ABSTRACT
Health systems attempt to measure an ever-increasing number of clinical measures when it’s about providing the best treatment to patients. Historically, agencies relied on lab tests such as blood panels and urine analysis instead of what the patients really felt; hence, they often missed the mark of what matters to patients. But with the growing importance of patients’ voices in health care system, the FDA (Food and Drug Administration) and other regulatory agencies are now practicing Patient-Reported Outcomes (PROs) more frequently in the drug approval process. This study presents a descriptive outline of PROs, followed by the instruments used to measure these outcomes called Patient-Reported Outcome Measures (PROMs) in clinical trial protocols. A few examples of PROM applications are provided, along with some methodological ways to evaluate PROM data. The process of instrument creation and implementation are discussed, along with the illustration of measurement tools intended to be used in the area of disorders related to neurological communication. This article examines the requirements for the PRO instruments used for evaluation of medical devices and the methods recommended by the FDA’s PRO guidance. The discipline of current psychometric measurement has the capacity to assist for the development of tools that agrees to look around the suitable places that is basically from the point of view of patient. Practicing of PROs in clinical trial protocols will expand the area of concepts that has ample of limelight already for the healthcare providers to strive for solutions regarding the clinical outcomes.
INTRODUCTION
In the clinical trials which are particularly conducted by the pharmaceutical industry, the application of patient reported outcomes has become more prevalent in the current times.1 The practice of these kind of outcomes are specifically customary for the products assisting treatment of various severe, disabling ailments where instead of seeking for a remedy or a way to heal the situation, the intent is to target the symptoms and restore the condition by facilitating the performance, or eventually directing towards enhancing the quality of life. Such kind of circumstances call out for an escalating need of patient reported outcomes which plays a very significant role in supplementing the old-style traditional clinical evidences for setting up a product’s spirited competitive benefit.2
A medical device is said to be any instrument or appliance including any material that is manufactured with the motive of diagnosing, analyzing, monitoring or healing the condition of a patient.3 The consolidation of the voice of the patient during the course of the developmental lifecycle of a medical device has a scope to support the enquiry for information related to the evaluation and the surveillance data of medical device. One such way is to methodically gather valuable information associated to the influence a medical device has on patient from the patient’s perspective with the help of the use of PROs.4
The patients must share information about the way they are feeling, any symptoms as such, or side effects of the treatment that has been prescribed with the intention of receiving exalted clinical care and quality of life. The research of the clinical outcomes was on the lead with the notion according to the patient outcome study and also marked the differences in physician, care and the style of communication for the patient and for the medical outcomes too. The focus on the participation of patients in research studies has elevated which caused to get a lead on other patient-oriented programs like Patient and Public Involvement in the United Kingdom.5 Such an attempt draws on the Health Technology Assessment program’s commissioned study, which looks out for opportunities to identify the boons and impediments of patient engrossment in clinical research along with the complementary involvement of the government research activities.6
The data that contains patient-reported outcomes is not interpreted by any other party and comes directly from the patients.7 These measures have a key role of being a part of a clinician’s assessment arsenal and are very crucial in assisting the establishment of patient-centered techniques of intrusion. Hence, a legacy of such approach to obtain these measures along with its significance in clinical research and practice has been provided through the inauguration of assessment tools utilized to mark patient reported outcomes.
The FDA has created a number of materials to aid sponsors in selecting, changing, or designing a PRO instrument. Examples of PRO instruments which are assisted to support the regulatory submissions of medical device has been taken up by the PRO Compendium, though it lists only some PRO instruments that are already in utilization or are reported publicly in the premarket clinical investigations of medical devices throughout the extensive range of instruments and indication. Furthermore, PRO instruments have been qualified as tools that the sponsors of the medical device can assist in developing and evaluating these devices under the program Medical Device Development Tools (MDDT).8
DEFINITION
The US FDA (FDA 2009) has issued a recent guidance document which states patient-reported outcomes as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patients response by a clinician or anyone else”.4 PROs therefore may comprise of a wide range of subjective outcomes for example symptoms such as nausea, vomiting or pain, operational working like physical, social or emotional functioning, health-related quality of life or might also include an inclination towards a specular therapy.
