two phases.23
In a study by Birari et al., the anti-inflammatory activity of ethanolic extract of Hibiscus rosa-sinensis L. flower was evaluated by using carrageenan induces paw edema, cotton pellet induces granuloma and xylene induces mice ear edema.24 In this experiment, indomethacin was used as the standard drug that exhibited a significant anti-inflammatory effect. Inflammation induced by carrageenan was significantly reduced after 3 hr by 250 mg and 500 mg per kg extract doses of hibiscus resulting in blocking of prostaglandin synthesis and release, thus inhibited the paw edema. Significant inhibition was also exhibited by 250 mg and 500 mg per kg of ethanolic extracts of the hibiscus in xylene induce ear edema test as the mean edema weight was reduced for 8.66±0.4944 mg and 7.33±0.6146mg respectively from 11.16±0.60 mg of control. In the cotton pellet induced granuloma formation test, the weight of granuloma was greatly influenced by fluids absorbed into the pellet and dry weight correlate with the amount of granulomatous tissue formed.24 250 mg and 500 mg per kg ethanolic extract of the plant produced a significant anti-inflammatory by the inhibition of the mean granuloma weight 22.67±0.6667 mg and 19.83±0.6009 mg respectively.24 The summary of anti-inflammatory activity of Hibiscus rosa-sinensis active constituents is shown in Table 2.
| Part Used | Extract | Active constituents | Anti-inflammatory test | References |
|---|---|---|---|---|
| Flower | Ethanol | Flavonoids, steroids, saponins | Carrageenan induced Paw Edema in Rats. | 20 |
| Leaf | Ethanol | Flavonoids, steroids, saponins | Carrageenan induced Paw Edema in Rats. | 20 |
| Flower | Tea extraction | Phenolic, flavonoid | Inhibition protein denaturation process | 22 |
| Leaf | Methanol | Carbohydrates, flavonoids, and glycosides. | Carrageenan and dextran induced rat paw edema. | 23 |
| Flower | Ethanol | Flavonoids, alkaloids, steroids, carbohydrates, glycosides | Carrageenan induces paw edema; cotton pellet induces granuloma and xylene induce mice ear edema. | 24 |
Antioxidant activity
An antioxidant is a substance that inhibits or slows the progression of cell damage caused by free radicals or unstable molecules that are present in the body.25 Antioxidants exert their effect to stop the cell damage by preventing the formation of radicals, exhibit free radical scavenging action or increase the decomposition of free radicals.26 Free radicals are associated with many chronic diseases such as cancer, cardiovascular diseases and cataract.26 An endogenous antioxidant is produced by the body while exogenous antioxidant comes from food, dietary supplements, or plant-based antioxidants.
In a study conducted by Garg et al., several tests were used to evaluate the antioxidant activity of Hibiscus rosa-sinensis: 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging assay, Nitric Oxide (NO) radical scavenging assay and Hydrogen Peroxide (H2O2) radical scavenging assay.9 Methanolic and aqueous extract of Hibiscus rosa-sinensis leaves and stem were used in these evaluations.9 In DPPH radical scavenging assay, aqueous leaf and stem extract of Hibiscus rosa-sinensis exhibited superior scavenging activity compared to the methanolic extract of the leaf and stem which is said to be associated with the ability to donate hydrogen molecule.9 Compared to the Hibiscus rosa-sinensis stem, the leaves exhibited more antioxidant activities in DPPH scavenging assay in both methanolic and aqueous extracts.9 Nitric oxide can be endogenously produced and when it’s produced in excess amount concurrently with Reactive Oxygen Species (ROS), it can cause several harmful effects such as neurotoxicity and apoptotic cell-death induced in different types of neuronal cells.27 Both methanolic and aqueous extract of the plant exhibit almost the same level of scavenging activity when evaluated by the use of NO radical scavenging activity assay, however, the hibiscus leaves in aqueous extract exhibit the most significant effect compared to other parts of the plant used in this test.9 H2O2 was commonly used procedure to induce oxidative stress in a cellular model.28 Both the leave and stem of Hibiscus rosa-sinensis in methanolic and aqueous extracts exhibit a significant result in inhibiting the oxidative stress damage and the activities were due to the presence of β-sitosterol, stigmasterol, taraxeryl acetate and three cyclopropane compounds in these extract that contributes to its antioxidant activity.9
