ABSTRACT
ABSTRACT
Introduction
SLNs have a surfactant-stabilized solid lipid core. They circumvent liquid lipid constraints and increase drug stability. Lipids promote oral medication absorption through the lymphatic pathway and replace hepatic first-pass metabolism. Using a 2-factor, 3-level, 32 full factorial design, the researchers optimized percentage Encapsulation Efficiency (%EE), drug content, and particle size to enhance the candesartan cilexetil-loaded SLN formulation.
Materials and Methods
The BCS class II low soluble Candesartan was used to formed as SLN and optimized utilizing independent variables Imwitor 900K and tween 60 concentrations and dependent factors %EE, drug content, and particle size. FTIR showed no drug-excipient interaction. Along with in vitro drug release.
Results
The optimized 13 formulations had %EE from 78.47 to 94.61, drug concentration from 57 to 107%, and particle size from 357 to 705 nm. SEM showed that certain particles were coagulated and near-spherical, supporting particle size and dispersion. C-SLN-7 released 60.39% and C-SLN-12 46.4% during 24 hr in vitro. BCS class II low soluble CC’s calibration curve was a straight line with an R2 value of 0.9978, indicating the drug’s standard curve. The improved SLN formulation may improve oral bioavailability of poorly soluble medicines.
Conclusion
The enhanced SLN formulation of candesartan cilexetil increased encapsulation efficiency, drug content, particle size, and in vitro drug release. Changes in lymphatic absorption may improve oral absorption of highly lipophilic medicines. The work sheds light on SLNs’ lipophilic drug delivery potential.
INTRODUCTION
The invention of new medications is not enough to assure improvement in drug treatment. Treatments may fail due to insufficient drug content due to fast metabolism, poor absorption, and excretion.1 Solid Lipid Nanoparticles (SLNs) are composed of a stable solid lipid core at the interface which is stabilized by a surfactant. Solid lipids are employed make SLNs to reduce the problem associated with liquid lipids and to improve the stability. The methods viz., solvent evaporation, high-pressure homogenization, ultrasonication, solvent emulsification etc., are claimed to be employed in the formulation of solid lipid nanoparticles.1–5 Lipids enhance oral drug absorption by altering absorption through the lymphatic pathway. For highly lipophilic medicines, the lymphatic pathway is recommended as a substitute to overcome first-pass metabolism in the liver. Lipophilic drugs are integrated into lipid particles, absorbed into the lymphatic circulation. Eventually, directed to the systemic circulation. The development of a novel oral lipid formulation of candesartan cilexetil is to improved bioavailability. SLNs are lipid nanoparticles that are submicron colloidal carriers with sizes ranging from 10-1000 nm. They have been suggested to be a promising carrier system for medications, with advantages such as lymphatic uptake, tolerability, and biodegradability.6 Because of their ability to overcome the constraints of earlier colloidal carriers, SLNs have attracted the interest of many researchers.7,8 SLNs are considered an excellent alternative to polymeric nanoparticles, nano emulsions, microemulsions, self-emulsifying and liposomes drug delivery systems.9–12 Candesartan Cilexetil (CC) is an BCS class II (low soluble, highly permeable) angiotensin II receptor blocker that acts on ACE inhibitors and is primarily used in the treatment of hypertension and heart disease, 32g per day is ideal. It has poor oral absorption; hence the drug candesartan cilexetil solid lipid nanoparticles is prepared.13 Studies show that formulating candesartan as solid lipid nanoparticles enhances the drug’s pharmacodynamic and pharmacokinetic profile and enhance oral bioavailability.3,14-16 In this study, the 2-factor, 3-level 32 full factorial design was used to optimize the SLN formulation. with independent variables as the concentration of polymer and surfactant and dependent variables as percentage Encapsulation Efficiency (%EE), drug content, and particle size. The optimized preparation is evaluated for %EE, drug content, particle size and in vitro release.
MATERIALS AND METHODS
Materials
Lipid-Imwitor 900K is kindly gifted from IOI Oleo GmbH, Germany. Drug-Candesartan cilexetil has been used, which is gifted by Hetero Drug Limited, Hyderabad. Polysorbate 60 (Tween 60), Sodium Hydroxide (NaOH), Phosphate buffer, and ethanol was obtained from SRL chem, Chennai, India. All materials utilized in the study are analytical grade.
