ABSTRACT
ABSTRACT
Phyllanthus acidus (L.), a medicinal plant, shows numerous pharmacological properties like antioxidant, anti-inflammatory, hypoglycemic effects, neuroprotective, anti-diarrheal, anti-pyretic, and analgesic and anaesthetic effects which may be attributed to the bioactive compounds produced by Phyllanthusacidus or due to associated endophytic fungi. In the current work, Phyllanthus acidus L. leaf samples were analysed for the presence of endophytic fungi and to produce quercetin, a beneficial chemical, from them using an optimal surface sterilising process and analytical techniques., five fungal endophytes were isolated and they were preliminarily identified by morphology. According to morphological features of isolated endophytes identified as Aspergillus sp. Aff and Chaetomium sp. Aff. Interestingly, only leaves were used to isolate all five fungus, and in response to selected plant samples, the overall colonisation frequency from surface sterilised leaves was found to be 7.5%. Shake flask culture approach used for fermentation followed by extraction and purification process. Analytical and thin-layer chromatography, HPLC of ethyl acetate extract of isolated fungal endophytes PAEF3 showed a distinct phytochemical fingerprinting profile.The fact that quercetin, a key component of Phyllanthusacidus L., is used for a variety of properties like Antiviral, Anticancer, and Antidiabetes, makes this study of practical significance as well.
INTRODUCTION
Endophytes are microbes that grow intracellularly in plant tissues and are a significant source of biological natural product synthesis.1 Bacteria or fungus colonise the interior plant tissues underneath the epidermal cell layers known as endophytes, which do not appear to harm host which show symbiotic association. The endophytic fungi of plants were a significant source of secondary metabolites, bioactive substances that are beneficial for the pharmaceutical industry and other sectors like Food and textile industries. These molecules include insecticides, antimicrobials, antioxidants, and antipyretics.2 Numerous distinctive bioactive metabolites, such as alkaloids, benzoquinones, flavanoids, phenols, stereoid, terpenoids, tetralones, and xanthones, have been discovered to be produced by endophytic fungi.3,4 Botanical characteristics of Phyllanthus acidus Linn. explained in Table 1.
| Phyllanthus acidus Linn is a small, glabrous tree up to 10 m | ||
| Branch | Phyllanthoid bark rough, grey, lenticels prominent. | |
| Leaf [Selected for isolation of endophytic fungi] | Pinnate, 20-40 cm long.Leaflets alternate, simple, entire, shortly petiolate, broadly ovate to ovate-lanceolate, apex acute, petiole 2.5-4 mm long, stipules triangular-acuminate. | |
| Flowers | Small, pink. | |
| Fruit | Drupaceous, oblate, 6 to 8 lobed, greenish yellow. | |
| Seeds | Grooved stone. | |
MATERIALS AND METHODS
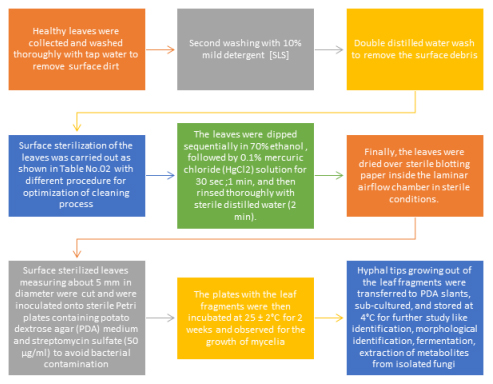
In the present study, endophytic fungi were isolated from healthy/ disease free leaf tissue of Phyllanthus acidus Linn. or Acidus distichus [phyllanthaceae family] endophytic from 18.5204° N, 73.8567° E and A survey to the fields were done and healthy-looking plants explat: leaf part were collected and in sterile bags, they were transported to the lab and processed there for a second round of isolation within two hours. A voucher specimen of the plant species has been deposited at Agharkar Research Institute Pune, Maharashtra, India.