A previous study by Ghaffar et al. highlighted the potency of natural antioxidant activity of Hibiscus rosa-sinensis leaves in 70% ethanol/water extraction. The presence of bioactive compounds such as carbohydrates, glycosides, steroids, triterpenes, flavonoids and tannins were responsible for the antioxidant activities of this plant.29 In Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) scavenging activity, Hibiscus rosa-sinensis showed the capability of performing H2O2 scavenging activity depending on the concentration of the extract used. At concentration 500 µg/mL, the extract significantly scavenged the H2O2 up to 48.5%. Other than H2O2, Hibiscus rosa-sinensis leaves extract also inhibited superoxide radical in a concentration dependent manner. At the concentration of 500 µg/mL, the extract was capable of scavenging the superoxide radical up to 60.4% and it was believed to be dued to the antioxidant power gained by the presence of flavonoid in the extract composition.29
Based on the study by Ghaffar et al., Hibiscus rosa-sinensis leaf extract inhibited the lipid and protein damage induced by oxidative stress in concentration depending manner.30 Lipid peroxidation is a process in which the free radicals attack lipids containing carbon-carbon double-bonds especially in the polyunsaturated fatty acids.31 Protein oxidation is a protein covalent modification that is induced by ROS or other indirect reactions with secondary by-products of oxidative stress.30 The presence of phenolics and flavonoids content in this plant were attributed to the inhibition of lipid peroxidation activity.29,32 At concentration the concentration of 500 µg/mL, 31% of lipid and protein oxidative damage was inhibited.29
Mak et al. highlighted the antioxidant activity of Hibiscus rosa-sinensis flower in aqueous and ethanolic extracts using Ferric Reducing Antioxidant Power (FRAP) assay.33 In FRAP assay, antioxidant activity was measured based on the capability of the Hibiscus rosa-sinensis extract to reduce Ferric tripyridyltriazine (Fe (III)-TPTZ) complexes to Ferrous tripyridyltriazine (Fe (II)-TPTZ). The higher reading of FRAP value from the result indicated the greater the antioxidant activity of the hibiscus extract. From this study, Hibiscus rosa-sinensis flower in aqueous extract exhibited greater reducing power compared to ethanolic extract.33
A previous study by Prasad et al. measured the antioxidant activity by calculating the percentage of DPPH radical inhibition in DPPH scavenging assay.34 The methanolic extract of the hibiscus petal showed more significant inhibition of DPPH free radical compared to the ethanolic extract of the plant. This may be due to the presence of different phytochemicals in both, methanol and ethanol extracts.34 Furthermore, methanol was proved as the best solvent to extract the maximum phytochemicals from the leaves of Hibiscus rosa-sinensis as compared to ethanol.34
Rengarajan et al. highlighted that the phenolic and flavonoid contents such as Hibiscetin-3- glucoside in the Hibiscus rosa-sinensis petal exhibited greater antioxidant activity compared to the standard antioxidant, ascorbic acid.35 In lipid peroxidation assay, the isolated compounds present in hibiscus showed an efficient lipid peroxidation activity depending on the concentration of the extract used. At the concentration of 20 µg/mL, Hibiscus rosa-sinensis extract exhibited greater antioxidant action compared to vitamin C.35 The summary of antioxidant activity of Hibiscus rosa-sinensis active constituents is shown in Table 3.
