ABSTRACT
Background
Genetic metabolic illnesses called Glycogen Storage Disease type I (GSD I) are caused due to abnormalities in the glucose-6-phosphatase enzyme complex involved in the metabolism of glycogen.
Objectives
The current study explores the induction and management of GSD I manifestations.
Materials and Methods
Chlorogenic Acid (CGA), a hydroxycinnamic acid found in various fruits and abundant in green coffee, can inhibit the enzyme glucose-6- phosphatase complex and metformin can alter the lactic acid levels.
Results
The in silico and in vivo approach unveiled, that β-carotene showed the highest binding affinity with glucokinase, forming 7 hydrogen bonds. Riboflavin exhibited the highest binding affinity with protein phosphatase 1, forming 4 hydrogen bonds. Vitamin C interacted at the active site of the control ligand. Vitamin E also displayed significant binding affinity with glucokinase. Overall, these bioactive micro molecules show promising interactions with their respective target proteins. In vivo results revealed the significant impact of Macronutrients and Micronutrients, particularly when combining Cassava starch with the micronutrients. This combination showed notable improvements in various parameters, including liver glycogen levels, liver weight, fasting blood glucose levels, and liver histology. The findings suggest the potential of this synergistic approach in enhancing liver function and glucose regulation. To conclude this work, it was evident that the combination of Macro and Micronutrients revealed distinct advantages. UCCS and Cassava starch effectively maintained normoglycemia due to their sustained glucose release from complex carbohydrates. Remarkably, Cassava starch, both alone and combined with micronutrients, outperformed UCCS in promoting better outcomes. This highlights the potential for comprehensive research on Cassava starch’s effects in GSD subjects, exploring its impact on other manifestations such as lactic acidosis, hypertriglyceridemia, and hyperuricemia.
Conclusion
These findings offer promising avenues for further investigation and potential therapeutic applications.
INTRODUCTION
The Glycogen Storage Diseases (GSD) or glycogenoses are a group of inherited metabolic diseases caused due to deficiency of the enzymes required to regulate glycogenolysis or gluconeogenesis.1,2 Hypoglycemia is the prominent manifestation of hepatic GSD, whereas weakness and muscle cramps are the predominant features of muscle GSD. The overall incidence of these diseases is approximately 1 in 20,000 to 43,000 live births. Types I, III, and IX represent 80% of hepatic GSDs, of which GSD type I is most prevalent.3
The GSD type I, also known as Von Gierke’s disease, is further categorized into type Ia and Ib. Deficient activity of Glucose-6- phosphatase – α (G6Pase-α) causes GSDIa and deficient activity of Glucose-6-Phosphate Transporter (G6PT) causes GSD Ib.2 Both disorders results in hypoglycemia, hyperlipidemia, hyperuricemia, lactic acidosis, and excessive accumulation of glycogen primarily in the liver and kidney leading to progressive hepatomegaly and nephromegaly.4
The incidence of GSD I is 1 in 100000, 80% of the patients represent GSD Ia and 20% by GSD Ib.5 In addition to the clinical symptoms and findings seen in GSD type Ia, recurrent infections, neutropenia, and neutrophil dysfunction are observed in type Ib6 due to impaired glucose transport across the cell membrane of the polymorphonuclear leukocytes. It has been suggested that microsomal Glucose-6-Phosphate (G6P) transportation has a role in the antioxidant protection of neutrophils and that the genetic defect of the transporter leads to the impairment of cellular functions and apoptosis, which may be a potential explanation for neutrophil dysfunction. Long term complications like renal calculi and progressive renal disease, inflammatory bowel disease, hepatic adenomas, and hepatocellular carcinoma7 can develop in older children and adults.8 In most GSD patient’s liver biopsy remains the gold standard diagnostic tool to determine the glycogen accumulation in hepatocytes, and other diagnostic measures include enzyme assay, mutation analysis, estimating the biochemical parameters, which reveal hypoglycemia, hyperlipidemia, hyperuricemia, lactic acidosis, and neutropenia specifically in case of GSD type Ib.9
The management of manifestation of GSD is a daunting task. The GSD patient needs to meticulously depend on frequent carbohydrate enriched diet (every 3-4 hr ) and uncooked corn starch (UCCS),9 devoid of simple sugars like sucrose (refined sugar), fructose (fruits) and lactose (dairy products) and other macronutrients. Special care should be taken to avoid excessive consumption of non-utilizable sugars, which may lead to accumulation of glycogen in tissues and endogenous production of lactate, triglycerides, and uric acid. Due to such diet constraints there is possibility of diminished macronutrient and micronutrient nourishment.9
There is evidences that certain micronutrients like Beta Carotene, Riboflavin, Vitamin C, Vitamin E,10 thiamine, Vitamin D,11 selenium,12 etc. influence the glucose metabolism and improve a few GSD manifestations.