A PRO is defined by Content Management System (CMS) to be any statement or testimony of a patient regarding its health condition or behavior obtained straight from the patient, with no elucidation of the health situation of the patient by any clinician or someone else. Patient information that is self-reported is a crucial source to obtain medical outcomes. Hence, the above-mentioned definition comprises of the following chief domains (as given in Figure 1).9,10
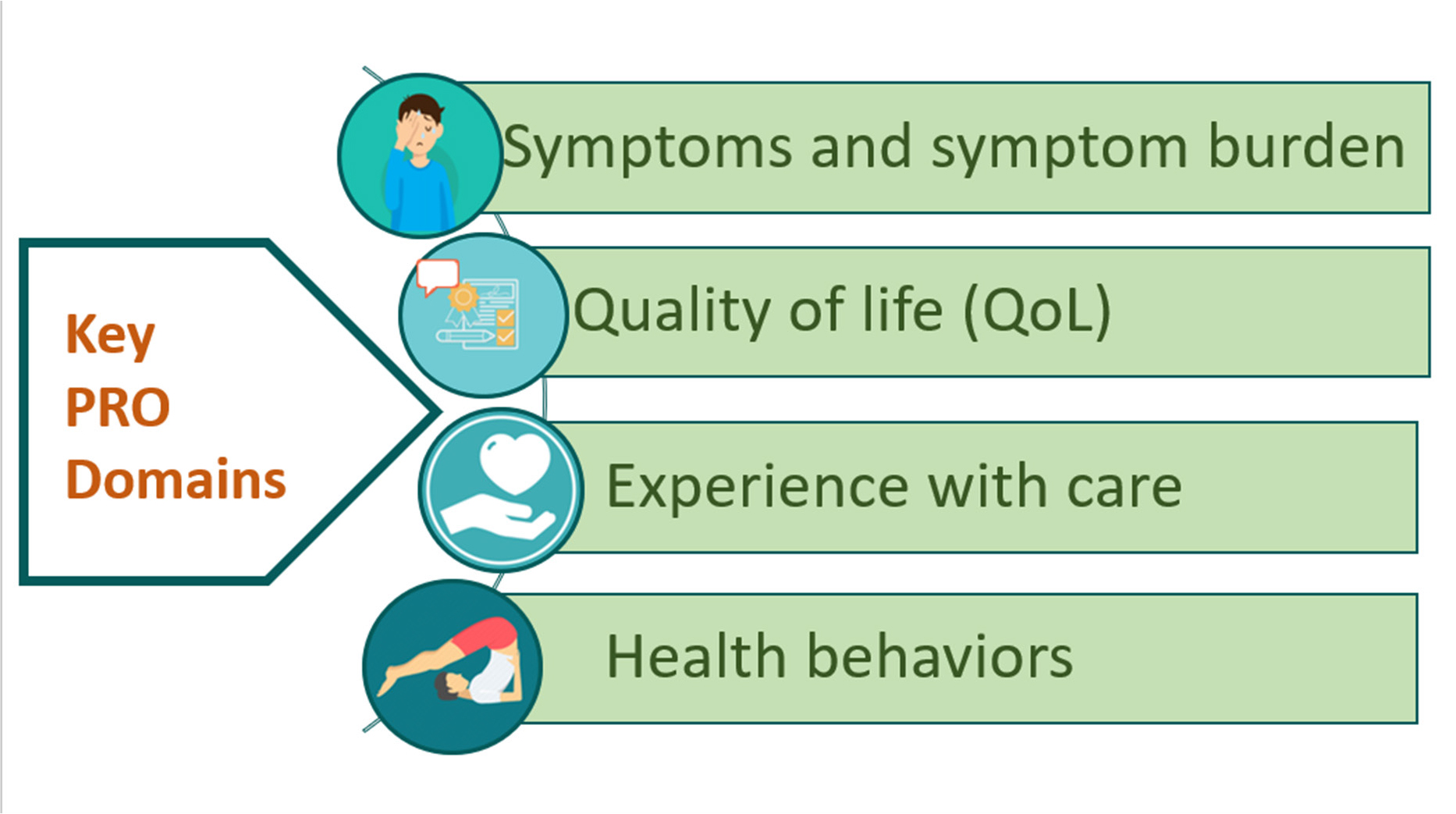
Figure 1:
The chief patient reported outcome domains.
Health quality i.e., life-related (functional status being included).
Symptoms and symptom burden (exhaustion, pain).
Behavioral health (exercise, diet, smoking).
Instead of using individual, the term “patient” corresponds health-associated attributes of the provided report, whereas the term “outcomes” are elaborated as variables which are anticipated to be modified due to the cause of the treatment.
DISCUSSION
Patient-Reported Measures (PROMs)
The instruments or tools which are intended to be utilized to measure the patient-reported outcomes are called PROMS.11 Mainly used to evaluate and measure the status of patient’s health i.e., Health-related Quality of Life (HQoL), these PROMS are also recurrently used as simple questionnaires12 which are generally patient accomplished bunch of queries. PROMs are tools or tests that examine health ability, health-related quality of life, symptom and symptom burden, personal experience with health care, and health-related habits including anxiety and depression.12,14 They might be generic as well as related disease.13 Broader PROMs analyses characteristics that relate to a wide range of medical diseases and permit comparisons across all of these issues to facilitate in the examination and implementation of novel approaches for providing healthcare and delivery of services equity.13,15
On the contrary, PROMS which are disease-specific are created to recognize symptoms associated and their implications on the function of disorders. Although disease-specific PROMs have greater validity and reliability and credibility as compared to other general PROMs, cross-disease comparisons are not always available.13 A composite of generic and disease-specific PROMs is widely used in clinical trials. For instance, the study which includes asthma patients, it may supposedly involve an ‘asthma-control’ patient-reported outcome measure and the same for a generic as well for an improved quality of life, like EuroQoL (EQ-5D).14
Some of the instruments which are used for the collection of self-reported patient information may include:9
Patient-Reported Outcomes Measurement Information System (PROMIS) — These tools, which are sponsored by the National Institutes of Health (NIH), assess patients’ state of well-being that is reported by the patient themselves.