| Part Used | Extract | Active constituents | Antioxidant test | References |
|---|---|---|---|---|
| Leave | Methanolic | Polyphenols, carbohydrates, flavonoids, tannins, β-sitosterol, stigmasterol, taraxeryl acetate and cyclopropane. | DPPH, NO, H2O2 radical scavenging activity | 9 |
| Stem | Aqueous | Polyphenols, carbohydrates, flavonoids, tannins, β-sitosterol, stigmasterol, taraxeryl acetate and cyclopropane. | DPPH, NO, H2O2 radical scavenging activity | 9 |
| Leave | 70% ethanol/water | Carbohydrates, glycosides, steroids, triterpenes, flavonoids and tannins. | ROS and RNS scavenging activity, lipid peroxidation (LPO) and protein oxidation (PO) assay | 29 |
| Flower | Aqueous and ethanolic | Phenolics, flavonoid and proanthocyanidins. | FRAP assay | 33 |
| Flower | Methanolic and ethanolic | Alkaloids, tannins, carbohydrates, terpenoids, flavonoids, cardiac glycosides and quinones. | DPPH scavenging activity | 34 |
| Flower | Aqueous | Phenolic and flavonoid (Hibiscetin-3- glucoside). | Lipid peroxidation (LPO) assay | 35 |
Anti-microbial activity
Several studies have revealed anti-microbial activities of Hibiscus rosa-sinensis. A study by Ruban et al. revealed that the methanolic and ethanolic extracts of Hibiscus rosa-sinensis flower have effects against the human pathogens such as Staphylococcus aureus, Streptococcus sp. Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Salmonella sp.36 Active constituents in the extracts such as flavonoids, tannins, alkaloids and triterpenoids may contribute to the antibacterial activities.36 However, no inhibition was observed for the methanolic flower extract of Hibiscus rosa-sinensis in both disc diffusion and agar diffusion methods.36
Patel et al. highlighted the activity of methanolic and ethyl acetate extracts of Hibiscus rosa-sinensis leaves exhibiting significant anti-microbial activity against pathogenic bacteria.37 The hibiscus extracts were tested against different gram-positive bacteria, such as Staphylococcus aureus, Bacillus subtilis, Streptomyces alboniger, Micrococcus luteus and Staphylococcus epidermis and gram-negative bacteria, Pseudomonas aeruginosa and Bordetella bronchiseptica.37 Ethyl acetate extract of the Hibiscus rosa-sinensis leaves exhibited the greatest antibacterial activity as the zone of inhibition measured was the highest compared to the zone of inhibition measured in methanolic extract of the hibiscus for the gram-positive bacteria; Staphylococcus aureus, Bacillus subtilis and Streptomyces alboniger. Bioactive metabolites such as phenols, tannins, alkaloids, steroids, glycosides and flavonoids are postulated to be responsible for the effective inhibitory potency against the tested pathogenic bacterial.37
A study by Ramesh et al. revealed the anti-microbial activity of ethanolic (hot and cold) and aqueous (hot and cold) Hibiscus rosa-sinensis leaves extracts by using the agar well diffusion method. Pathogenic microorganisms such as Streptococcus mutans and Lactobacillus acidophillus were used in the test.38 For Streptococcus mutans, hot and cold ethanolic extracts and hot aqueous extract of the hibiscus plant exhibited anti-microbial activity at minimum concentration 50 μg/mL and 75 μg/mL, respectively, while there was no growth inhibition observed for cold aqueous extract of Hibiscus rosa-sinensis. This might be due to the nature of the chemical composition of the plant leaves.38 Hot aqueous and cold ethanolic extracts of hibiscus exhibited anti-microbial activity at a minimum concentration 50 μg/mL, while for cold aqueous and hot ethanolic extracts of hibiscus plant required 25μg/mL as the minimum concentration for Lactobacillus acidophilus growth inhibition.38 Preliminary chemical constituents analysis revealed that there was presence of secondary metabolites in the Hibiscus rosa-sinensis leaves which responsible for anti-microbial activity such as sterols, cardiac glycosides and tannins.38
Vijayakumar et al. conducted a study that highlighted the anti-microbial effects of methanolic and chloroform Hibiscus rosa-sinensis flower extract by using pathogenic bacteria and fungi such as Neisseria gonorrhoeae and Candida albicans.39 Preliminary phytochemical analysis was carried out by using Gas-Chromatography Mass Spectrometry (GC-MS). The results of the analysis revealed several active chemical constituents which were responsible for the growth inhibition of microbes such as 1, 2-benzenedicarboxylic acid, octadecanoic acid, 3- N-hexylthiolane, 1-iodoundecane, 2, 2, 4 – trimethyl 3-pentanone, 2-propenamide and amyl nitrite.39 Methanolic extract of the hibiscus exhibited a greater inhibition effect on the gram-negative bacteria Neisseria gonorrhoeae compared to the chloroform extract of the plant. The growth of Candida albican fungus was also significantly inhibited by the methanolic flower extract of Hibiscus rosa-sinensis.39
A study conducted by Al-Alak et al. revealed the anti-bacterial activity of hot and cold aqueous extraction of Hibiscus rosa- sinensis flower on five different pathogenic bacterial, Pseudomonas aeruginosa, Serratia sp. Micrococcus sp. Enterobacter sp. and Salmonella sp.40 The test was conducted by using agar well diffusion method and antibiotic amoxicillin was used as positive control.40 It is clear from the findings of the investigation that Hibiscus rosa-sinensis flower consists of chemical constituents that are responsible for the anti-bacterial activity that can be used for pathological treatment caused by the isolated bacteria. The hot aqueous extract of the plant showed more effective effects with 10 mm to 32 mm of inhibition zones compared to cold watery extract with 10 mm to 27 mm inhibition zones recorded.40 The summary of anti-microbial activity of Hibiscus rosa-sinensis active constituents is shown in Table 4.