The literature suggests that the designing and establishing of disease model for GSD is an uphill task, though the gene knockout animals and genetically mutated cells serve as disease models. The stability, survivability, maintenance, and the cost of these models is a big challenge. As per literature review, certain chemical derivatives like Chlorogenic Acid (CGA), a type of hydroxycinnamic acids, occurs in many types of fruits and in high concentration in green coffee12 and its derivatives are known to inhibit the particular enzyme glucose-6-phosphatase complex, along with Metformin (Met), which can simulate the manifestations similar to GSD type I. The present work is an attempt to determine how best these chemical derivatives mimic the characteristic manifestations of GSD and to assess the role of macro and micronutrients in the management of the manifestations. Based on literature review Uncooked corn starch and Cassava Starch (CS) were used as macronutrients and based on in silico trial few micronutrients were selected for the study (viz. Beta Carotene,13 Riboflavin, Vitamin C, Vitamin E).10 In GSD, it is necessary to maintain normoglycemia throughout the day and night, which can be achieved by either frequent meals, continuous glucose therapy and/or uncooked corn starch (UCCS).12 However, frequent meals can be fed during day, but during night the patient needs to rely on UCCS,14 which has its own time bound limitations, like lack of nutrition, fiber, complex carbohydrate, which arises the chance to pursue better alternative starch, like Cassava starch/tapioca starch to help maintain the nutritional value of the diet and at the same time sustain the normoglycemia better than UCCS.13 The next major manifestation of GSD type 1 is glycogen accumulation in hepatocytes which leads to progressive hepatomegaly and hepatic dysfunction in advanced conditions,14 to overcome this issue we attempted to inhibit the process of Glycogenesis, by inactivation of major enzymes involved in the glycogen synthesis pathway, viz, Glycogen synthase,15 Glucokinase and standby enzyme called Protein phosphatase-1 enzyme,16 with the help of few selected micronutrients. So, to sum up, the macronutrients are expected to maintain the normoglycemia for the maximum period possible and on the other hand the micronutrients are expected to reduce the glycogen accumulation in the liver, thus maintaining the normal architecture and physiology of the liver.
MATERIALS AND METHODS
In silico study
Molecular Docking
The molecular docking was performed under the three main steps i.e., ligand preparation, macromolecule preparation and ligand-protein docking.
Ligand Preparation
The 3D structure of each ligand was retrieved from PubChem database in .sdf format and converted into .pdb format using Discovery Studio 2021. The energy minimized using uff force field under conjugation gradient as an optimum algorithm. After energy minimization each ligand was converted into .pdbqt format.
Macromolecule preparation
The x-ray 3D crystallographic protein of Protein Phosphatase-1 (PDB:6DNO), GSK3β (PDB:4J1R), glucokinase (PDB: 1SZ2) were retrieved from RCSB protein databank. All the hetero atoms were removed using Discovery Studio 2021. Molecules were then added with kollman charges. Later, all the ligands were saved in .pdb format.
Ligand-protein docking
Each ligand was docked against Protein Phosphatase-1 (center x, y, z=15.33, 80.65, 181.65, and size x, y, z=46.56, 46.15, 56.039), GSK3β (center x, y, z=34.11, 32.72, 18.41 and size x, y, z=61.01, 54.50, 60.32), and glucokinase (center x, y, z=10.133, -3.79, 12.89 and size x, y, z=63.16, 53.23, 57.57) using AutoDock Vina. After docking 10 different poses were obtained. The ligand’s pose with minimum binding energy was chosen to visualize the ligand-protein interaction using Discovery Studio 2021.
Chemicals and Drugs
The chlorogenic acid (Sigma Aldrich, USA) was procured and maintained at 8 to 25°C as per the manufacturer’s instructions and metformin tablets (USV India), corn starch (Brown and Polson), Casava starch (Koko’s), and vitamins were maintained at room temperature. All other chemicals were of analytical grade and procured from various manufacturers
Experimental design to induce and treat the GSD type-I manifestations in rats
Animal ethical clearance
The study design was performed after prior approval by the Institutional Animal Ethics Committee (IAEC) of KLE College of Pharmacy, Vidyanagar, Hubballi, Karnataka, India. (Proposal No. :01/KLECOPH/19). All the experimental procedures were carried out in accordance with the committee for the purpose of control and supervision of experiments on animal (CPCSEA) guidelines.
Experimental animal selection and housing
The study includes the use of male albino rats of Wistar strain weighing (150-200) g obtained from the animal house of KLE College of Pharmacy, Hubballi, Karnataka. They were housed in polypropylene cages and maintained at 27 ± 2°C under 12 hr dark and light cycles. They were fed with standard rat feed (Gold Mohur Lipton India Ltd.) and water ad libitum was provided. The proposed animal study was reviewed by the Committee for Control and Supervision of Experiments on Animals (CCSEA) in India and was approved (Approval No. 01/KLECOPH/19) by the Institutional Animal Ethics Committee (IAEC) and ARRIVE guidelines were followed for all the experimental methods.
Experimental design to assess the role of macro and micronutrients in modulating the GSD type 1 manifestations