Optimization of surface sterilization methodology for endophytic fungi isolation
Optimization of Surface sterilization of Phyllanthus acidus Linn. Leaf and isolation of endophytic fungi
As per shown in above Figure 1 different surface sterilizing agents like 70% ethanol, 4% sodium hypochlorite and 0.1% mercuric chloride solution were used, from above surface sterilizing agents and various time of treatment; 1% mercuric chloride (HgCl2) solution for 30 sec after 70% ethanol was chosen. due to no evidence found for contamination when experiment caried out as shown in Table 4.7
Statistical analysis for isolated endophytic fungi colony
Colonization frequency
It was done as follows to calculate the colonisation frequency percentage (CF%).
This formula was used to determine the EIR percentage:
Morphological identification of isolated endophytic fungi by microscopy methodology9,10 isolates of fungi The morphological characteristics of PAEF1, PAEF2, PAEF3, PAEF4, and PAEF5 have been identified, comprising colony growth, the presence or absence of aerial mycelium, colony colour, the presence of wrinkles and furrows, and pigment production etc. in reference to Barnett, 1992.
With the use of lactophenol with a 5 min heating treatment, isolated endophytic fungi were examined under a microscope with 40X and 100X resolution using Labomed microscope.
Preservation of isolated endophytic fungi
Fermentation and metabolites extraction13-16
The isolated endophytic fungi PAEF1, PAEF2, PAEF3, PAEF4, PAEF5 were cultivated in Potato Dextrose Broth (PDB) fermentation further used for extraction process details as shown in Figure 2.

Figure 2:
Stepwise procedure for fermentation and extraction of isolated endophytic fungi.
After the pre-determined incubation period, the culture broth was filtered using sterile Whatman filter paper to remove the mycelia. In a solvent extraction process, the metabolites of the endophytic fungi PAEF1, PAEF2, PAEF3, PAEF4, and PAEF5 were extracted using ethyl acetate as an organic solvent. Equal parts of ethyl acetate were added to the filtrate, shaken vigorously for 15–20 min, and then set aside until two distinct clear immiscible layers arose. The upper layer i.e organic layer containing the extracted biochemicals was separated using a separating funnel. The solvent was evaporated to yield the crude metabolite, and the product that was extracted was then dried in a rotator vacuum evaporator.
Preliminary phytochemical screening for isolated endophytic fungi extract for PAEF1, PAEF2, PAEF3, PAEF4, PAEF517,18
The formed Endophytic fungi extract for PAEF1, PAEF2, PAEF3, PAEF4, PAEF5 utilised for phytochemical analysis of alkaloids, flavonoids, glycosides, volatile oils, saponins.
A survey of the literature revealed that Phyllanthus acidus has a wide range of phytoconstituents, including alkaloids, tannins, flavonoids, and terpenoids,all of which have been demonstrated to have advantageous biological effects, such as antioxidant, anti-inflammatory, hypoglycemic effects, neuroprotective, anti-diarrheal, anti-pyretic, and analgesic and anaesthetic effects.19–24
Numerous potential bioactive molecules like quercetin, kaempferol, epicatechin, coumaric, hypogallic acid, gallic acid, adenosine and cinnamic acids were identified in Phyllanthus acidus leaves.25,26
Characterization of extracts27-29
Therefore the extract obtained after fermentation process further used for UV analysis, Infrared Spectroscopy (IR), Thin Layer Chromatography (TLC), High Performance Thin Layer Chromatography.
PAEF3 extract was used for further processing since it contains Quercetin, according to preliminary testing and UV analysis. Steps for separation as follow:
- Concentration and hydrolyzation by 7% H2SO4 (5 hr).
- Filteration and extraction with ethyl acetate (1:1/ 3 times).
- Concentration to get the crude form Quercetin.
- 10% ethanol utilized for Crystallization.
UV Spectroscopy
Quercetin solution: made by dissolving 10 mg of quercetin in 10.0 mL of ethanol in a volumetric flask. Which used for comparison with extract prepared from isolated endophytic fungi.
Infrared Spectroscopy (IR)
The sample was made by combining the separated portion of quercetin with KBr (1:100), and then it was analysed using infrared spectroscopy.