| Part Used | Extract | Active constituents | Anti-microbial test | References |
|---|---|---|---|---|
| Flower | Methanolic and ethanol | Flavonoids, tannins, alkaloids, and triterpenoids. | Disc diffusion and Agar well diffusion | 36 |
| Leaves | Methanolic and Ethyl acetate | Phenols, tannins, alkaloids, steroid, glycoside, and flavonoids. | Agar Ditch diffusion | 37 |
| Leaves | Ethanolic and Aqueous | Sterols, cardiac glycosides, and tannins. | Agar well diffusion | 38 |
| Flower | Methanolic and chloroform | 1, 2-Benzenedicarboxylic acid, Octadecanoic acid, 3-N-Hexylthiolane, 1-Iodoundecane, 2, 2, 4 – Trimethyl 3-pentanone, 2-Propenamide and Amyl nitrite. | Agar well diffusion | 39 |
| Flower | Hot and cold aqueous extract | – | Agar well diffusion | 40 |
Antidiabetic activity
Diabetes mellitus is a disorder in which the body is unable to produce adequate amounts of insulin or cannot respond normally toward insulin which consequently elevates sugar concentration in the blood and produces a condition called hyperglycemic. In Malaysia, diabetes mellitus is one of the major concerns in public health and type 2 diabetes affects 20.8% or 2.8 million adults above 30 years old.41 Herbal medicines have been the source for antidiabetic medicine due to the presence of active phytoconstituents that mimic insulin activity and exhibit hypoglycemic properties.42
A study by Moqbel et al. revealed a significant anti-diabetic effect exhibited by the chloroform fraction of the ethanolic extract of Hibiscus rosa-sinensis leaves on the Non-Obese Diabetic (NOD) mouse which spontaneously developed type 1 diabetes or Insulin-Dependent Diabetes Mellitus (IDDM).43 Before the administration of the hibiscus extract, the fasting blood glucose levels of the mice were 290 mg/dl and 278.6 mg/dl. After the mice were treated with 100 mg/kg and 200 mg/kg per body weight of chloroform fraction of Hibiscus rosa-sinensis, the blood glucose level significantly decreased to 94.5 mg/dl and 90.2 mg/dl which fell within the normal range.43 Moqbel et al. further the investigation by using preserved blood from the mice for biochemical analysis. The reading of plasma insulin level from the preserved blood increased with the administration of the chloroform fraction of the Hibiscus rosa-sinensis and the insulin secretion level on par with the mice intraperitoneally injected with insulin.43
A study by Pillai et al. highlighted the antidiabetic effect of ethyl acetate fraction of Hibiscus rosa-sinensis flower.44 The fraction at dose 25 mg/kg was administered to a streptozotocin-induced diabetic rat and the effect was compared with metformin, which is an antidiabetic agent.44 The elevated sugar blood concentration and glycated hemoglobin of the diabetic rat were significantly reduced from 398.56 ± 35.78 mg/dL and 12.89 ± 1.89% to 156.89 ± 14.45 mg/dL and 6.12 ± 0.49%, respectively upon the administration of Hibiscus rosa-sinensis extract. The antidiabetic effect exhibited by the plant was due to the presence of flavonoid compounds.44