Chlorogenic Acid (CGA) and Metformin (Met) were chosen to induce GSD I manifestations for this study, considering their safety, availability, and effectiveness. For this experimental setup, the study design was divided into two categories, to explore the influence of macronutrients and micronutrients respectively. For macronutrient study the animals were divided into six groups each group consisting of six rats, where group I control group were subjected to normal food and water ad libitum, group II negative control/ disease control chlorogenic acid 200 mg/kg and metformin 500 mg/kg were administered, group III (CGA+Met)+UCCS fed, group IV (CGA+Met)+CS fed, group V (CGA+Met)+UCCS+micronutrients and (CGA+Met)+CS+micronutrients administered.
Macro and Micronutrient selection
The normal chow pellets were crushed into fine powder consistency using pestle and mortar, and in the ration of 50:50 (10 gms + 10 gms per animal) corn flour and the crushed pellet chow were mixed with the gradual addition of purified water, similarly the Cassava flour and the pellet powder were also mixed, no additional binding agent was required, as the starch itself acts as a binding agent.
Estimation of Glycogen in hepatocytes
Immediately after the autopsy the glycogen was estimated as described by Montgomery (1957).
Estimation of serum Lactic acid levels by measuring Lactate dehydrogenase
The serum lactic acid was estimated as per the method adopted by Barker S. B. et al. 1941. Clinical significance: The enzyme Lactate dehydrogenase (LDH) is concentrated in the heart, kidney, liver, muscle, and body tissues. Consequently, damage to these results in increased serum levels of LDH. Elevated levels are associated with myocardial infarction, renal damage, hepatitis, anaemias, malignancies, and muscular disease or damage.
Estimation of serum Uric acid levels
Uricase converts uric acid into allantoin and hydrogen peroxide. In the presence of peroxidase, hydrogen peroxide oxidatively couples with a phenolic chromogen to form a red coloured compound which has maximum absorbance at 505 nm. The concentration of red coloured compound is proportional to the amount of uric acid in specimen (Barham, D. et al., 1972).
Liver, weight and histological examination of liver tissue
Histopathological studies of liver tissues were carried out using Periodic acid-Schiff (PAS), all the slides were observed for changes in histopathological characteristics and reported in the result section.
Management of the manifestations using Macronutrients
Preparation of starch-enriched diet.
Uncooked Corn Starch admixed with normal Chow pellets.
Cassava Starch admixed with normal Chow pellets.
The normal chow feed was churned into the pestle and mortar and equal quantity of starch (UCCS and Casava Starch Individually) (i.e. 10 gms of each per animal) was admixed and small quantity of distilled water was added to mix well, now the mixture was pressed into pellets and dried overnight.
Management of the manifestations using Micronutrient
Oral administration of Beta carotene (6 mg/kg/day), Riboflavin (10 mg/kg/day), Vitamin C (100 mg/ kg/day) and Vitamin E (60 mg/kg/day).
Data analysis
The collected data was subjected to statistical analysis using statistical GraphPad Prism version 7. The results were expressed as the mean ± SEM (Standard Error of the Mean). The results obtained were analysed using one-way ANOVA followed by Bonferroni’s multiple comparison post-hoc test.
RESULTS
In silico study
Molecular Docking
Among all the bioactive micro molecules, β-carotene showed the highest binding affinity with glucokinase (Figure 2A) (binding energy -8.5 kcal/mol) and however, it had no H- bond interaction vs control ligand (-6.1 kcal/mol); showed 7 H-bond interactions with Ala107, Ala103, Thr102, His312, Ser153, Trp151. However, the control ligand had the highest binding affinity with GSK3β (binding energy -8.4 kcal/mol, 3 H-bond interactions with Val135 and Pro136) (Figure 1A, 2A, and 3A). Among the test ligands, β-carotene showed the maximum binding affinity compared to rest of test ligands with GSK3β (binding energy -8 kcal/mol) (Table 1). Likewise, among all the test ligands, riboflavin showed the highest binding affinity with protein phosphatise 1 (Figure 3B) (binding energy -7.4 kcal/mol 4 H-bond interactions with Tyr69, Arg74, Asp71 vs control ligand (Figure 4) (binding energy -9.9 kcal/mol, 6 H-bind interactions with Cys278, Arg221, His248, Asp92, Arg96, Lys98. The binding affinity of each ligand with a respective target is presented in (Table 1). Among all the test ligands, these compounds i.e., Riboflavin (Figure 2B), β-carotene (had no H-interactions) and vitamin E (Figure 2D) interacted on different site compared to the control ligand for glucokinase (Figure 1D). However, vitamin C had the interaction with all the amino acids that control ligand has meaning it acts within active site where control ligand binds (Figure 1C, 2C, and 3C). Likewise, riboflavin had the common Val 135 interacting residue with GSK3β (Figure 1B) compared to its control ligand, meaning its probable GSK3β inhibitory activity within the given site. Similarly, Vitamin C had the common 2 amino acid residues i.e., Arg221 and Arg96 to interact with protein phosphatase 1 (Figure 3 C) reflecting its probable inhibitory activity within the site of control ligand’s interaction (Figure 4).

