ABSTRACT
Background
Capecitabine (CAP), a BCS class-I drug which is used for the treatment of Colorectal Cancer (CRC). Site specific and enzyme activated drug delivery was achieved by albumin coated nanoparticles. Co-administration of Proton Pump Inhibitors (PPI) with the tablet dosage form of CAP lowers the dissolution of the CAP due to elevated gastric pH, resulting in decreased CAP absorption. To overcome this absorption, issue the albumin coated CAP NPs were administered through rectal route as suppositories dosage form.
Materials and Methods
The capecitabine nanoparticles were prepared using different concentrations of Poly Lactic-co-Glycolic Acid (PLGA) polymer by salting-out technique and were characterized. Further, the optimized polymeric nanoparticles were coated with Egg Albumin (EA) using a glutaraldehyde cross-linker and characterized for their particle size, zeta potential, polydispersity index, drug content, SEM, and in vitro drug release. The suppositories were prepared by fusion moulding method using different grades of PEG as a base and evaluated for various parameters and in vitro drug release.
Results and Discussion
The particle size of the optimized CAP NPs and the EA coated CAP NPs were determined to be 171.7 nm and 239.6 nm respectively. The in vitro release of CAP from the EA coated NPs and the suppository formulation shows 88.3% and 81.0% respectively.
Conclusion
Capecitabine suppositories were formulated and the quality control parameters were assessed for the site specific and enzyme activated drug delivery in the management of colorectal cancer with help of literature references.
INTRODUCTION
Colorectal Cancer (CRC) is one of the leading causes of death and morbidity worldwide, making it a major public health issue. CRC is the second most frequent cancer in women and the third most common cancer in men worldwide. It accounts for almost 9% of all cancer incidences.1,2 About 41% of all colorectal malignancies are found in the proximal colon, 22% in the distal colon, and 28% in the rectum.3 The mortality rate of colorectal cancer has dropped by roughly 35% between 1990 and 2007 and is currently about 50% lower than peak mortality rates as a result of efficient screening procedures, early treatments, and better treatment options.4
The FDA approved the use of capecitabine, a prodrug of 5-fluorouracil that is classified as a BCS class-I drug, for the adjuvant treatment of Dukes’ stage C colorectal cancer. It is also used as monotherapy, or in combination with other agents for advanced or metastatic disease, and with concurrent radiation for the neoadjuvant treatment of rectal cancer.5 Following oral absorption, capecitabine undergoes its initial metabolism to 5-DFCR by carboxyl-esterase, which is primarily carried out in the liver, this enzyme is also present in colon and tumour tissues. Cytidine deaminase in the liver and also in tumour tissue converts the metabolite to 5-DFUR, and thymidine phosphorylase, an enzyme that is present in tumour tissue, converts it to5-FU intracellularly.6
The term “Nanoparticles” (NP) refers to a class of colloidal drug delivery methods that constitutes particles with diameters ranging from 10 to 1000 nm.7 Improved bioavailability, controlled drug release from a single dose, increasing residence time in the body, and the capacity to protect the drug until delivery to the desired site led to the investigation of polymeric nanoparticles.8,9A polymeric matrix that creates nano sized particles allows for the uniform and physical retention or adsorption of the medicament.10 Particularly Polylactic Acid (PLA), Polyglycolic Acid (PGA), and Poly Lactic-co-Glycolic Acid (PLGA) derived polymers that display properties of biodegradability and biocompatibility are systems used for the controlled and targeted distribution of pharmaceuticals.11
Enzyme-Activated Drug Delivery System (EADDS) is a class of rate-controlled systems that is biochemically activated. This kind of drug delivery is used to treat a variety of illnesses, including cancer. Chemotherapy, a typical sort of conventional cancer treatment, however, rapidly becomes toxic due to its non-specificity. The EADDS may resolve this kind of disadvantage. The protease enzyme found in cancer cells selectively targets proteins like albumin therefore the drug-coated with albumin can target the cancer cells. The protease enzyme works in this system to break down the albumin coat.12 Due to its selective distribution, non-toxicity, and non-immunogenicity, albumin is one of the biomolecules employed for targeted delivery and act as a carrier system.13