Thin Layer Chromatography (TLC)27-29
The 3.0 × 8.0 cm precoated TLC plate was activated in a hot air oven for 30 min at 105°C before being cooled to room temperature. Along with extracted Quercetin, standard Quercetin was dissolved in ethanol and applied 1 cm above the edge of the pretreated TLC plate. In an airtight chromatography chamber with optimised solvent mixture of ethyl acetate, toluene, and formic acid (4:3.5:0.5), this plate was run for identification process. The developed plates 1% ethanolic aluminum chloride solution were air dried and visualized under UV. The TLC plates were further sprinkled by reagents for flavonoids such as ferric chloride and aluminium chloride. Report the Rf value and compare with standard quercetin. Optimization of solvent system explained in Table 2.
| Solvent system used for TLC development | Ration for solvent |
|---|---|
| N butanol-Acetic Acid-Water | 4:1:5 |
| Ethyl Acetate Saturated with Acetic Acid: Water | 6:4 |
| Acetic Acid, HCl, Water | 10:3:30 |
| Butanol, Acetic Acid, Water | 4:1:5 |
| Ethyl Acetate, Toluene, Formic Acid | 4:3.5:0.5 |
High Performance Thin Layer Chromatography (HPTLC)
The prepared extract was subjected to HPLC studies. HPLC conditions are given in Table 3.
| Sl. No. | Parameter | Set Value |
|---|---|---|
| 1 | Injection Volume | 10 ul |
| 2 | Run Time | 30 min |
| 3 | Column name | C185 u |
| 4 | Flow Rate | 1 mL/min |
| 5 | Detection | PDA 220nm |
RESULTS AND DISCUSSION
Optimization of Surface sterilization of Phyllanthus acidus Linn. Leaf and isolation of endophytic fungi
Different surface sterilizing agents like 70% ethanol, 4% Sodium hypochlorite and 0.1% Mercuric chloride (HgCl2) solution were used, and their efficacy of action shown in Table 4.
| Sl. No. | Surface sterilization agents used for isolation of endophytic fungi | Time of treatment [sec] | Observations (In term of successful treatment for isolation of endophytic fungi with no contamination in petriplate which compare with control) |
|---|---|---|---|
| 1 | 0.1% mercuric chloride (HgCl2) solution | 30 sec | + |
| 2 | 0.1% mercuric chloride (HgCl2) solution | 60 sec | + |
| 3 | 4% sodium hypochlorite (NaOCl) | 180 sec | + |
| 4 | 70% ethanol +4% sodium hypochlorite (NaOCl) | 60 sec + 180 sec | + |
| 5 | 70% ethanol + 0.1% mercuric chloride (HgCl2) solution | 30 sec each | – |
| 6 | 70% ethanol | 30 sec | + |
70% ethanol for 30 sec followed by 0.1% mercuric chloride (HgCl2) solution 30 sec was selected due to no evidence found for contamination when experiment caried out as shown in Table 4.
Statistical analysis for isolated endophytic fungi colony
Relative frequency and colonisation were computed as mentioned in Table 5.
| Sl. No. | Name of the Plant | Number of infected segments | EIR (%) | CF (%) | Total number of segments | Number of sporulative species | Endophytic fungi isolated |
|---|---|---|---|---|---|---|---|
| 1 | Phyllanthus acidus (L.) | 25 | 25/35*100= 71.42 | 5/35*100=17.5 | 35 | 5 | 5 |
Morphological identification of isolated endophytic fungi by microscopy methodology
Total 05 endophytic fungi isolated from Phyllanthus acidus fresh leaves as per above mentioned optimised procedure and code given PAEF 01, PAEF 02, PAEF 03, PAEF 04, PAEF 05.