Figure 1 (A):
Beta carotene docked with glycogen synthase kinase 3b.

Figure 1 (B):
Riboflavin docked with glycogen synthase kinase 3b.

Figure 1 (C):
Vitamin C docked with glycogen synthase kinase 3b.

Figure 1 (D):
Vitamin E docked with glycogen synthase kinase 3b.
Selected Micronutrients having Good Binding Affinity with Glycogen Synthase Kinase 3b (GSK3b)

Figure 2 (A):
Beta Carotene docked with Glucokinase.

Figure 2 B:
Riboflavin docked with Glucokinase

Figure 2 C:
Vitamin C docked with Glucokinase

Figure 2 D:
Vitamin E docked with Glucokinase
Micronutrients having good binding affinity with Glucokinase

Figure 3 A:
Beta carotene docked with Protein Phosphatase-1

Figure 3 B:
Riboflavin docked with Protein Phosphatase-1

Figure 3 C:
Vitamin C docked with Protein Phosphatase-1

Figure 3 D:
Vitamin E docked with Protein Phosphatase-1

Figure 4 (A, B and C):
Showing the interactions of the control ligands with GSK3β, Protein Phosphatase 1, and Glucokinase
Micronutrients having good binding affinity with Protein Phosphatase-1

Figure 5:
Values are expressed as mean±standard error of the mean (n=6). Negative control group showed significant increase in the liver glycogen content (***p<0.001), (CGA+Met)+UCCS+micronutrients showed the significant reduction in glycogen (ΨΨp<0.01) and (CGA+Met)+cassava starch+micronutrients (ΨΨΨp<0.001) when compared to Negative control, whereas, (CGA+Met)+cassava starch+micronutrients group showed (γγp<0.01) when compared to (CGA+Met)+UCCS and (δp<0.05) when compared to (CGA+Met)+CS.
In vivo study
Liver glycogen estimation
Liver Glycogen Estimation
The negative control group exhibited a substantial increase in liver glycogen content (***p<0.001). However, the (CGA+Met)+UCCS+micronutrients group showed significant reduction in liver glycoen (ΨΨp<0.01), and the (CGA+Met)+cassava starch+micronutrients group displayed even higher significance (ΨΨΨp<0.001) when compared to the negative control. Furthermore, the (CGA+Met)+cassava starch+micronutrients group exhibited statistical significant reduction in liver glycogen (γγp<0.01) when compared to (CGA+Met)+UCCS, and (δp<0.05) when compared to (CGA+Met)+CS (Figure 5).