PPI withcapecitabine co-administration has recently generated controversy and studies indicate that they might lower capecitabine effectiveness and results in poor survival rates. The mechanism behind the drug-drug interaction is elevated gastric pH by the PPI use, that reduces the dissolution of CAP tablets, which in turn reduces the CAP absorption. Therefore, to overcome this interaction the rectal delivery of drug was focused.14
The rectal route of administration is beneficial for infants and children who have difficulty in swallowing oral medication. Suppository drug administration has the potential to provide both local and systemic effects. Both hydrophilic and lipophilic bases can be used to make suppositories. These suppositories disintegrate or melt in body fluids and releases the medication.15Suppositories are drug-filled solid bodies designed for rectal administration. Rectal drug delivery has several benefits, including decreased hepatic first-pass elimination of high clearance drugs, improved enzymatic drug stability, high drug loading capacity and avoidance of gastric irritation associated with some drugs in cases of nausea, vomiting and when the patient is unconscious.16
In this current investigation, the capecitabine nanoparticles were prepared using PLGA polymer, then coated with egg albumin and then loaded into suppository dosage form for rectal administration in order to target the drug to CRC specifically without affecting the normal cells and also to overcome the drugs first-pass metabolism.
MATERIALS AND METHODS
Materials
Capecitabine was received as a gift sample from Valary labs Pvt. Ltd., Visakhapatnam, Andhra Pradesh. PLGA (Sigma Aldrich, USA), PVA (Hi Media Labs, Mumbai), acetone (Lobachemie, Mumbai), egg albumin (Lobachemie, Mumbai), glutaraldehyde (Reachem Lab. Pvt. Ltd., Chennai), PEG 4000 (Lobachemie, Mumbai), and PEG 600 (SD Fine Chem., Mumbai) were used.
Methods
Preparation of Capecitabine nanoparticles
CAP nanoparticles were prepared by the salting-out technique. In the organic phase, CAP and different concentrations of PLGA (polymer) were dissolved in acetone. The aqueous phase is prepared by dissolving stabilizing agent (polyvinyl alcohol) and salting-out agent (magnesium chloride tetrahydrate) in the ratio of 1:3. The organic phase is gradually added to the aqueous phase while stirring which leads to the formation of nanoparticles.17
Coating of CAP NPs with egg albumin
The optimized CAP NPs were coated with egg albumin, 50 mg of CAP NPs were added to egg albumin solution which is prepared by 250 mg of egg albumin dissolved in 10 mL of water and stirred at room temperature for 1 hr. For cross-linking and stabilization, 50 μL of 0.25% v/v glutaraldehyde solution was added and stirred overnight at room temperature.18
Preparation of Suppositories by fusion method
In a preheated china dish, the base was melted at a controlled temperature with vigorous stirring, the egg albumin-coated CAP NPs were added and then thoroughly mixed with the base (PEG 4000 and PEG 600 in the ratio of 40:60). The resulting mixture was then filled into the mould to the point of overflowing, and let to cool on ice for 15 min to solidify. The mould was opened, and formulations were gently removed from the moulds and packed in aluminium foil.19
Characterization of CAP NPs
Particle size and Zeta potential
The particle size and zeta potential for the formulated CAP NPs were analysed using Malvern zeta sizer.20
Polydispersity Index (PDI)
The PDI is dimensionless and indicates that the sample is monodisperse or polydisperse in nature.21
Equivalent weight of capecitabine in preparation
10 mg of optimized CAP NPs (F2) were weighed and dissolved in 10 mL of pH 6.0 phosphate buffer, and the absorbance was noted using a UV-visible spectrophotometer at 239 nm after suitably diluted.22
Entrapment efficiency
Drug entrapment was determined for the optimized formulation (F2), the amount of drug present in the supernatant after centrifugation at 14000 rpm for 30 min was analysed by using UV-visible spectrophotometer at 239 nm.23
Characterization of Egg Albumin coated CAP NPs
Particle size, zeta potential and Polydispersity Index (PDI)
Equivalent weight of CAP in EA coated NPs
10 mg of EA coated CAP NPs were weighed and dissolved in 10 mL of pH 6.0 phosphate buffer, which determines the amount of drug present in the formulation. The solution was appropriately diluted, was measured using UV-visible spectrophotometer at 239 nm.22
Morphological evaluation by SEM
The morphology of the formulated nanoparticles was investigated using Field Emission Scanning Electron Microscopy (FE-SEM) from Carl Zeiss (USA), equipped with in lens detector, SE2 detector and BSD detector under a resolution of 1.5 nm.