Methods
Preparation of standard curve
10mg of CC is dissolved in 10mL on 0.1N NaOH in a standard flask and labeled as primary stock. From this 1 mL solution was taken in a separate standard flask and the volume was made to 10 mL with 0.1N NaOH. Successive dilutions comprise the strength of CC for 2μg/mL, 4μg/mL, 6μg/mL, 8μg/mL, and 10μg/mL were prepared by the same procedure furthermore observed under Ultraviolet-visible Spectroscopy (UV) absorbance at 249 nm using 0. 1N NaOH as blank for the preparation of standard curve.
Optimization of the formula
Formulation development by Design of Experiments (DoE) is a logical practice for planning the experiments to achieve the desired goal with proper rationalization. Commercially available software (Design Expert, version 11.0, Stat-Ease Inc, MN). The experimental design was chosen based on response surface, 2 factors, 3-level factorial, quadratic with no blocks, and 13 run formulations were formulated, out of which five as replicates (Table 1). The run orders were kept randomized to prevent the time-related variables and satisfy the independent variables statistical analysis. Analysis of Variance (ANOVA) and all other statistical analysis were performed in the same software for the suitable model selection. Calculations of the response variable or independent variables were performed; normal and response surface plots were plotted.
| Variable type | Variable | Levels | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| Independent | Concentration of Imwitor 900K (X1). | 0.12 | 2 | 4.12 |
| Concentration of Tween 60 (X2). | 0.79 | 1.5 | 2.21 | |
| Dependent | % Entrapment efficiency (Y1). | 78.47 | 84.28 | 94.68 |
| Drug content (Y2). | 57.44 | 78.04 | 107.94 | |
| Particle size (Y3). | 357 | 527.9 | 705 | |
The two factors-concentration of Imwitor (X1) and the concentration of Tween 60 (X2) were considered in the formulation of SLN. Using these concentration 13 formulations, Candesartan cilexetil Solid Lipid Nanoparticles formulations 1 to 13 (C-SLN 1-13) was developed and evaluated for the dependent variables of “% entrapment efficiency (Y1), drug content (Y2), and particle size (Y3)”. Based on the results the optimized formulation was selected.
Preparation of CC-loaded SLN
Aqueous-based formulation approach was used to prepare SLN, which is aimed not to use of any alcohols and other organic solvents. Firstly, a precisely weighed quantity of Imwitor 900 K was heated at 80-85°C separately in a beaker to melt the lipid phase. An accurately weighed quantity of drug CC was added to this molten lipid phase to dissolve the lipophilic drug to get a clear lipid drug mixture. At the same time, water was heated along with accurately weighed quantity Tween-60 at 80-85°C separately for 5 min to get the equilibrium temperature of both the mixture. Then hot melted lipid phase containing the drug was transferred slowly using a hypodermic syringe to (80-85°C) the aqueous mixture containing nonionic surfactant Tween 60. The entire mixture was furthermore positioned on the magnetic stirrer and stirred continuously for 10 min at 600 rpm to get a milky like mixture. After this, mixture has ultrasonicated using a probe sonicated at 6:3 pulse sorts with 25% amplitude for 20 min in an ice bath. The sonicated mixtures were kept in the pre-freezer for 6 hr at -20°C and then subjected to freeze drying for 6 hr. The developed CC-loaded SLNs were kept in the refrigerated condition using Eppendorf tubes for further in vitro evaluations. The formulated SLN compositions are given in Table 2.
| Sl. No | Formulation Code | Formulation Code | ||
|---|---|---|---|---|
| Candesartan Cilexetil (mg) | Imwitor 900 K | Tween 60 | ||
| 1 | C-SLN-1 | 1 | 2 | 0.79 |
| 2 | C-SLN-2 | 1 | 0.5 | 1 |
| 3 | C-SLN-3 | 1 | 0.12 | 1.5 |
| 4 | C-SLN-4 | 1 | 0.5 | 2 |
| 5 | C-SLN-5 | 1 | 3.5 | 2 |
| 6 | C-SLN-6 | 1 | 2 | 1.5 |
| 7 | C-SLN-7 | 1 | 3.5 | 1 |
| 8 | C-SLN-8 | 1 | 4.12 | 1.5 |
| 9 | C-SLN-9 | 1 | 2 | 1.5 |
| 10 | C-SLN-10 | 1 | 2 | 1.5 |
| 11 | C-SLN-11 | 1 | 2 | 1.5 |
| 12 | C-SLN-12 | 1 | 2 | 1.5 |
| 13 | C-SLN-13 | 1 | 2 | 2.21 |
Drug excipient compatibility studies by FTIR
Excipients may interact with drug molecules and lead to chemical changes. The compatibility of excipients interaction resulting the justification for the selection of suitable excipients in the developmental stage of formulation. The reciprocal action among the lipids and drug was determined from the Fourier Transform-Infrared (FTIR) spectroscopic studies. The FTIR spectrum of pure drug, lipid, surfactant and combination of all were obtained by using a Shimadzu FT-IR Spectrophotometer. The range for scanning is 4000-400 cm1. Measurements were performed by using compression of potassium bromide KBr pellets with samples.