Figure 6:
Results are expressed as mean±standard error of the mean (n=6), there was notable increase in the liver size of Negative control when compared to Control group (***p<0.001), (CGA+Met)+cassava starch group showed significant decrease (Ψp<0.05) when compared with negative control and both micronutrient treated groups showed significant decrease in the liver weight when compared to Negative Control (ΨΨΨp<0.001) similarly (CGA+Met)+UCCS+micronutrients and (CGA+Met)+cassava starch+micronutrients, decreased the liver weight significantly (γγγp<0.001) when compared to (CGA+Met)+UCCS and (δδδp<0.001) when compared to (CGA+Met)+cassava group.
Liver weight
The liver weight varied across groups. The negative control group had significantly increased liver weight (***p<0.001) compared to the control group, while the (CGA+Met)+cassava starch group decreased liver weight (Ψp<0.05) compared to the negative control. Both micronutrient-treated groups significantly reduced liver weight (ΨΨΨp<0.001) compared to the negative control.

Figure 7:
The statistical significance was analysed by one-way ANOVA followed by Bonferroni’s multiple comparison post-hoc test, (A) showed no significant changes, whereas (B) showed significant decrease in blood glucose level (**p<0.01 at 30 and 60 min) and (***p<0.001 at 90 and 120 min). (C) showed significant elevation (***p<0.001) at 30, 60, 90 min, whereas (*p<0.05) at 120 min, On the other hand, (D) showed significant elevation in blood glucose levels (***p<0.001) at 30, 60 and 120 min and (**p<0.01 at 90 min) when compared to 0.0 min reading.
Additionally, combining (CGA+Met)+UCCS+micronutrients and (CGA+Met)+cassava starch+micronutrients led to a significant decrease in liver weight (γγγp<0.001) compared to (CGA+Met)+UCCS and (δδδp<0.001) compared to (CGA+Met)+cassava starch (Figure 6). These findings illustrate the treatment effects on liver weight in our study.
Fasting Blood Glucose levels
The normal group exhibited no significant changes in blood glucose levels. Conversely, the negative control group in Figure B displayed a substantial decrease in blood glucose levels (**p<0.01 at 30 & 60 minutes) and (***p<0.001 at 90 & 120 minutes). In Figure C, the treatment group (CGA+Met+UCCS) demonstrated significant elevations in blood glucose levels (***p<0.001) at 30, 60, and 90 minutes, with a (*p<0.05) increase at 120 minutes. In contrast, in Figure D, the treatment group (CGA+Met+CS) showed remarkable elevations in blood glucose levels (***p<0.001) at 30, 60 & 120 minutes and (**p<0.01 at 90 minutes) when compared to the 0.0 min reading.