Venkatesh et al. revealed the hypoglycemic effect of ethanolic extract of Hibiscus rosa-sinensis flower on Wistar albino rats.45 The rats were injected with 100 mg/kg of alloxan intraperitoneally in order to a induce diabetic state with a blood glucose level of more than 150 mg/dl.45 Alloxan is a diabetogenic agent which has almost the same action as streptozotocin which causes the partial destruction of the pancreatic β-cell, thus affecting the quality and quantity of insulin produced by this cell.46 The effects of the ethanolic extract of the plant was compared with an antidiabetic available in the market, glibenclamide, which is used in the treatment of type 2 diabetes. The rats were administered with two different concentrations the extract, 250 mg/kg and 500 mg/kg respectively. For the first 1 hr upon administration, only rats that were administered with 500 mg/kg of hibiscus extract exhibited a significant reduction in the blood glucose, while the rat treated with 250 mg/kg of the plant extract showed its effect after 3 hr of administration. Compared to the rat administered with glibenclamide, 500 mg/kg Hibiscus rosa-sinensis ethanolic extract exhibited a better and significant reduction of plasma glucose level.45
Mandade et al. revealed the antidiabetic activity of the aqueous-ethanolic aerial part extract of Hibiscus rosa-sinensis on the streptozotocin-induced diabetes mellitus Wistar albino rats.47 A preliminary A phytochemical study was conducted on the plant extract and several active constituents such as sterols, glycosides, carbohydrates, tannins and flavonoids were present in the plant.47 The hibiscus aqueous-ethanolic extract at dose 500mg/kg was administered intraperitoneally to the rats and the results were compared to the rats injected with insulin 6 units/kg of the body weight. Administration of the hibiscus extract significantly reduced the blood glucose level and increased the amount of insulin produced in the rats. The possible hypoglycemic mechanism of the extract may be due to the potency of the pancreatic β-cell of islets to secrete insulin in response to the enhanced glucose transportation to the peripheral tissue.47,48
The antidiabetic effect of ethanolic Hibiscus rosa-sinensis flower extract was further studied by Sankaran et al. against the streptozotocin-induced diabetic rats in a dose dependent manner.49 A phytochemical investigation was conducted and the presence of active constituents such as flavonoids and phenolic compounds were detected. The flower plant extract at doses 125 mg/kg, 250mg/kg and 500 mg/kg were administered respectively to the rats, and it was given orally every day for 4 weeks.49 The doses 125 mg/kg, 250 mg/kg and 500 mg/kg significantly attenuated the streptozotocin-induced elevated blood glucose concentration of the rats. The rat administered with 250 mg/kg of the hibiscus plant extract showed a significant effect in the reduction of plasma glucose level compared to the rats administered with 125 mg/kg and 250 mg/kg.49
The hyperglycemic effect of ethanolic extract of Hibiscus rosa-sinensis flower was studied by Sachdewa et al. in streptozotocin-induced diabetic rats and the result was compared to rats injected with anti-diabetic medication, glibenclamide.50 After 7 and 21 days, there was significant reduction in blood glucose level by 21.30 ± 6.33% and 30.94 ± 4.45% and serum
insulin level were elevated by 10.39 ± 4.34% and 14.58 ± 5.58% in rats administered with ethanolic extract of Hibiscus rosa-sinensis flower. It is evident from the data that both the flower extract and glibenclamide have a comparable result on glycemic suppression. However, changes in insulin levels with the administration of plant ethanol extract are insufficient to account for the obvious improvement in the glucose profile.50 The summary of antidiabetic activity of Hibiscus rosa-sinensis active constituents is shown in Table 5.