In vitro drug release
The in vitro release of EA-coated CAP-loaded nanoparticles was performed at 37°C. In short, the CAP-loaded nanoparticle dispersion was dialyzed against a 100 mL buffer solution while being retained in a dialysis membrane (pH 6.0, receiver solution). 1 mL of the receiver solution was removed and replenished at regular intervals with the same amount of solution. With a UV-visible spectrophotometer, the absorbance was measured at 239 nm.24
Evaluation of Suppositories
Appearance
The physical appearance of the EA-coated CAP NPS-loaded suppositories on their outer surface were examined for smoothness or gritty condition.19
Weight variation (IP)
Briefly, the average weight of 10 suppositories was determined after each one was weighed individually. Not more than two individual weights differ by more than 5% from the average weight, and none differ by more than 10%.
Hardness
The prepared suppositories were tested for its hardness using Erweka hardness tester equipment. The amount of force needed to cause a suppository to break is used as a gauge for the suppository’s hardness. To establish whether the suppositories could endure the risks of packaging and shipment, a hardness test or fracture point test was conducted to ascertain their tensile strength.[25]
Disintegration test (IP)
The disintegration time of the suppository was determined using Lab India tablet disintegrator, type 1 apparatus. In a nutshell, the test suppository was put in a cylindrical glass container with perforated ends, submerged in 1000 mL of buffer solution, and kept at a temperature of 37.5°C. The glass cylinder was positioned in the buffer and moved up and down. The amount of time needed for the suppository to completely melt in the medium was the time for disintegration. Six distinct suppositories’ mean values were calculated.
Macro melting range test
The capillary technique was used to determine the macro melting range, one end of the tube was inserted into the suppository formulation, and enough was packed to fill capillary tubes with a 1 cm column and submerged in a water-filled beaker. The temperature was gradually increased, and it was noted at which point the mass liquefies.26
Liquefaction time and temperature
Using a constructed instrument, liquefaction time and temperature were determined. A large pipette was used, which had a wide entrance on one side and a narrow opening on the other. In hot water that was kept at 37°C, the pipette was submerged. The narrow end is now towards the hot water. The sample suppository was gently pushed down the pipette’s length from the top via the pipette’s broad end until it reached the narrow end. After that, a glass rod was inserted so that it was resting on top of the suppository. It was observed that the liquefaction temperature corresponds to the temperature at which the glass rods have just started to fall. The liquefaction time is the point at which the glass rod reaches the narrow end after the suppositories have completely melted.27
Drug content
Drug content were determined for suppositories by dissolving in 100 mL of pH 7.4 phosphate buffer and slowly stirred with a magnetic stirrer at 37°C for 1 hr. After filtering the solution, the filtrate was appropriately diluted, and analyzed at 239 nm.28
In vitro drug release
EA coated CAP NPs loaded suppositories were tested for in vitrodissolution in a USP dissolution apparatus, Type-I (rotating basket apparatus) and the dissolution medium was phosphate buffer (pH 7.4) with 100 rpm at 37 ± 0.4°C. 5 mL of sample were taken at predefined intervals and filtered using Whatman filter paper. The same amount of fresh medium was replaced, in order to maintain sink conditions. The samples were analyzed at 239 nm using a UV-visible spectrophotometer.25
In vitro drug release kinetics
The in vitro release data were fitted into various kinetic models of zero order, first order, Higuchi diffusion models, Korsmeyer-Peppas model, Hixson-Crowell model, and Weibull model in order to determine the drug kinetics using DD solver software.