Determination of Particle Size
The size of the developed C-SLNs was demonstrated by using Nano-S90 Zeta Sizer (Malvern Instruments, UK). The analysis was achieved with a constant scattering angle of 90°C and an equilibrated temperature of 25°C. Prior to the testing, every sample was thoroughly diluted with distilled water.
Determination of Surface morphology
The surface morphology of the developed C-SLNs was performed using Scanning Electron Microscopy (SEM). 1 mg of sample was taken, dispersed in the solvent, and prepared in different dilutions. Samples were prepared as a thin film on aluminum foil, and images were taken by FEI Quanta FEG 200-High Resolution Scanning Electron Microscope (HRSEM).
Drug Content
1 mg of C-SLNs was transferred into 5 mL of ethanol to dissolve it, and the volume was made up to 10 mL with ethanol in the standard flask. Then take 1 mL of this solution and make up the volume to 10 mL with 0.1N NaOH and observe under Ultraviolet Visible Spectroscopy (UV) at 249nm using the same bank in reference cell.
Entrapment Efficiency
Entrapment efficiency in C-SLNs was determined using centrifugation technique for the separation of non-entrapped Drug from SLNs. Briefly, 1.5 mL of C-SLN was taken in Eppendorf tube and centrifuged at 15,000 rpm for 30min at 4°C. 100μL of the supernatant was taken and made up to 10 mL with 1:1 ethanol and 0.1N NaOH, respectively. The absorbance was noted in UV at 249 mm.
In vitro Drug release
Drug release from the developed SLN was carried out by dialysis bag technique using dialysis membrane with phosphate buffer of pH 7.4 as dissolution media. The dialysis bag was prepared by soaking the dialysis membrane at the required size in the phosphate buffer for 2 hr and tied at one end with the nylon thread. 1 mL of C-SLNs was transferred carefully to the previously prepared dialysis bag and tied it carefully with nylon thread. The residues of the Candesartan cilexetil were washed with phosphate buffer pH 7.4, and the drug-containing dialysis bag was immediately transferred to the beaker that contains100 mL of phosphate buffer pH 7.4 and stirred at low rpm using a magnetic stirrer. At specified intervals (0.5, 1, 1.5, 2, 3, 4, 6, 8, and 12 hr), the entire setup was kept at 37°C. 1 mL of the sample was taken out and replaced with an equal volume of fresh phosphate buffer at pH 7.4 to maintain the sink condition.
RESULTS
The standard graph of pure drug CC was obtained as linear in the concentration range of 2 to 10 μg/mL, as shown in Table 3 and Figure 1. The regression coefficient of the calibration curve was found to be 0.9978.
| Drug concentration (μg/mL) | Absorbance 249nm |
|---|---|
| 2 | 0.092 |
| 4 | 0.194 |
| 6 | 0.297 |
| 8 | 0.421 |
| 10 | 0.542 |
The optimization of C-SLN formulation was performed using response surface methodology based on the criteria for achieving the highest drug content, %EE and minimum particle size. The optimization process suggests three formulations having the dependent variable with a greater desirability value (close to 1), indicating the suitability of the SLN preparation. Table 4 contains the summary of the results of regression analysis from the optimized design for the SLN formulation. The contour plot of %EE, drug content, particle size and response surface plot of %EE, drug content and particle presented in Figure 2.