Morphological characterisation of isolated endophytic fungal colonies mentioned in Table 6.
| Sl. No. | Endophytic fungi code | Front view Isolated endophytic fungal colonies | Back View Isolated endophytic fungal colonies | Isolated endophytic fungi colony Description |
|---|---|---|---|---|
| 1 | PAEF1 | Filamentous fungus, smooth, hair-like soft tufts, filiform margin, translucent, cottony texture. | ||
| 2 | PAEF2 | Whitish black color colony, irregular and lobate margin. | ||
| 3 | PAEF3 | Brown/yellow color round granular/ powdery colony, entire margin. | ||
| 4 | PAEF4 | White color velvetycolony and red color backside, entire margin,red pigment observed at bottom of colony. | ||
| 5 | PAEF5 | White color cottony colony, smooth and entire margin. Black coloration develop at centre of colony [backside].Rised elevation, unbonated. |
Isolated endophytic fungi used for microscopic observation by use of lactophenol with description mentioned in Table 7.
| Sl. No. | Endophytic fungi code | Microscopic view | Microscopic Observation | |
|---|---|---|---|---|
| 1 | PAEF1 | Hyaline and smooth-walled conidiophore stipes. Conidia have smooth walls, are globose to ellipsoidal in shape, and range in size from 1.5 to 2.5 m. | ||
| 2 | PAEF2 | Ascospores are limoniform and 9–12 x 8–10 × 6–8 m in size. One lateral face is noticeably flattened, and the ends are weakly apiculate. | ||
| 3 | PAEF3 | Conidial heads are small, columnar, biseriate, and have a diameter of up to 500 x 30–50 m.Hyaline and smooth-walled conidiophore stipes.Conidia have smooth walls, are globose to ellipsoidal in shape, and range in size from 1.5 to 2.5 m. | ||
| 4 | PAEF4 | Ascomata 160-300 mm in diameter, spherical to ovoid. | ||
| 5 | PAEF5 | Asci 30-40 x 11–16 mm, clavate, long-stalked, 8-spored. | ||
Based on the preliminary morphological features of all isolated endophytic fungi, were selected for further confirmation for Microscopical characteristics utilized for identification of endophytic fungi by Agharkar Research Institute, department of science and technology, Pune 04, Maharashtra, India. And identification data mentioned in Table 8.
| Sl. No. | Endophytic fungi code | Identification Remarks | Family |
|---|---|---|---|
| 1 | PAEF1 | Aspergillus sp. Aff A. Terreus | Aspergillaceae |
| 2 | PAEF2 | Chaetomium sp. Aff C. Globosum | Chaetomiaceae |
| 3 | PAEF3 | Aspergillus sp. Aff A. Terreus | Aspergillaceae |
| 4 | PAEF4 | Chaetomium sp. Aff C. Globosum | Chaetomiaceae |
| 5 | PAEF5 | Chaetomium sp. Aff C. Globosum | Chaetomiaceae |
Fermentation and metabolites extraction
The isolated endophytic fungi PAEF1, PAEF2, PAEF3, PAEF4, and PAEF5 were grown in 200 mL of Potato Dextrose Broth (PDB) and incubated at 28°C in a BOD shaking incubator for two weeks at 120 rpm. The filtrate was then used for extraction and phytochemical research, which are detailed in Table 6 below.
Preliminary phytochemical screening for Isolated Endophytic fungi extracts for PAEF1, PAEF2, PAEF3, PAEF4, PAEF5
Endophytic fungi extract for PAEF1, PAEF2, PAEF3, PAEF4, and PAEF5 is used for phytochemical analysis, which reveals the presence of glycosides, phenolic compounds, flavonoids, proteins, amino acids, carbohydrates, and saponins. Results of analysis mentioned in Table 9.
| Sl. No. | Constituent | Test/Reagent | PAEF1 | PAEF2 | PAEF3 | PAEF4 | PAEF5 |
|---|---|---|---|---|---|---|---|
| Alkaloids | Dragendroffs reagentMayers reagent | – | – | + | + | + | |
| 1 | Carbohydrate | Molish’s test Barfoeds test | + | – | + | + | + |
| 2 | Flavonoids | Shinoda test | – | + | + | + | – |
| 3 | Tannins | Ferric Chloride test | – | – | + | + | – |
| 4 | Proteins | Biuret test Xanthoproteic test | + | + | + | + | – |
| Saponins | Foam test | – | – | + | – | – | |
| Steroids | Liebermann Burchard test Salkowski reaction | – | + | + – | + | + |
Characterization of extracts
UV Spectroscopy
The formed Endophytic fungi extract for PAEF1, PAEF2, PAEF3, PAEF4, PAEF5 utilised for UV analysis from data obtained regarding λmax, PAEF3 showed presence of quercetin. Quercetin and the isolated extract PAEF3 λmax were found to be identical as shown in Figure 5.