The Medicare Health Outcomes Survey (HOS) was the foremost technique to measure the medical outcomes which have been utilized in Medicare Advantage plans. The primary goals of the program are to assemble the effective and moreover consistent and dependable information regarding the condition of health in Medicare-managed care to support in events indulged in the enhancing of quality, planning the accountability, public coverage and reporting along with health development. It is mandatorily required for all the managed care plans to contribute in the Medicare Advantage contracts.
Focus on Therapeutic Outcomes (FOTO)—Using self-reported health status surveys, this instrument assesses the operational state of patients whoever collected the restoration of the specific outpatient. The FOTO tool evaluates the operative position change by associating and comparing the measurements made in the beginning, throughout, and at the end of the therapy. Despite of this, the outcomes gathered by the instruments are deficient and lack in the gauging routine on their own, and they cannot be used by the accountability programs unambiguously. Quality measures are ought to be developed by measure developers that use the result data obtained by the instruments to assess the quality of care (Figure 2).
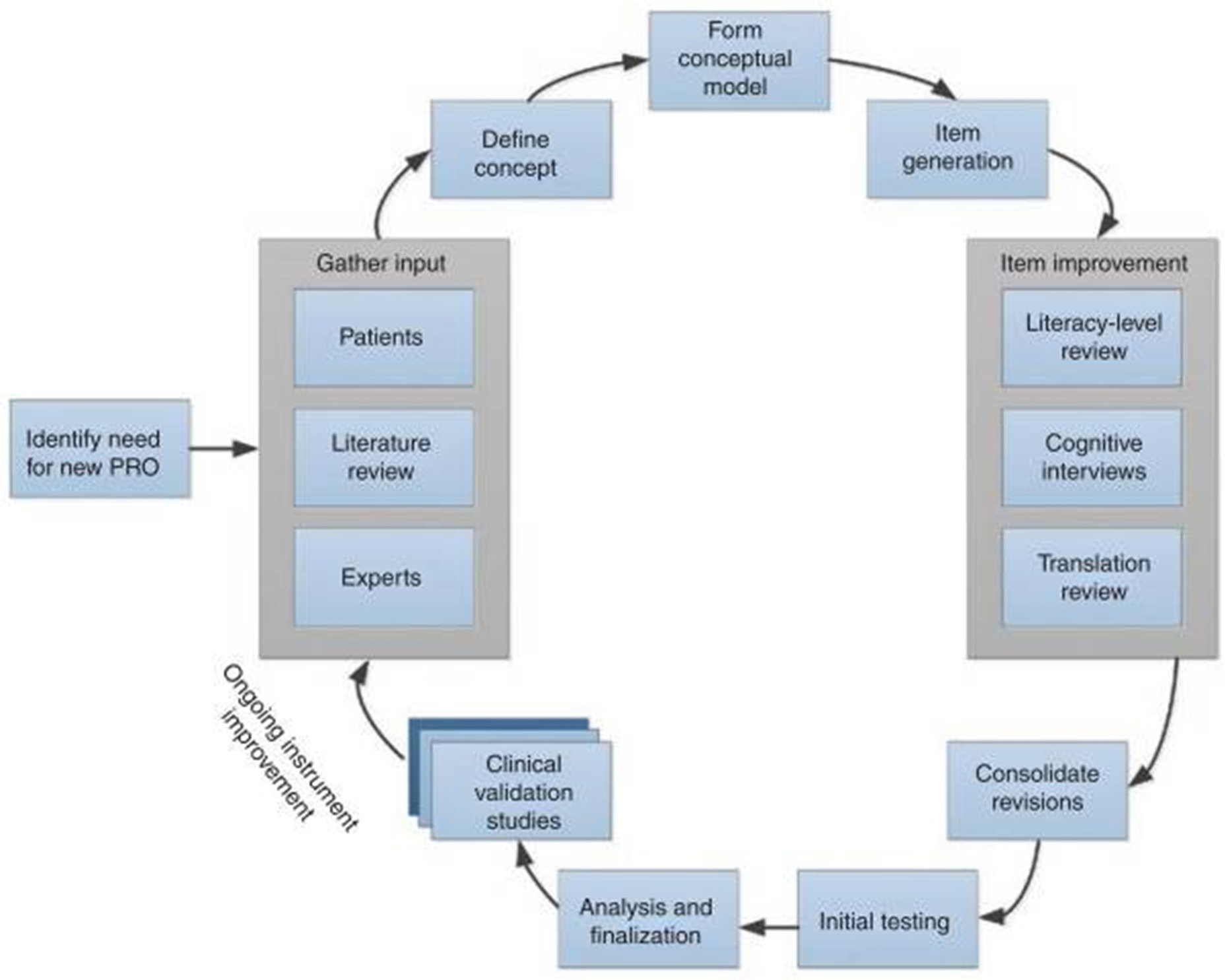
Figure 2:
The development process of patient reported outcomes.16
History
PROM development in research PROMs were originally established for its utilization in pharmaceutical research13 and investigation in the services of healthcare and were particularly limited to the areas of England, Sweden and some parts of the United States as a means of improving patient clinical care.14 In 1975, the Swedish medical profession developed the statewide use of PROMs through the use of quality registers; defined as clinical databases for disease-specific information. The PROMs were established in several segments of the United States by the year 2000, with a sole purpose to expand the PROMs as a means to obtain compensations required for the liability and accountability inside the care organizations. PROMs are being used outside of clinical research because of their promise to alter health care and enhance quality and safety by placing patients in the middle of the decision-making process.11
Approaches to developing Patient-Reported Outcome-Based Performance Measures
Pick and Specify a Patient-Reported Outcome
Many different types of data are reported by patients, some of which are self-reported data without clinical interpretation. Measure creators must first decide which patient-reported outcomes will be used as quality metrics for a target or beginning group. A suitable result is relevant from a clinical or policy perspective. A poor PRO would be, for instance, when the patient experienced a surgical site infection following cataract surgery. A patient could complain of redness, swelling, and discharge without truly knowing if they are infected. A clinically significant measure of visual improvement may be a preferable outcome measure in this case. Additionally, outcome quality metrics must be applicable to the target population and usable by the entities that are liable for being monitored. When defining suitable and relevant outcomes, wherever feasible, measure makers should seek the advice of clinical professionals.
Choose the Correct Method for Collecting the PRO Utilizing a PROM (Tool)