| Part Used | Extract | Active constituents | Anti-diabetic test | References |
|---|---|---|---|---|
| Leaves | Chloroform fraction of ethanolic extract. | Alkaloids, carbohydrates, tannins and flavonoids. | Test on the NOD type 1 diabetes mice insulin and blood sugar level. | 43 |
| Flower | Ethyl acetate fraction | Flavonoid | Test on the streptozotocin-induced diabetic rat. | 5 |
| Flower | Ethanolic | Alkaloids, carbohydrates, tannins, and flavonoids. | Test on the Wistar albino rats alloxan-induced diabetic. | 45 |
| Aerial parts | Aqueous-ethanolic | Sterols, glycosides, carbohydrates, tannins, and flavonoid. | Test on the Wistar albino rats streptozotocin -induced diabetic. | 47 |
| Flower | Ethanolic | Flavonoid and phenolic compound | Test on the streptozotocin-induced diabetic rats. | 49 |
| Flower | Ethanolic | Flavonoid | Test on the streptozotocin-induced diabetic rats. | 50 |
CONCLUSION
According to information obtained from previous studies, Hibiscus rosa-sinensis exhibited several pharmacological activities such as anti-inflammatory, antioxidant, anti-microbial and antidiabetic depending on the extracts used and the part of the plant involved in the study. Each part of the plant consists of a wide range of chemical constituents that have been determined in phytochemical analysis and the chemical constituents are responsible for the pharmacological action shown in the studies. The extracts of Hibiscus rosa-sinensis flowers and leaves are available in the market as traditional treatments for ailments however more studies are required to develop this plant into a formulation for therapeutic purposes.
References
- Yuan H, Ma Q, Ye L, Piao G.. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559 [PubMed] | [CrossRef] | [Google Scholar]
- [PubMed] | [CrossRef] | [Google Scholar]
- Jadhav VM, Thorat RM, Kadam VJ, Sathe NS. Traditional medicinal uses of . J Pharm Res. 2009;2(28):1220-2. [PubMed] | [CrossRef] | [Google Scholar]
- Khan IM, Rahman R, Mushtaq A, Rezgui M.. Array. Int J Chem Biochem Sci. 2019;12:147-51. [PubMed] | [CrossRef] | [Google Scholar]
- Al-Snafi AE. Chemical constituents, pharmacological effects and therapeutic importance of : a review virtual learning view project medicinal plants with CNS activity view project chemical constituents, pharmacological effects and therapeutic. IOSR J Pharm. 2018;8 [PubMed] | [CrossRef] | [Google Scholar]
- Missoum A.. An update review on phytochemistry and medicinal uses. J Ayu Her Med. 2018;4(3):135-46. [CrossRef] | [Google Scholar]
- . [Jun 13 2021];Infopedia. [CrossRef] | [Google Scholar]
- Patel R, Patel A, Desai S, Nagee A.. Study of secondary metabolites and antioxidant properties of leaves, stem and root among cultivars. Asian J Exp Biol Sci.. 2012;3(4):719-25. [CrossRef] | [Google Scholar]
- Garg D, Shaikh A, Muley A, Marar T.. Array. Free Radic Antioxid. 2012;2(3):41-6. [CrossRef] | [Google Scholar]
- Al-Snafi AE. Chemical constituents, pharmacological effects and therapeutic importance of : a review plants with antiparasitic effect view project medicinal plants with anticancer effects view project chemical constituents, pharmacological effects. IOSR J Pharm. 2018;8 [CrossRef] | [Google Scholar]
- Goutam M, Prabir DK, Pranjal D, Akhsay S, Kundu S, Pintu S, et al. Authentication and photochemical screening of . Int J Res Anal Rev.. 2018;5(4):112-8. [CrossRef] | [Google Scholar]