Figure 3:
Fermentation process for Isolated Endophytic fungi for PAEF1, PAEF2, PAEF3, PAEF4, PAEF5.
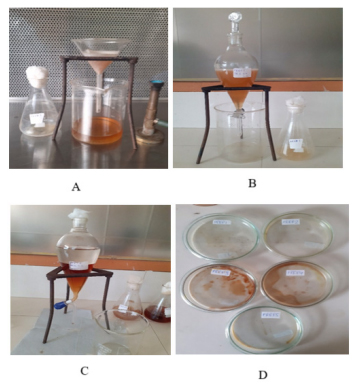
Figure 4:
Extraction process for Isolated Endophytic fungi for PAEF1, PAEF2, PAEF3, PAEF4, PAEF5 [A: Filtration of Fermented broth in aseptic condition ;B: Solvent Extraction mechanism ; C: Distinct two clear immiscible layers formed after Solvent Extraction mechanism];D: Extract formation after evaporator treatment
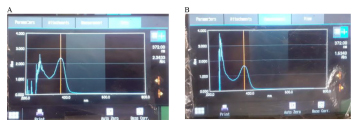
Figure 5:
UV-spectroscopy of standard quercetin[A] and PAEF3 extract[B].
Infrared Spectroscopy (IR)
It was discovered that the characteristic IR peaks matched those of their respective standard reference component of quercetin, IR graphs as mentioned in Figure 6.
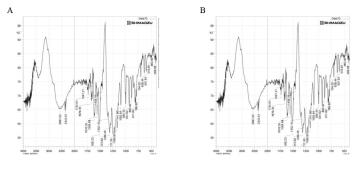
Figure 6:
IR spectra of standard quercetin [A] and PAEF3 extract [B].
Thin Layer Chromatography (TLC)
For PAEF3 extract quercetin, a solvent mixture of ethyl acetate, toluene, and formic acid (4:3.5:0.5) produced the best results. The standard quercetin appears in spots on the TLC plate that was generated under UV light. The Rf value of the isolated quercetin from the sample was identical to the Rf value of the standard quercetin i. 0.43. Set put for TLC shown in Figure 7.
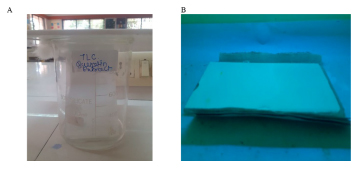
Figure 7:
Thin Layer Chromatography for PAEF3.
High Performance Thin Layer Chromatography (HPTLC)
Comparable HPTLC spectra were obtained for isolated and standard quercetin. HPLC analysis of separated quercetin extract revealed retention times of 12.18 and 12.19 min, which were identical to those of standard quercetin as shown in below Figure 8.
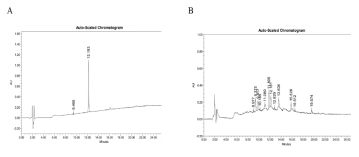
Figure 8:
HPLC of standard quercetin [A] and PAEF3 extract [B].
CONCLUSION
Quercetin is a key component that has numerous benefits, including those for preventing cancer, controlling diabetes, protecting the liver, and fighting free radicals. was identified using a quick, easy, and simple isolation technique from the endophytic fungus PAEF03. The structure of standard and isolated quercetin was confirmed after the separated fraction was characterised using sophisticated methods of investigation from physicochemical, spectroscopic, and chromatographic studies.
Cite this article: Chaudhari SP, Powar PV. Bioprospecting of Endophytic Fungi from Phyllanthus acidus Linn Leaf Identification of Secondary Metabolites Quercetin. Int. J. Pharm. Investigation. 2023;13(4):817-27.