An environmental scan and literature research are always the first steps in the development of a measure (as given in Figure 3) to determine whether the outcome may have already been collected in the target population using existing methods. Developers of measures may take into account using instruments with proven psychometric features (e.g., adequate data element and tool reliability and validity). Although the tools themselves are not measures, measure creators may use the data from these tools to create and test the construct of a PROM with additional testing in clinical settings. The measure developer should assess the viability for the pertinent clinical applications to assist establish whether the tool offers high-quality performance data. It’s crucial to test these tools on the population that the measurement targets. Also keep in mind that although most PRO tools have only been tested in controlled settings, there may be differences between the reliability and validity of a PRO tool when used in more controlled settings (such as clinical trials or academic research projects) and when used in actual practice settings.
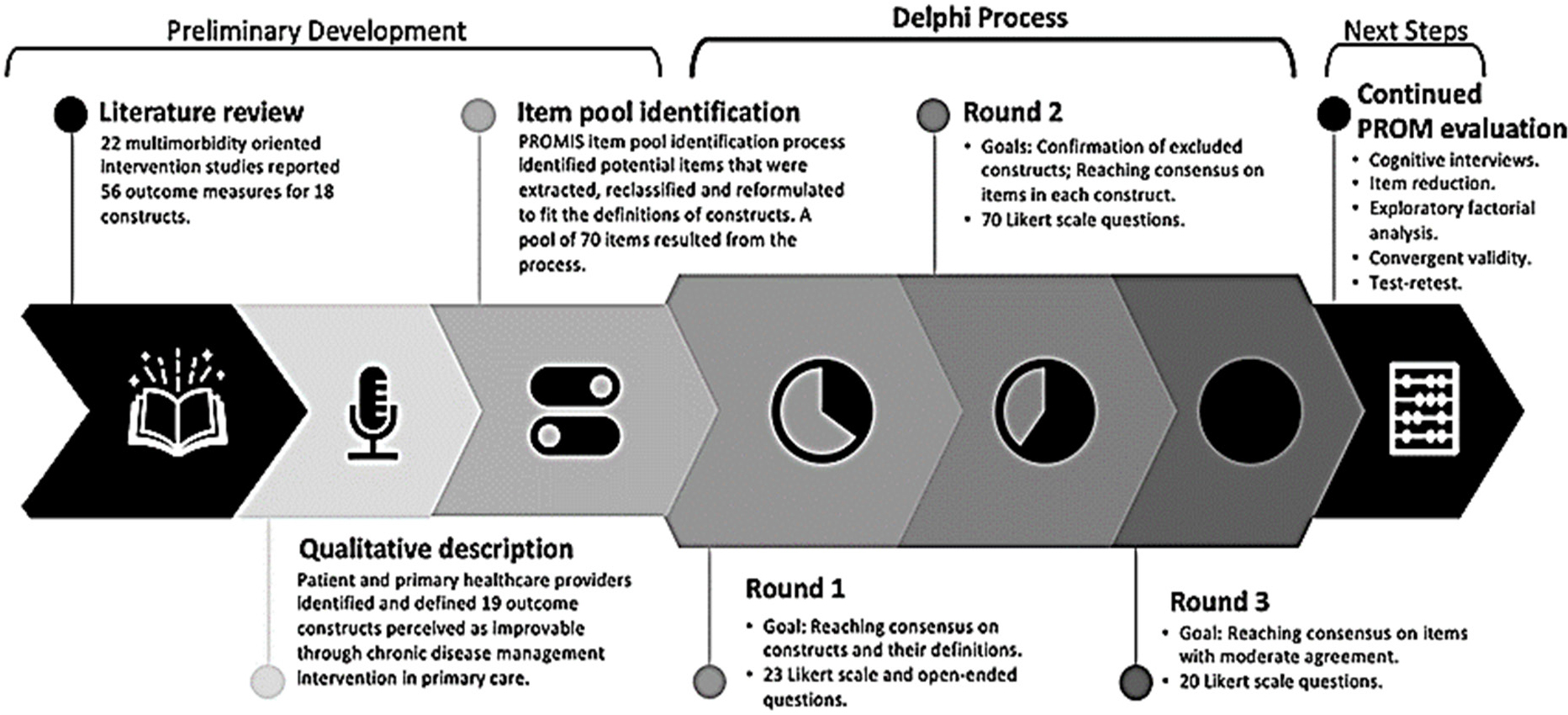
Figure 3:
Flowchart of the development of the patient-reported outcome measurement for people with multimorbidity.17
Choose the PRO-PM as the appropriate performance metric
According on the issue of interest, the measure creator should present the results for the target/initial populations as an average change or percentage improvement. All measurements must undergo reliability, usability, usage, feasibility, validity, and threats to validity testing by the measure creator, who must also determine how to manage missing data and make the necessary risk adjustments. The outcome must accurately reflect the effects of the care provided and not the influence of comorbidities or other extraneous variables in order to distinguish variances in performance between measured entities. However, the measure creator shouldn’t permit risk modification to hide inequalities, just like with any other outcome measurement. Assessing the Need for Risk Adjustment and the Development and Evaluation of Risk Adjustment Models is covered in the extra material, Risk Adjustment in Quality Measurement.9
Regulatory aspect
The increased use of PROMs has resulted in increased trust among regulatory authorities seeking for standardization in the application and during the clinical studies, the need for understanding of the outcomes. For example, both the US Food and Drug Administration and the European Medicines Agency have issued recommendations requiring the usage of PROMs in order to support the labelling claims.4,18 Ever since 2009, the United Kingdom has been demanding for PROMs for reporting results related to certain elective surgical situation for patients which indeed turns out to be used as a method to gather valuable data associated with the impact and influences of the therapy applied on the patient from the patient’s point of view in the National Health Service (NHS) itself. The UK government has pushed for the mandatory use of PROMs in order to compare health services, recognizing and categorizing strengths and shortcomings in the provision of health care, steer for quality improvement, notifying about the charges, and choice encouragement. Moreover, there is a guidance issued by the Department of Health in the NHS on national standards for prerequisite routine of PROM assortment during specific elective surgical procedures.