Figure 8:
(A) Normal Group Periodic Acid-Schiff stain (PAS) showing normal architecture with intact hepatocytes and no Glycogen accumulation. (B) CGA (200mg/kg) treated group PAS stained, showed significant Mosaic pattern, Glycogen nuclei [a], Glycogen [b] swollen hepatocytes [c], ballooning degeneration [d] and sinusoidal congestion [e]. (C) (CGA+Met)+UCCS treated group. (D) (CGA +Met)+Cassava Starch treated group PAS stained, showed notable Glycogen [b], swollen hepatocytes [c], and ballooning degeneration [d]. (E) UCCS+micronutrients treated group. (F): Cassava starch+micronutrient treated group showed near normal architecture with intact hepatocytes and no Glycogen accumulation.
| Ligand | Glucokinase (PDB: 1SZ2) | GSK3β (PDB: 4J1R) | Protein Phosphatase-1 (PDB: 6DNO) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Binding Affinity | NHBI | HBR | Binding Affinity | NHBI | HBR | Binding Affinity | NHBI | HBR | |
| Control ligand | -6.1 | 7 | Ala107, Ala103, Thr102, His312, Ser153, Trp151 | -8.4 | 3 | Val135, Pro136 | -9.9 | 6 | Cys278, Arg221, His248, Asp92, Arg96, Lys98 |
| Riboflavin | -6.8 | 2 | Asp100, Thr137 | -7.3 | 3 | Val135, Ile62, Asn64 | -7.4 | 4 | Tyr69, Arg74, Asp71 |
| Vitamin C | -5.9 | 4 | Thr102, Ala103, Ala107, Trp151 | -5.3 | 4 | Glu283, Met284, Thr235 | -5.6 | 5 | His125, Arg96, Tyr272, His248, Arg221 |
| β-carotene | -8.5 | – | – | -8 | – | – | -7.9 | – | – |
| Vitamin E | -5.9 | 1 | Gly134 | -7 | – | – | -6.6 | 1 | Lys98 |
Histopathological analysis
The typical histopathological features of rat livers for all the groups by Periodic Acid-Schiff stain (PAS) staining are represented in Figure 7. The CGA treated group showed significant Mosaic pattern, Glycogen nuclei, Glycogen, swollen hepatocytes, ballooning degeneration & sinusoidal congestion when compared to normal group. On the other hand (CGA+Met) + UCCS treated group and (CGA +Met) + Cassava Starch treated group showed notable Glycogen, swollen hepatocytes, & ballooning degeneration. Whereas, the UCCS + micronutrients and Cassava starch + micronutrients treated group showed near normal architecture with intact hepatocytes and no Glycogen accumulation (Figure 8).
Statistical analysis
All the data were analysed using the statistical program GraphPad Prism version 6.0 and expressed as mean SEM. Where, Analysis of Variance (ANOVA) was used to examine the data, followed by Bonferroni’s multiple comparison post-hoc test, where p values of <0.05) were set to be considered as statistically significant.
DISCUSSION
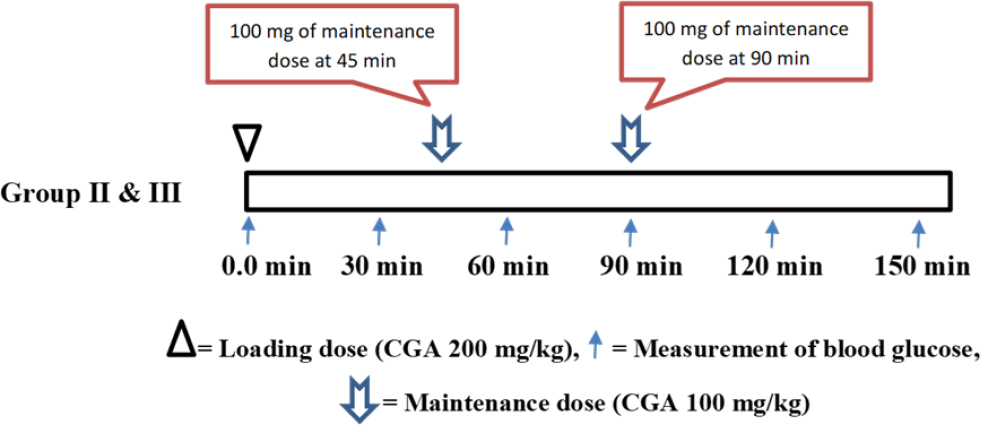
The aim of the study was to explore the predominant manifestation of GSD type I i.e., hypoglycemia, A phytochemical called CGA was considered optimum at 200 mg/ kg/ po. Which showed some promising capability to induce fasting hypoglycemia in albino wistar rats. Several in vitro studies have claimed that CGA can inhibit glucose-6-phosphate enzyme complex (glucose-6- phosphatase and glucose-6-phosphate translocase).18 Thus, in this study the green coffee extract,19 consisting of 36 % CGA was admixed in the drinking water and was fed continuously to the animals in the treatment groups, with an expectation of maintain the CGA effect, along with that the animals received a daily dose of 200 mg of 95% CGA via oral gavage. Glycogen levels are slightly elevated in CGA treated rats which can be due to the specific inhibition of glucose-6-phosphatase and it is also one of the contributing factors for hepatomegaly. Throughout the experiment the animals were subjected to overnight fasting and 1 hr before the experiment, all the groups except Normal group were subjected to 95% CGA at 200 mg/kg via oral gavage as a loading dose, followed by a 100 mg/kg dose of 95% CGA after every 45 min interval to maintain the bioavailability of the CGA. In addition to 95% CGA animals were subjected to 36% CGA, continuously in drinking