RESULTS AND DISCUSSION
Preparation of CAP NPs
The CAP NPs were prepared by using salting-out technique, which has a salting-out agent, polymer and a stabilizing agent.
Characterization of CAP NPs
Particle size and Zeta potential
The particle size of the optimized formulation (F2) of CAP NPs was determined to be 171.7 nm. This value makes it evident that the nanoparticle’s in nanometer range. The stability of the nanoparticles determined by the surface charge. The zeta potential for the formulation was found to be -20.8 mV as shown in Table 1.
| Formulation code | Drug (%) | Polymer (%) | Stabilizing agent (%) | Salting-out agent (%) | Particles size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|---|---|---|---|
| F1 | 1 | 1 | 0.5 | 1.5 | 164.7 | 0.245 | -18 |
| F2 | 1 | 1.5 | 0.5 | 1.5 | 171.7 | 0.160 | -20.8 |
| F3 | 1 | 2 | 0.5 | 1.5 | 194.2 | 0.317 | -26.2 |
Polydispersity Index (PDI)
The polydispersity index of the optimized formulation (F2) of CAP NPs was found to be 0.160, which indicates that the NPs are monodisperse in nature.
Entrapment efficiency
The entrapment efficiency for the CAP NPs (F2 formulation) were estimated to be 69.07%.
Equivalent weight of capecitabine in preparation
10 mg of CAP NPs (F2 formulation) was dissolved in buffer and absorbance was noted in UV-Visible spectrophotometer at 239 nm appropriately diluted and found that 10 mg of CAP was present in 43.66 mg of preparation.
Coating of CAP NPs using egg albumin
The optimized CAP NPs (F2 formulation) was coated using egg albumin with the help of a cross-linking agent.
Characterization of EA coated CAP NPs
Particle size, zeta potential and Polydispersity Index (PDI)
Particle size, zeta potential, and polydispersity index for egg albumin coated capecitabine nanoparticles were reported to be 239.6 nm, -26. 2mV and 0.191 correspondingly.