Pharmaceutical businesses and regulatory bodies place a high value on PRO assortment, evaluation, analysis, and reporting. PROs are important since there is an increasing emphasis on healthcare systems which are patient-oriented and completely revolves around the patient’s perspective. The US FDA understands well-enough that the patient outcomes obtained from a well-developed and steadfast PRO instrument during a structured inquiry might have the potential to come handy for supporting a label claim in a medical device (FDA 2009).19 Walking through the other lane down, the European Medicines Agency (EMA) states that “the most important segment of any evaluation conducted for studying the therapeutic benefits of new medicines is how well is the experience of the patient and in what ways the treatment has enhanced the patient’s well-being and everyday regime” in the European Union (EU) (EMA 2016). PROs can also be utilized to assist in Health-Technology Assessment (HTA) preferences. As a result, essentially PROs have become a fundamental part of most of the drug-development programs, frequently beginning with early-phase trial designs.
FDA’s perspective on PROs in evaluation of Medical Devices
Recently, in August 2020 a draft guidance was issued by the Food and Drug Administration (FDA) namely “principles for selecting, developing, modifying, and adapting patient-reported outcome instruments for use in medical device evaluation” which gives an overview of the prerequisites for PRO tools consumed in the evaluation of medical devices in clinical trials and in the pharmaceutical industry. Throughout the process of conducting a clinical trial the patients go through various assessments which gives data of how they feel and function. This data has to be collected in a systematic arrangement and in order to assort them precisely and with ease, the healthcare attendants work with PRO instruments. This data has been recognized by the FDA as influential and valuable because by picking up and combining all the patients’ voices throughout the lifecycle of the total sum product, the notions and perceptions which are important to patients are taken into consideration for the assessment, observation and evaluation of medical devices.20
These tools permit the collection of a particularly specific information which they find scientifically as a valid proof of safety and efficacy that is complementary to the other indications obtained by clinical outcomes and/or the supportive biomarkers used. The application of instruments used to collect PROs are generally intentional in most of the cases, but in certain situations, there is a scope that these instruments are specially recommended in specific standards and guidelines. PRO instruments may incorporate tools like patient diaries, signals of visual analogue and the common numeric rating scales to measure the extent of severity of the pain, symptom assessment and multidomain survey forms assessing traits of health-related quality of life (HRQoL). By the assistance of self-reports or interviews, as long as the interviewer records the patient’s response solely, a PRO can be obtained, measured and reported. Some of the unnoticeable symptoms and constructs which are only known to the patients cannot be directly observed by an interviewer and hence PRO helps in overcoming such issues by detecting and measuring such outcomes like the intensity of pain and anxiety. These instruments are also indulged in clinical research to detect changes in the influences of any medical treatment or intervention or any kind of changes observed in the health of the patient.