References
- Shekhawat KK, Rao DV, Batra A. Morphological study of endophytic fungi inhabiting leaves of L. Int J Pharm Sci Rev Res. 2010;5(3):177-80. [Google Scholar]
- Firáková S, Šturdíková M, Múčková M. Bioactive secondary metabolites produced bymicroorganisms associated with plants. Biologia. 2007;62(3):251-7. [CrossRef] | [Google Scholar]
- Shukla ST, Habbu PV, VH Array, Kulkarni KS, Aprajita RP, Sutariya VN, et al. Endophytic microbes: A novel source for biologically/pharmacologically active secondary metabolites. Asian J Pharmacol Toxicol. 2014;2(3):1-16. [CrossRef] | [Google Scholar]
- Nishanthi R, Gowrie SU, Chathurdevi G. An evaluation of potential bioactive metabolites of endophytic fungi isolated from medicinal plant. Eur J Pharm Med Res. 2016;3(4):450-8. [CrossRef] | [Google Scholar]
- Tan S-P, Tan EN-Y, Lim Q-Y, Nafiah MA. Array. J Ethnopharmacol. 2020:253 [CrossRef] | [Google Scholar]
- Manogaran S, Kannan KP, Mathiyalagan Y. Fungal endophytes from (L.) and (L.). Int Res J Pharm. 2017;8(10):86-9. [CrossRef] | [Google Scholar]
- Carvalho CR, Gonçalves VN, Pereira CB, Johann S, Galliza IV, Alves TMA, et al. The diversity, antimicrobial and anticancer activity of endophytic fungi associated with the medicinal plant (Mart.) Coville (Fabaceae) from the Brazilian savannah. Symbiosis. 2012;57(2):95-107. [CrossRef] | [Google Scholar]
- Petrini O, Stone J, Carroll FE. Endophytic fungi in evergreen shrubs in western Oregon: a preliminary study. Can J Bot. 1982;60(6):789-96. [CrossRef] | [Google Scholar]
- Damm U, Johnston PR, Damm U. R. The Colletotrichum gloeosporioides species complex. Stud Mycol. 2012;73(1):115-80. [PubMed] | [CrossRef] | [Google Scholar]
- Lu Y, Chen C, Chen H, Zhang J, Chen W. Isolation and identification of endophytic fungi from and investigation of their bioactivities. Evid Based Complement Alternat Med. Array [PubMed] | [CrossRef] | [Google Scholar]
- Espinel-Ingroff A, Montero D, Martin-Mazuelos E. Long-term preservation of fungal isolates in commercially prepared cryogenic microbank vials. J Clin Microbiol. 2004;42(3):1257-9. [PubMed] | [CrossRef] | [Google Scholar]
- Ferrari BC, Zhang C, Van Dorst J. Recovering greater fungal diversity from pristine and diesel fuel contaminated sub-Antarctic soil through cultivation using both a high and a low nutrient media approach. Front Microbiol. 2011;2:217 [PubMed] | [CrossRef] | [Google Scholar]
- Song Z, Sun YJ, Xu S, Li G, Yuan C, Zhou K, et al. Secondary metabolites from the endophytic fungi Fusarium decemcellulare F25 and their antifungal activities. Front Microbiol. 2023. 2023;14 [PubMed] | [CrossRef] | [Google Scholar]
- Wen J, Okyere SK, Wang J, Huang R, Wang Y, Liu L, et al. Endophytic fungi isolated from Ageratina adenophora exhibits potential antimicrobial activity against multidrug resistant . Plants (Basel). 2023;12(3):650 [PubMed] | [CrossRef] | [Google Scholar]
- Deepika M, Gomathi N. Isolation of endophytic fungi from green pepper () and analysis of phytochemicals and their antioxidant and antibacterial activity. Int J Sci Res. 2023;12(2):359-66. [PubMed] | [CrossRef] | [Google Scholar]