Equivalent weight of capecitabine in preparation
10 mg of EA-coated CAP NPs were determined in pH 6.0 phosphate buffer and the absorbance was noted using a UV-visible spectrophotometer at 239 nm. 10 mg of capecitabine was found in 124.68 mg of coated NPs.
Scanning Electron Microscopy (SEM)
The SEM image for the EA coated CAP NPs were shown in Figure 1.
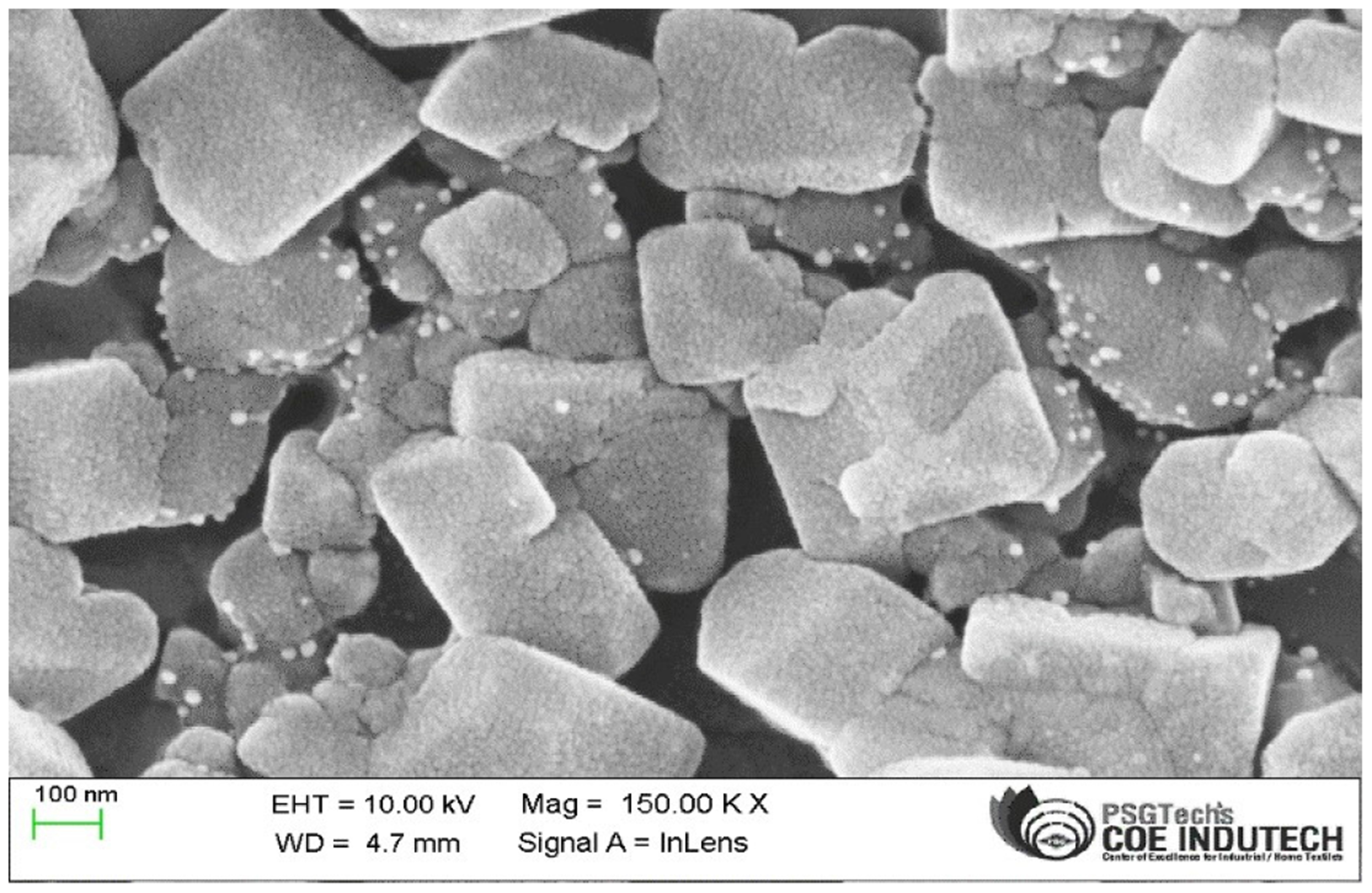
Figure 1:
SEM images of EA-coated CAP NPs.
In vitro drug release
The in vitro drug release for the EA-coated CAP NPs were found to be 88.34% in 180 min as depicted in Figure 2.
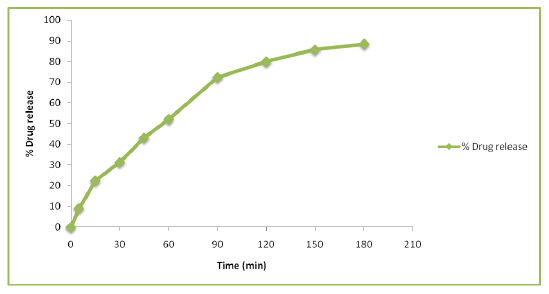
Figure 2:
In vitro release study of EA-coated CAP NPs.
Preparation of suppositories
The EA coated CAP NPs was incorporated into suppository bases using fusion method to make a suppository dosage form.
Evaluation of suppositories
The suppositories were well-formed and had a smooth glimmering surface with creamy white colour. After being longitudinally cut, they exhibited no fissures, cracks, contraction holes, or air bubbles trapped inside.
All 10 suppositories were found to be within the IP limit (1.48g-1.51g with an average weight of 1.49g) as none deviates from the acceptance range, it passes the test.
The hardness for EA-coated CAP NPs loaded suppositories was found to be 2.04 kg/cm2.
The disintegration time of the EA-coated CAP NPs loaded suppositories was found to be 7 min 20 sec, which was within the IP acceptance range, therefore it passes the test.
The macro melting range test was performed for the EA-coated CAP NPs loaded suppositories and the temperature was found to be 56°C.
The time required for the suppository to liquefy under pressure is known as the liquefaction time. The formulated suppositories using PEG 4000 and PEG 600 (40:60) have liquefaction time and temperature in the range of 18-22 min and 52-56°C respectively.