Several assets and funds are proposed by the FDA to support the sponsors of these tools for their selection, modification and development. These resources include guidance documents titled, “Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labelling Claims”, a sequence of documents associated with Patient-Focused Drug Development and the following resources that were posted to FDA’s website as part of CDRH’s (Center for Devices and Radiological Health) 2016-2017 Strategic Priorities:21 “Value and Use of Patient-Reported Outcomes (PROs) in Assessing Effects of Medical Devices,”9 “Clinical Outcome Assessment (COA) Case Studies,”10 and “PRO Compendium.”11 The COA Case Studies include examples of PRO instruments used to support medical device regulatory submissions and the PRO Compendium lists some, but not all, of the PRO instruments that have been used and publicly reported in medical device premarket clinical investigations across a wide variety of devices and indications.
The general considerations for PRO instruments which are used in medical device evaluation
Principles to consider
According to the FDA,22 the following principles are very essential while including PRO tools in the assessment protocol of medical devices:
The concept of interest (COI) of the instruments has to be defined and pre-established.
The role of the instrument has to be precisely identified in the clinical trial protocol.
The evidence that the instrument has reliably been assessed to the COI shall be provided; and,
Appropriate and clear communication of the results of the instrument during labelling has to be done to ease the process of decision making of the patient and to update to the healthcare provider.
To ensure that PRO instruments are understandable to the patients
FDA recommends the fundamentals of these instruments; which usually incorporates guidelines, directives, articles, recall period and response options; to be composed by the usage of simple and plain language to support the patients with varying levels of literacy so that majority could understand and able to respond to the provided information.
The options of response to the particular item must be coherent with the wording of that item in particular, for instance, provided that through the evocation interviews the frequency of itching has to be recognized, then the response options are supposed to be measures of frequency (for example, always, often, sometimes, seldom, never) and the response options and articles defined with the use of cognitive interviews.
FDA endorses sponsors to offer PRO instruments in variety of languages in order to assess the patient experience, wherever appropriate, for patients having poor English speaking and reading skills; hence trying to make the clinical study more generalizable to the intended population.
FDA suggests the sponsors to consider the influence on patients after responding to the instruments in order to lessen the unnecessary burden by using well-developed, short-form version of instruments.
The role of PRO tools in clinical study protocol and statistical analysis plan
Defining the endpoint of the instrument being used to take hold of the clinical trial along with a clear statement of what is being measured and how is it interpreted to clearly convey the Concept of Interest (COI) and Context of Use (COU) in the clinical study protocol and statistical analysis plan.
Before submission of the Investigational Device Exemption (IDE), the sponsors are persuaded to retain FDA to purport a benefit-risk assessment for the suitable PRO instrument.
While showcasing the clinical study reports of these tools during a medical device submission, the sponsors should make sure that the concept measurements match the COI stated in the COU and if any changes are present, a clinically meaningful justification should be provided for the specified change.
Next Generation PROs
The PROMIS Project
With the increasing usage of PROs, a much greater attention was held by the inefficacy and inappropriate quality of the instruments, incapacity to equate the results amidst the instruments and increased burden for patients from lengthy sessions of assessments. The Patient-Reported Outcome Measurement Information System (PROMIS)23 is known to be launched in 2005 through a National Institutes of Health (NIH) Roadmap Initiative for addressing such kind of issuances. This project has an objective to offer clinicians to retrieve to the effective, detailed, exact and rational PRO measures. PROMIS opts for extensively rigorous qualitative and quantitative methods, which integrates definition constructing, reviewing literatures, obtaining inputs from experts and focus groups, review of literacy and translation, and cognitive interviews.24,25
Moreover, PROMIS also offers equal participation26 for patients suffering from a range of different impairments like visual, motor or reading impairments to extend and maximize the generalization of clinical research. It utilizes severe qualitative and quantitative methodologies which ropes in to build definitions, review literature, feedback received from specialists and focus troops, translation reviews and cognitive interviews.27
PROMIS, for a long period of time. through its assessments has been measuring physical, emotional and social health of adults, children and parent proxies (parents who achieve a measure for 5-18 aged children).