- Oktiansyah R, Elfita E, Widjajanti H, Setiawan A, Mardiyanto M, Nasution SS, et al. Antioxidant and antibacterial activity of endophytic fungi isolated from the leaves of Sungkai Peronema canescens. Trop J Nat Prod Res. 2022;7(3):2596-604. [PubMed] | [CrossRef] | [Google Scholar]
- Kokate CK, Purohit AP, Gokhale SB. Text book pharmacognosy Nirali Prakashan. 57. 2004;93:132 [PubMed] | [CrossRef] | [Google Scholar]
- Khandelwal KR. Practical pharmacognosy techniques and experiments. Pune: Nirali Prakashan. 2004:149-53. [PubMed] | [CrossRef] | [Google Scholar]
- Manikandan R, Beulaja M, Thiagarajan R, Palanisamy S, Goutham G, Koodalingam A, et al. Biosynthesis of silver nanoparticles using aqueous extract of L. fruits and characterization of its anti-inflammatory effect against HO exposed rat peritoneal macrophages. Process Biochem. 2017;55:172-81. [CrossRef] | [Google Scholar]
- Angamuthu J, Ganapathy M, Evanjelene VK. Evaluation of antioxidant activity of . World J Pharm Pharm Sci. 2016;8(11):1011-6. [CrossRef] | [Google Scholar]
- Zheng XH, Yang J, Lv JJ, Zhu HT, Wang D, Xu M, et al. Phyllaciduloids A-D: Four new cleistanthane diterpenoids from (L.) Skeels. Fitoterapia. 2018;125:89-93. [PubMed] | [CrossRef] | [Google Scholar]
- Angamuthu J, Ganapathy M, Evanjelene VK. Evaluation of antioxidant activity of . World J Pharm Pharm Sci. 2016;5(10):1011-6. [PubMed] | [CrossRef] | [Google Scholar]
- Chaimum-aom N, Chomko S, Talubmook C. Toxicology and Oral glucose Tolerance Test (OGTT) of Thai Medicinal Plant Used for Diabetes controls, L. (Euphorbiaceae). Pharmacogn J. 2017;9(1):58-61. [PubMed] | [CrossRef] | [Google Scholar]
- Uddin MS, Mamun AA, Hossain MS, Ashaduzzaman M, Noor MAA, Hossain MS, et al. Neuroprotective effect of L. on learning and memory impairment in scopolamine-induced animal model of dementia and oxidative stress: natural wonder for regulating the development and progression of Alzheimer’s disease. Adv Alzheimers Dis. 2016;5(2):53-72. [PubMed] | [CrossRef] | [Google Scholar]
- Hossain M, Akter S, Begum Y, Bulbul I. Analgesic and anti-inflammatory activities of ethanolic leaf extract of L. on Swiss albino mice. Eur J Med Plants. 2016;13(1):1-10. [CrossRef] | [Google Scholar]
- Abd Ghafar SZA, Mediani A, Maulidiani NS, Ramli NS, Antioxidant Abas F. α-glucosidase, and nitric oxide inhibitory activities of and LC–MS/MS profile of the active extract. Food Biosci. 2018;25:134-40. [CrossRef] | [Google Scholar]
- Duong TH, Bui XH, Le Pogam PL, Nguyen HH, Tran TT, Nguyen TAT, et al. Two novel diterpenes from the roots of (L.) Skeel. Tetrahedron. 2017;73(38):5634-8. [CrossRef] | [Google Scholar]
- Sousa M, Ousingsawat J, Seitz R, Puntheeranurak S, Regalado A, Schmidt A, et al. An extract from the medicinal plant and its isolated compounds induced airway chloride secretion: A potential treatment for cystic fibrosis. Mol Pharmacol. 2007;71(1):366-76. [PubMed] | [CrossRef] | [Google Scholar]
- Vijayalakshmi A, Ravichandiran V, Malarkodi V, Nirmala S, Jayakumari S. Screening of flavonoid ”quercetin” from the rhizome of Linn. for anti-psoriatic activity. Asian Pac J Trop Biomed. 2012;2(4):269-75. [PubMed] | [CrossRef] | [Google Scholar]
- Sanghavi N, Srivastava R, Malode Y. Isolation and identification of the flavonoid ”quercetin” from Linn. Int J Pharm Sci Res. 2014;5(4):1454-9. [PubMed] | [CrossRef] | [Google Scholar]