water throughout the study. The UCCS and Cassava starch were used as Macronutrients whereas Beta-Carotene, Riboflavin, Vitamin- C and Vitamin-E were selected as Micronutrients based on drug likeness using in silico approach, where, β-carotene exhibited the highest binding affinity with glucokinase and formed 7 hydrogen bonds with specific amino acids, while the control ligand showed lower binding affinity with no hydrogen bonds. β-carotene also displayed significant binding affinity with GSK3β. Riboflavin demonstrated the highest binding affinity with protein phosphatase-1 and formed 4 hydrogen bonds with specific amino acids. The control ligand showed stronger binding with protein phosphatase-1 and formed 6 hydrogen bonds. Riboflavin, β-carotene, and Vitamin E interacted at different sites compared to their respective control ligands for glucokinase. Vitamin C interacted with all the amino acids targeted by the control ligand, indicating potential inhibitory activity in the active site. Riboflavin shared a common interacting residue with GSK3β, suggesting a probable inhibitory activity at that site. Similarly, Vitamin C had two common amino acid residues with the control ligand, reflecting its potential inhibitory activity at the site of control ligand’s interaction with protein phosphatase-1. On the other hand, in vivo results demonstrated the influence of Macronutrients and Micronutrients, especially Cassava starch in combination with the micronutrients showed significant improvement in the parameters such as liver glycogen, liver weight, fasting blood glucose levels and liver histology, and to address the glycogen accumulation in hepatocytes, leading to hepatomegaly and hepatic dysfunction, we attempted to inhibit the process of glycogenesis. Our approach involved inactivating key enzymes, including glycogen synthase, glucokinase, and protein phosphatase-1, using selected micronutrients.
CONCLUSION
Inclusion of Macro and Micronutrients demonstrated their beneficial effect in their own ways, UCCS and Cassava Starch showed gradual maintenance of normoglycemia, due to the presence of complex carbohydrates, which is believed to convert into glucose in sustained manner. Among these two, Cassava starch alone as well as in combination with the micronutrients showed better results when compared to UCCS alone and in combination with micronutrients. The micronutrients were pivotal in preventing excess glycogen accumulation in hepatocytes which is the major cause for the classical GSD manifestations especially hepatomegaly. Further there is considerable scope to study the beneficial role of macro and micronutrients extensively in GSD I subjects, targeting other manifestations such as lactic acidosis, hypertriglyceridemia, and hyperuricemia.
Cite this article
Patil SB, Gadad PC. Novel Approaches in Glycogen Storage Disease Type I Management: Harnessing the Potential of Micronutrients and Macro Molecules. Int. J. Pharm. Investigation. 2024;14(1):141-50.
ACKNOWLEDGEMENT
We gratefully acknowledge the Principal, KLE College of Pharmacy, Hubballi, Karnataka for providing research facilities.
ABBREVIATIONS
| GSD | Glycogen Storage Disease |
|---|---|
| CGA | Chlorogenic acid |
| UCCS | Uncooked corn starch |
| CS | Cassava Starch |
| Met | Metformin |
| PAS | Periodic Acid-Schiff stain |
References
- Özen H. Glycogen storage diseases: new perspectives. World J Gastroenterol. 2007;13(18):2541-53. [PubMed] | [CrossRef] | [Google Scholar]
- Chou JY, Jun HS, Mansfield BC. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase/glucose-6-phosphate transporter complexes. J Inherit Metab Dis. 2015;38(3):511-9. [PubMed] | [CrossRef] | [Google Scholar]
- Hiraiwa H, Pan CJ, Lin B, Moses SW, Chou JY. Inactivation of the glucose 6-phosphate transporter causes glycogen storage disease type 1b. J Biol Chem. 1999;274(9):5532-6. [PubMed] | [CrossRef] | [Google Scholar]