Project Patient Voice
Project Patient Voice is a cyber podium for patients and caretakers as an initiative by the FDA to look after the “patient-reported symptom data collected from cancer clinical trials”. It aims to provide patient-reported symptom data constantly from the cancer clinical trials of approved products. Such information is generally not available in the US Prescribing Information (drug label) but delivers extra data to patients and caregivers. Comprising FDA, a lot of groups seek for the patient experience data to be provided publicly, but the drug label lacks the space that an online platform can provide.28
The data on this system comes from clinical trials supporting FDA approval of a particular drug for cancer. Although it intends to be used by healthcare providers for cancer-related treatment; one should not completely rely on Project Patient Voice only to form decisions for patient care since the conclusions and deductions formed from the experience of the patients may be limited, because there is a possibility that not all symptoms have been collected via survey.
Neuro-QOL
Using the same methodology as that of PROMIS, Neuro-QOL established Computer Adaptive Test (CAT) and build several short forms to measure and evaluate various realms. These areas incorporate many behavioral and physical notions which cover up emotional distress, substantial and bodily function, relative applied intuition or cognition, inability to control emotions and behaviors, positive impact, traumatized or interrupted sleep cycle, social functioning as an individual and in group, maintenance of social associations and interactions, stigma, agony, communication, suicidal or death-related concerns and apprehension, bowel/bladder physiological working, and sexual function.29 For the usage of the adult population, adolescents and children, PRO tools are made available in various languages like English and Spanish.6 The development and primary validation assessments for the tools has been accomplished for impairments like stroke, muscular dystrophy and Parkinson’s disease. Other severe cases involved include multiple sclerosis, epilepsy and amyotrophic lateral sclerosis.
Some of the medical devices which have been approved or sanctioned by the PROs embodied by the FDA for instance, covers up devices like an implantable pulse generator used to activate the lumbar multifidus muscle in patients with failed therapy who are not appropriate nominee for the spine surgery, namely ReActiv8 Implantable Neurostimulation System and the OPRATM which basically stands for osseo-anchored prostheses for the rehabilitation of amputees; which have the intent to be supportive in cases of above-knee amputations amidst the adult population,30 especially the ones estimated with the come back issues i.e. convalescence barriers along with socket-leg prostheses or the ones who cannot use them. If for instance, the clinical outcomes data is summed up with the PRO information acquired from the medical device evaluation on the basis of SF-36 and the EQ-5D respectively,24,25 exhibited the quality of life to be much more enhanced comparatively, as per the patient-report. Additionally, PROs also established and reported labeling claims. The FDA summary incorporating the Safety and Efficacy Data (SED) and information related to labeling can be approached or could be the go-to-place for individuals and healthcare providers who are in search of detailed data.4
CONCLUSION
The PROs are considered and established to be a very strong tool to comprehend and recognize the health of the patient and their quality of life. Unlike the old-style traditional ways of clinical measuring and evaluation which involved operating with the blood samples and similar laboratory assessments, the PROs are known to be assisting to clinical professionals who keep a check on the patient’s well-being and help them in providing the optimized level of care that they require. The prevailing psychometric testing, which were implemented for the medical rehabilitation, acquire techniques and they also add novelty and inciting approachable pitch to this job. But still supplementary tasks are required to be done in order to enhance the usage of PROs and a routine check-in for clinical experiments.
It is interesting to know that the clinicians involved in hearing-out their patients understand the importance of this procedure and are always keen to be aware of the patient’s review after the treatment. The participation of patients has hence elevated and during the decision-making for treatment, patients have given preference to use of PRO tools. But despite of observing such support, PROs have to yet face many challenges in research and clinical practice like hindrance due to changeability in the quality of the tools, incapability of comparing the outcomes across the assisting reports and long session of examinations causing burden to patients. The establishment of PROMIS though, has brought in new optimism in the research area. It focusses on the barriers faced, which creates a range of general assessments, depletes the weightage of patients related to their response and simplifies the process of scoring with the use of item response theory and advancing technology in computers. These PRO instruments holding quality are generally taken up for efficient gathering of valuable and reliable information from the reports based on a comprehensive and detailed compilation of patient experiences. Moreover, it is important to realize that PROs are the ultimate tools which can be used to increase the ability of understanding and measuring in response to the patients’ symptoms, the events encountered and their quality of life.
Both physicians and researchers are experiencing an exciting time right now. It might be possible in the future to perform a battery of tests that will guide clinical practice. This may involve concepts like self-efficacy, adaptability, autonomy, social functioning, and others. Client-centered therapies require collaboration between researchers, patients, and clinicians. By utilizing PRO measures, physicians may be able to document the results of these interventions and concentrate treatment on problems that patients value. There isn’t a single treatment for communication difficulties that is so effective that it can cure all of the illnesses’ side effects. Instead, therapy may involve a range of activities, some of which may be aimed at lessening the disability, some at enhancing speech or language, and still others at concentrating on communication within the context of more general psychosocial problems. By describing patient perceptions, we’ll be able to record some of these varied strategies.