- Vastrad JV, Byadgi SA. Phytochemical screening and antibacterial activity of leaf extracts. Int J Curr Microbiol Appl Sci. 2018;7(3):3329-37. [CrossRef] | [Google Scholar]
- Sivaraman CM, Saju F.. Medicinal value of : a review. Int J Pharm Chem. 2021;2(1):1-11. [CrossRef] | [Google Scholar]
- Bhaskar A, Nithya V.. Evaluation of the wound-healing activity of L. (Malvaceae) in Wistar albino rats. Indian J Pharmacol. 2012;44(6):694-8. [PubMed] | [CrossRef] | [Google Scholar]
- Nithya AB. Phytochemical screening and antioxidant activities of the ethanolic extract of L. 2011;2(5):653-61. [PubMed] | [CrossRef] | [Google Scholar]
- Anbu Jeba Sunilson J, Anandarajagopal K, Vignesh M, Parkavi J, Anita Gnana Kumari A, Palavesam A., et al. Preliminary phytochemical and antiulcer studies of Linn. root extracts. Int J Green Pharm. 2010;4(1):41-3. [CrossRef] | [Google Scholar]
- Agarwal S, Prakash R, Alka Srivastava DRM, Mathur. Quantitative and qualitative analysis of phytochemicals, present in flower extract of . 2016;2277:78-9. [CrossRef] | [Google Scholar]
- Kate IE, Lucky OO. The effects of aqueous extracts of the leaves of Linn. on renal function in hypertensive rats. Afr J Biochem Res. 2015 [CrossRef] | [Google Scholar]
- Khristi V, Patel V.. Therapeutic Potential of : a review therapeutic potential of : a review. Chemistry. 2017 [CrossRef] | [Google Scholar]
- Raduan SZ, Hakim MN. Anti-inflammatory effects of L. and var. alba ethanol extracts. Int J Pharm Pharm Sci. 2013;5(4):754-62. [CrossRef] | [Google Scholar]
- Ginwala R, Bhavsar R, Chigbu DI, Jain P, Khan ZK. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants (Basel). 2019;8(2):1-30. [PubMed] | [CrossRef] | [Google Scholar]
- Singh KG, Sonia S, Konsoor N.. Array. Int J Pharm Sci Res. 2018;9(8):3543 [PubMed] | [CrossRef] | [Google Scholar]
- Tomar V, Kannojia P, Jain KN, Dubey KS. Antinociceptive, and anti-inflammatory activity of leaves of . Medicine. 2010;1(1):201-5. [PubMed] | [CrossRef] | [Google Scholar]
- Birari RB, Jalapure SS, Changrani SR, Shid SL, Tote MV, Habade BM, et al. Anti-inflammatory, analgesic and antipyretic effect of Linn. Flower. Pharmacologyonline. 2009;747:737-47. [PubMed] | [CrossRef] | [Google Scholar]
- [May 4 2022];Antioxidants: health benefits and nutritional information [internet]. [PubMed] | [CrossRef] | [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118-26. [PubMed] | [CrossRef] | [Google Scholar]
- Wei T, Chen C, Hou J, Xin W, Mori A.. Nitric oxide induces oxidative stress and apoptosis in neuronal cells. Biochim Biophys Acta. 2000;1498(1):72-9. [PubMed] | [CrossRef] | [Google Scholar]
- Ransy C, Vaz C, Lombès A, Bouillaud F. Use of H2O2 to cause oxidative stress, the catalase issue. Int J Mol Sci.. 2020;21(23):9149 [PubMed] | [CrossRef] | [Google Scholar]
- Ghaffar FRA, El-elaimy IA. Array. J Appl Pharm Sci. 2012;2(2):51-8. [PubMed] | [CrossRef] | [Google Scholar]
- Zhang W, Xiao S, Ahn DU. Protein oxidation: basic principles and implications for meat quality. Crit Rev Food Sci Nutr. 2013;53(11):1191-201. [PubMed] | [CrossRef] | [Google Scholar]
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438 [PubMed] | [CrossRef] | [Google Scholar]
- Jiang P, Burczynski F, Campbell C, Pierce G, Austria JA, Briggs CJ, Jiang P, et al. Rutin and flavonoid contents in three buckwheat species and and their protective effects against lipid peroxidation. Food Res Int. 2007;40(3):356-64. [CrossRef] | [Google Scholar]