- Chou JY, Jun HS, Mansfield BC. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase/glucose-6-phosphate transporter complexes. J Inherit Metab Dis. 2015;38(3):511-9. [PubMed] | [CrossRef] | [Google Scholar]
- Labrune P, Ullrich K, Smit P, Rake J, Visser G, Leonard J, et al. Guidelines for management of glycogen storage disease type I – European study on glycogen storage disease Type I (ESGSD I). Eur J Pediatr. 2002;161:S112-9. [CrossRef] | [Google Scholar]
- Calderwood S, Kilpatrick L, Douglas SD, Freedman M, Smith-whitley K, Rolland M, et al. Recombinant human granulocyte colony-stimulating factor therapy for patients with neutropenia and/or neutrophil dysfunction secondary to glycogen storage disease type 1b. 2016;97(2):376-83. [CrossRef] | [Google Scholar]
- Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: A case series. J Inherit Metab Dis. 2005;28(2):153-62. [PubMed] | [CrossRef] | [Google Scholar]
- Dieckgraefe BK, Korzenik ÆJR. Association of glycogen storage disease 1b and Crohn disease: results of a North American survey. 2002;161:S88-92. [PubMed] | [CrossRef] | [Google Scholar]
- Kishnani PS, Austin SL, Abdenur JE, Arn P, Bali DS, Boney A, et al. Diagnosis and management of glycogen storage disease type I: A practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16(11):e1 [PubMed] | [CrossRef] | [Google Scholar]
- Derks TGJ, Rodriguez-Buritica DF, Ahmad A, de Boer F, Couce ML, Grünert SC, et al. Glycogen storage disease type Ia: current management options, burden and unmet needs. Nutrients. 2021;13(11):1-17. [PubMed] | [CrossRef] | [Google Scholar]
- Bhattacharya K. Dietary dilemmas in the management of glycogen storage disease type I. J Inherit Metab Dis. 2011;34(3):621-9. [PubMed] | [CrossRef] | [Google Scholar]
- Melis D, Della Casa R, Parini R, Rigoldi M, Cacciapuoti C, Marcolongo P, et al. Vitamin E supplementation improves neutropenia and reduces the frequency of infections in patients with glycogen storage disease type 1b. Eur J Pediatr. 2009;168(9):1069-74. [PubMed] | [CrossRef] | [Google Scholar]
- Kalkan Ucar S, Coker M, Sözmen E, Goksen Simsek D, Darcan S. An association among iron, copper, zinc, and selenium, and antioxidative status in dyslipidemic pediatric patients with glycogen storage disease types IA and III. J Trace Elem Med Biol. 2010;24(1):42-5. [PubMed] | [CrossRef] | [Google Scholar]
- Ong KW, Hsu A, Tan BKH. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation. Biochem Pharmacol. 2013;85(9):1341-51. [PubMed] | [CrossRef] | [Google Scholar]
- Schnorr CE, Morrone M, Simões-Pires A, Bittencourt L, Zeidán-Chuliá F, Moreira JCF, et al. Supplementation of adult rats with moderate amounts of β-carotene modulates the redox status in plasma without exerting pro-oxidant effects in the brain: A safer alternative to food fortification with vitamin A?. Nutrients. 2014;6(12):5572-82. [PubMed] | [CrossRef] | [Google Scholar]
- Chen YT, Cornblath M, Sidbury JB. Cornstarch therapy in Type I glycogen-storage disease. N Engl J Med. 1984;310(3):171-5. [PubMed] | [CrossRef] | [Google Scholar]
- Bhattacharya K, Mundy H, Lilburn MF, Champion MP, Morley DW, Maillot F, et al. A pilot longitudinal study of the use of waxy maize heat modified starch in the treatment of adults with glycogen storage disease type I: A randomized double-blind cross-over study. Orphanet J Rare Dis. 2015;10(1):18 [PubMed] | [CrossRef] | [Google Scholar]
- Pursell N, Gierut J, Zhou W, Dills M, Diwanji R, Gjorgjieva M, et al. Inhibition of glycogen synthase II with RNAi prevents liver injury in mouse models of glycogen storage diseases. Mol Ther. 2018;26(7):1771-82. [PubMed] | [CrossRef] | [Google Scholar]
- Marr L, Biswas D, Daly LA, Browning C, Vial SCM, Maskell DP, et al. Mechanism of glycogen synthase inactivation and interaction with glycogenin. Nat Commun. 2022;13(1):3372 [PubMed] | [CrossRef] | [Google Scholar]