References
- [2023Jan05];Patient-reported outcomes measures: an introduction for clinicians [internet].
- [2023Jan05];Council Directive 93/42/EEC of 14 June 1993 concerning medical devices [internet]. Council Directive 93/42/EEC of 14 June 1993 concerning medical devices. Queen’s Printer of Acts of Parliament;.
- Oliver SR. How can health service users contribute to the NHS research and development programme?. BMJ. 1995;310(6990):1318-20. [PubMed] | [CrossRef] | [Google Scholar]
- Snyder CF, Jensen RE, Segal JB, Wu AW. Patient-Reported Outcomes (PROs): putting the patient perspective in patient-centered outcomes research. Med Care. 2013;51(8-S3):S73-9. [PubMed] | [CrossRef] | [Google Scholar]
- [PubMed] | [CrossRef] | [Google Scholar]
- . [2023Jan05];centers for Medicare and Medicaid. [Internet]. [PubMed] | [CrossRef] | [Google Scholar]
- Rahimi K, Malhotra A, Banning AP, Jenkinson C.. Outcome selection and role of patient reported outcomes in contemporary cardiovascular trials: systematic review. BMJ. 2010;341:c5707 [PubMed] | [CrossRef] | [Google Scholar]
- Weldring T, Smith SMS. [2023Jan05];Patient-reported outcomes (pros) and Patient-reported outcome measures (Proms) [internet]. Health Serv Insights. 2013;6:61-8. [PubMed] | [CrossRef] | [Google Scholar]
- [PubMed] | [CrossRef] | [Google Scholar]
- Black N.. Patient reported outcome measures could help transform health care. BMJ. 2013;346:f167 [PubMed] | [CrossRef] | [Google Scholar]
- Black N, Jenkinson C.. Measuring patients’ experiences and outcomes. BMJ. 2009;339:b2495 [PubMed] | [CrossRef] | [Google Scholar]
- [PubMed] | [CrossRef] | [Google Scholar]
- Rothrock NE, Kaiser KA, Cella D.. Developing a valid patient-reported outcome measure [internet]. Clin Pharmacol Ther. 2011;90(5):737-42. [PubMed] | [CrossRef] | [Google Scholar]
- Sasseville Maxime, Chouinard Maud-Christine, fortin Martin. [2023Jan05];Evaluating the content of a patient-reported outcome measure for people. [Internet]. [PubMed] | [CrossRef] | [Google Scholar]
- Bottomley A, Jones D, Claassens L.. [2023Jan05];Patient-reported outcomes: assessment and current perspectives of the guidelines of the Food and Drug Administration and the reflection paper of the European Medicines Agency [internet]. Eur J Cancer. 1990;45(3):347-53. [PubMed] | [CrossRef] | [Google Scholar]
- Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD, et al. [2023Jan05];Use of existing Patient-Reported Outcome (PRO) instruments and their modification: the ISPOR Good Research Practices for evaluating and documenting content validity for the use of existing instruments and their modification Pro Task Force Report [internet]. Value Health J Int Soc Pharmacoecon Outcomes Res. 2009;12(8):1075-83. [PubMed] | [CrossRef] | [Google Scholar]
- [PubMed] | [CrossRef] | [Google Scholar]
- . Strategic priorities center for devices and radiological health; 2016-2017. [PubMed] | [CrossRef] | [Google Scholar]
- [PubMed] | [CrossRef] | [Google Scholar]
- . Healthmeasures.net. 2019 [PubMed] | [CrossRef] | [Google Scholar]
- Carle AC, Cella D, Cai L, Choi SW, Crane PK, Curtis SM, et al. Advancing Promis’s methodology: results of the Third Patient-Reported Outcomes Measurement Information System (PROMIS®) Psychometric Summit. Array. 2011;11(6):677-84. [PubMed] | [CrossRef] | [Google Scholar]
- Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care.. 2007;45(5S):1:S3-S11. [PubMed] | [CrossRef] | [Google Scholar]
- Ader DN. Developing the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care.. 2007;45(10 S-2):S1-172. [PubMed] | [CrossRef] | [Google Scholar]
- DeWalt DA, Rothrock N, Yount S, Stone AA. PROMIS Cooperative Group. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;45(5S1):S12-21. [PubMed] | [CrossRef] | [Google Scholar]
- . [Jan 05 2023];Project Patient Voice. 2022 [PubMed] | [CrossRef] | [Google Scholar]
- Cella D, Nowinski C, Peterman A, Victorson D, Miller D, Lai JS, et al. The neurology quality-of-life measurement initiative. Arch Phys Med Rehabil. 2011;92(S10):S28-36. [PubMed] | [CrossRef] | [Google Scholar]
- Lai JS, Nowinski C, Victorson D, Bode R, Podrabsky T, McKinney N, et al. Quality-of-life measures in children with neurological conditions: pediatric Neuro-QOL. Neurorehab Neural Repair. 2012;26(1):36-47. [PubMed] | [CrossRef] | [Google Scholar]