The estimated drug in the formulations was found to be 96.5% and passed the test.
In vitro drug release
The formulated suppositories show drug release of 81% in phosphate buffer of pH 7.0 at the end of 180 min was shown in the Figure 3.
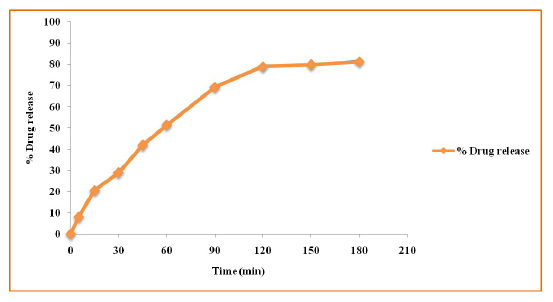
Figure 3:
In vitro drug release study for EA-coated CAP NPs loaded suppository.
In vitro drug release kinetics
To establish the best feasible mechanism for CAP release from suppositories loaded with EA-coated CAP nanoparticles, multiple models were utilised in the current experiment. From the reported data, it was found that the Weibull model fits CAP the best when compared to other models and exhibits better linearity (R2 = 0.9875) and shows a matrix type of release.
CONCLUSION
The EA coated CAP NPs was loaded into suppositories and the quality parameters were assessed. The optimized CAP NPs were found to have a particle size, zeta potential and PDI of 171.7 nm, -20.8 mV and 0.160 respectively and the optimized CAP NPs were coated using egg albumin. The EA coated CAP NPs was further characterized, the particle size, zeta potential and PDI was determined to be 239.6 nm, -26.2 mV and 0.191 respectively and shows the in vitro release of 88.3%. The EA coated CAP NPs were loaded into suppositories and were evaluated for various quality parameters, the in vitro release was found to be 81% in 180 min and drug release kinetics fits well in Weibull model which shows matrix type of release. Based on the literature references, the albumin coating might have EADD and administration through the rectal route might overcome the drug-drug interaction with PPI.
Cite this article
Mythili G, Subramanian S. Development of Albumin Coated Nanoparticulate Capecitabine Suppositories. Int. J. Pharm. Investigation. 2023;13(4):772-7.
ACKNOWLEDGEMENT
We are thankful to PSG College of Pharmacy for providing me with needed facilities and continuous support throughout and Valary Labs Pvt. Ltd., Visakhapatnam, Andhra Pradesh for providing me the Capecitabine drug as gift sample.
ABBREVIATIONS
| CAP | Capecitabine |
|---|---|
| CRC | Colorectal cancer |
| EA | Egg albumin |
| PPI | Proton pump inhibitors |
| EADDS | Enzyme activated drug delivery system |
| PLGA | Poly lactic-co-glycolic acid |
| PVA | Poly vinyl alcohol |
| PEG | Poly ethylene glycol |
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-917. [Google Scholar]
- Cheng L, Eng C, Nieman LZ, Kapadia AS, Du XL. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. J Clin Oncol. 2011;34(6):573-80. [Google Scholar]
- Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, Jemal A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177-93. [Google Scholar]
- Johnston PG, Kaye S. Capecitabine: a novel agent for the treatment of solid tumors. Anti-cancer drugs. 2001;12(8):639-46. [Google Scholar]
- Shimma N, Umeda I, Arasaki M, Murasaki C, Masubuchi K, Kohchi Y, et al. The design and synthesis of a new tumor-selective fluoropyrimidine carbamate, capecitabine. Bioorg Med Chem. 2000;8(7):1697-706. [Google Scholar]
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17-71. [Google Scholar]
- Hrkach J, Von Hoff D, Ali MM, Andrianova E, Auer J, Campbell T, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128):128ra39 [Google Scholar]
- Rizvi SA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharmaceut J. 2018;26(1):64-70. [Google Scholar]
- Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles-based drug delivery systems. Colloids Surf B. 2010;75(1):1-8. [Google Scholar]
- Jeevitha D, Amarnath K. Chitosan/PLGA nanoparticles as a novel carrier for the delivery of anthraquinone: synthesis, characterization and cytotoxicity evaluation. Colloids Surf B. 2013;101:126-34. [Google Scholar]
- Thornton PD, Mart RJ, Ulijn RV. Enzyme-responsive polymer hydrogel particles for controlled release. Adv Mater. 2007;19(9):1252-6. [Google Scholar]
- Yewale C, Baradia D, Vhora I, Misra A. Proteins: emerging carrier for delivery of cancer therapeutics. Expert Opin Drug Deliv. 2013;10(10):1429-48. [Google Scholar]
- Kitazume Y, Kawazoe H, Uozumi R, Yoshizawa T, Iihara H, Fujii H, et al. Proton pump inhibitors affect capecitabine efficacy in patients with stage II–III colorectal cancer: a multicenter retrospective study. Sci Rep. 2022;12:6561 [Google Scholar]
- Swarbrick J. Encyclopedia of Pharmaceutical Technology: Volume 6. 2013 [Google Scholar]
- Jain NK. Progress in controlled and novel drug delivery systems CBSS. Gopalakrishnan. J pharm sci technol. 2004;3(2):84-5. [Google Scholar]
- Eley JG, Pujari VD, McLane J. Poly (lactide-co-glycolide) nanoparticles containing coumarin-6 for suppository delivery: release profile and tissue distribution. Drug deliv. 2004;11(4):255-61. [Google Scholar]
- Sattarahmady N, Azarpira N, Hosseinpour A, Heli H, Zare T. Albumin coated arginine-capped magnetite nanoparticles as a paclitaxel vehicle: Physicochemical characterizations and evaluation. J pharm sci technol. 2016;36:68-74. [Google Scholar]
- Basavaraj BV, Devi S, Bharath S, Deveswaran R, Madhavan V. Design and evaluation of sustained release pro-pranolol hydrochloride suppositories. IJPSR. 2013;22:5-12. [Google Scholar]
- Pignatello R, Ricupero N, Bucolo C, Maugeri F, Maltese A, Puglisi G, et al. Preparation and characterization of eudragit retard nanosuspensions for the ocular delivery of cloricromene. Aaps Pharm sci tech. 2006;7:E192-8. [Google Scholar]
- Nidhin M, Indumathy R, Sreeram KJ, Nair BU. Synthesis of iron oxide nanoparticles of narrow size distribution on polysaccharide templates. Bull Mater Sci. 2008;31:93-6. [Google Scholar]
- Nagarajana E, Shanmugasundarama P, Ravichandirana V, Vijayalakshmia A, Senthilnathanb B, Masilamanib K, et al. Development and evaluation of chitosan based polymeric nanoparticles of an antiulcer drug lansoprazole. J Appl Pharm Sci. 2015;5(4):20-5. [Google Scholar]
- Das S, Banerjee R, Bellare J. Aspirin loaded albumin nanoparticles by coacervation: implications in drug delivery. Trends BiomaterArtif Organs. 2005;18(2):203-13. [Google Scholar]
- Gaihre B, Khil MS, Lee DR, Kim HY. Gelatin-coated magnetic iron oxide nanoparticles as carrier system: drug loading and drug release study. Int J Pharm. 2009;365(1-2):180-9. [Google Scholar]
- Baviskar P, Jaiswal S, Sadique S, Landged A. Formulation and evaluation of lornoxicam suppositories. Pharmainnov. 2013;2:20 [Google Scholar]
- Sah LM, Saini RT. Formulation development and release studies of indomethacin suppositories. Indian J Pharm Sci. 2008;70:498-501. [Google Scholar]
- Biyani DM, Ranjan P, Chandrashekhar A. Cow Ghee as a Base for Diclofenac Sodium Suppositories. World J Pharm Pharm Sci. 2012;1(3):1180-87. [Google Scholar]
- De MC, Lefebvre RA, Remon JP. Study of the bioavailability of four indomethacin suppository formulation in healthy volunteers. Int J Pharm. 1994;104(1):87-91. [Google Scholar]