- Mak YW, Chuah LO, Ahmad R, Bhat R.. Antioxidant and antibacterial activities of hibiscus ( L.) and Cassia ( L.) flower extracts. J King Saud Univ Sci. 2013;25(4):275-82. [CrossRef] | [Google Scholar]
- Prasad MP. Array. Int J Pure Appl Biosci. 2014;2(3):83-8. [CrossRef] | [Google Scholar]
- Rengarajan S, Melanathuru V, Govindasamy C, Chinnadurai V, Elsadek MF. Antioxidant activity of flavonoid compounds isolated from the petals of . J King Saud Univ Sci. 2020;32(3):2236-42. [CrossRef] | [Google Scholar]
- Ruban P, Gajalakshmi K.. Array. Asian Pac J Trop Biomed. 2012;2(5):399-403. [PubMed] | [CrossRef] | [Google Scholar]
- Patel R, Patel A, Vaghasiya D, Nagee A.. Antimicrobial evaluation of plant extracts against some pathogenic bacteria. Bull Environ Sci Res. 2012;1(1-2):14-7. [PubMed] | [CrossRef] | [Google Scholar]
- Nagarajappa R, Batra M, Sharda AJ, Asawa K, Sanadhya S, Daryani H, et al. Antimicrobial effect of L. and L. extracts against pathogenic oral microorganisms-an comparative study. Oral Health Prev Dent. 2015;13(4):341-8. [PubMed] | [CrossRef] | [Google Scholar]
- Vijayakumar S, Morvin Yabesh JE, Arulmozhi P, Praseetha PK. Identification and isolation of antimicrobial compounds from the flower extract of L: and approaches. Microb Pathog. 2018;123:527-35. [PubMed] | [CrossRef] | [Google Scholar]
- Shaymaa Khudhr A, Rasha Mohamed Sajet A, Basam Basim M, Abd-Alkhalik N.. Antibacterial activity of extract and synergistic effect with amoxicillin against some human pathogens. Am J Phytomed Clin Ther. 2015;3(1):20-7. [PubMed] | [CrossRef] | [Google Scholar]
- Hussein Z, Taher SW, Gilcharan Singh HK, Chee Siew Swee W.. Diabetes care in Malaysia: problems, new models, and solutions. Ann Glob Health.. 2015;81(6):851-62. [PubMed] | [CrossRef] | [Google Scholar]
- Patel DK, Prasad SK, Kumar R, Hemalatha Sz. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2(4):320-30. [PubMed] | [CrossRef] | [Google Scholar]
- Moqbel FS, Naik PR, Najma HM, Selvaraj S.. Antidiabetic properties of L. leaf extract fractions on Nonobese Diabetic (NOD) mouse. Indian J Exp Biol. 2011;49(1):24-9. [PubMed] | [Google Scholar]
- Pillai SS, Mini S.. Array. Plant Foods Hum Nutr. 2016;71(1):42-8. [PubMed] | [CrossRef] | [Google Scholar]
- Venkatesh S, Thilagavathi J, Shyam Sundar D. Antidiabetic activity of flowers of . Fitoterapia. 2008;79(2):79-81. [PubMed] | [CrossRef] | [Google Scholar]
- Ighodaro OM, Adeosun AM, Akinloye OA. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina (Kaunas). 2017;53(6):365-74. [PubMed] | [CrossRef] | [Google Scholar]
- Mandade R, Sreenivas SA. Antidiabetic effects of aqueous ethanolic extract of L. on streptozotocin-induced diabetic rats and the possible morphologic changes in the liver and kidney. Int J Pharmacol. 2011;7(3):363-9. [CrossRef] | [Google Scholar]
- Saravanan G, Pari L.. Hypoglycaemic and antihyperglycaemic effect of bark in streptozotocin-induced diabetic rats. J Pharmacol Toxicol. 2008;3(1):1-10. [CrossRef] | [Google Scholar]
- Sankaran M, Vadivel A.. Antioxidant and antidiabetic effect of flower extract on streptozotocin induced experimental rats-a dose response study. Not Sci Biol. 2011;3(4):13-21. [CrossRef] | [Google Scholar]
- Sachdewa A, Khemani LD. Effect of Linn. ethanol flower extract on blood glucose and lipid profile in streptozotocin induced diabetes in rats. J Ethnopharmacol. 2003;89(1):61-6. [PubMed] | [CrossRef] | [Google Scholar]