| Models | R2 | Adjusted R2 | Predicted R2 | SD | %CV | Remarks |
|---|---|---|---|---|---|---|
| % Entrapment Efficiency | ||||||
| Linear | 0.7110 | 0.6532 | 0.3819 | 2.68 | ||
| 2FI | 0.7728 | 0.6971 | 0.4195 | 2.50 | ||
| Quadratic | 0.9242 | 0.8701 | 0.4612 | 1.81 | 1.64 | Suggested |
| Drug Content % | ||||||
| Linear | 0.7239 | 0.6686 | 0.3917 | 8.74 | 9.99 | Suggested |
| 2FI | 0.7258 | 0.6344 | -0.0530 | 9.18 | ||
| Quadratic | 0.7432 | 0.5598 | -0.8262 | 10.07 | ||
| Particle Size | ||||||
| Linear | 0.4715 | 0.3658 | 0.0963 | 104.80 | ||
| 2FI | 0.4835 | 0.3113 | -0.0209 | 109.21 | ||
| Quadratic | 0.9076 | 0.8415 | 0.3426 | 52.39 | Suggested | |
The FTIR spectra of pure drug CC, CC combination with excipients, and C-SLNs formulations were studied to determine the compatibility of the drug and excipients. FTIR studies were performed, and the graphs are represented in Figure 3. The result indicateds that the prominent peak of CC was retained when combined with excipients and SLN formulation, indicating no interaction with excipients. Hence the selected excipients are used in the formulation development of SLN.
The physico-chemical characterizations such as %EE, drug content, and particle size of the developed candesartan cilexetil-loaded SLN were represented in Table 5. All the developed C-SLN were shown between 357 to 705 nm particle size and shows greater drug content ranging from 57 to 107%, good entrapment efficiency from 78.47 to 94.61. This may achieve by employing variable concentration of surfactant and lipid concentration along with optimum sonication. The result indicates that the increase in the surfactant concentration as well as the lipid concentration leads to a decrease in particle size and an increase in entrapment efficiency and drug content. Formulation C-SLN-8 has the highest %EE of 94.68, with drug content of 107.94 and a particle size of 705 (Figures 4 and 5). The surface morphology of the developed C-SLNs was studied DLS, and SEM analysis shows the particle size and distribution. The photomicrograph of formulation C-SLN-8 shows the spherical nature of the lipid particle (Figure 5). All the analysis was done for triplicate times (Table 5).
| Formulation code | % EE | Drug content | Particle Size |
|---|---|---|---|
| C-SLN-1 | 89.48 | 57.44 | 676.2 |
| C-SLN-2 | 84.28 | 77.14 | 403 |
| C-SLN-3 | 78.47 | 68.94 | 457.7 |
| C-SLN-4 | 91.18 | 78.04 | 394.3 |
| C-SLN-5 | 93.40 | 102.04 | 527.9 |
| C-SLN-6 | 91.64 | 86.64 | 357 |
| C-SLN-7 | 94.34 | 105.74 | 636.6 |
| C-SLN-8 | 94.68 | 107.94 | 705 |
| C-SLN-9 | 91.64 | 86.64 | 357 |
| C-SLN-10 | 91.64 | 86.64 | 357 |
| C-SLN-11 | 91.64 | 86.64 | 357 |
| C-SLN-12 | 91.64 | 86.64 | 357 |
| C-SLN-13 | 94.61 | 106.34 | 391.9 |
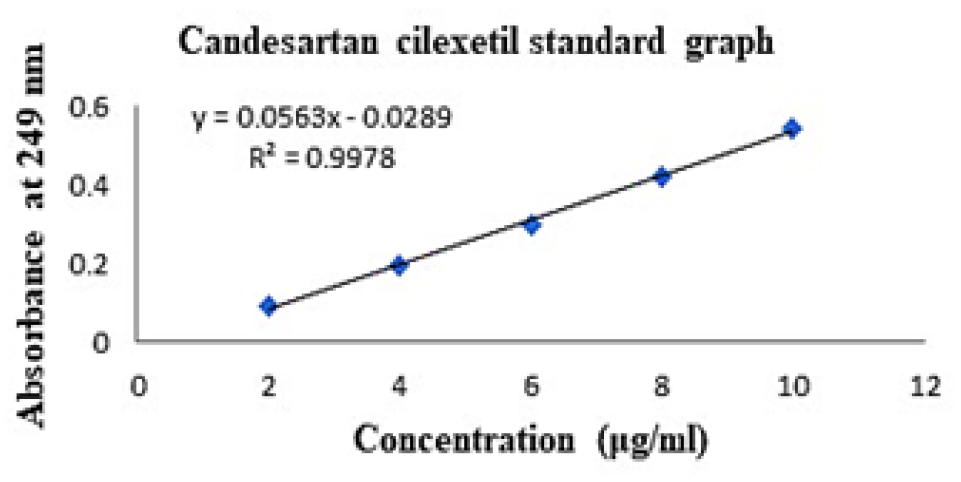
Figure 1:
Candesartan cilexetil standard curve.
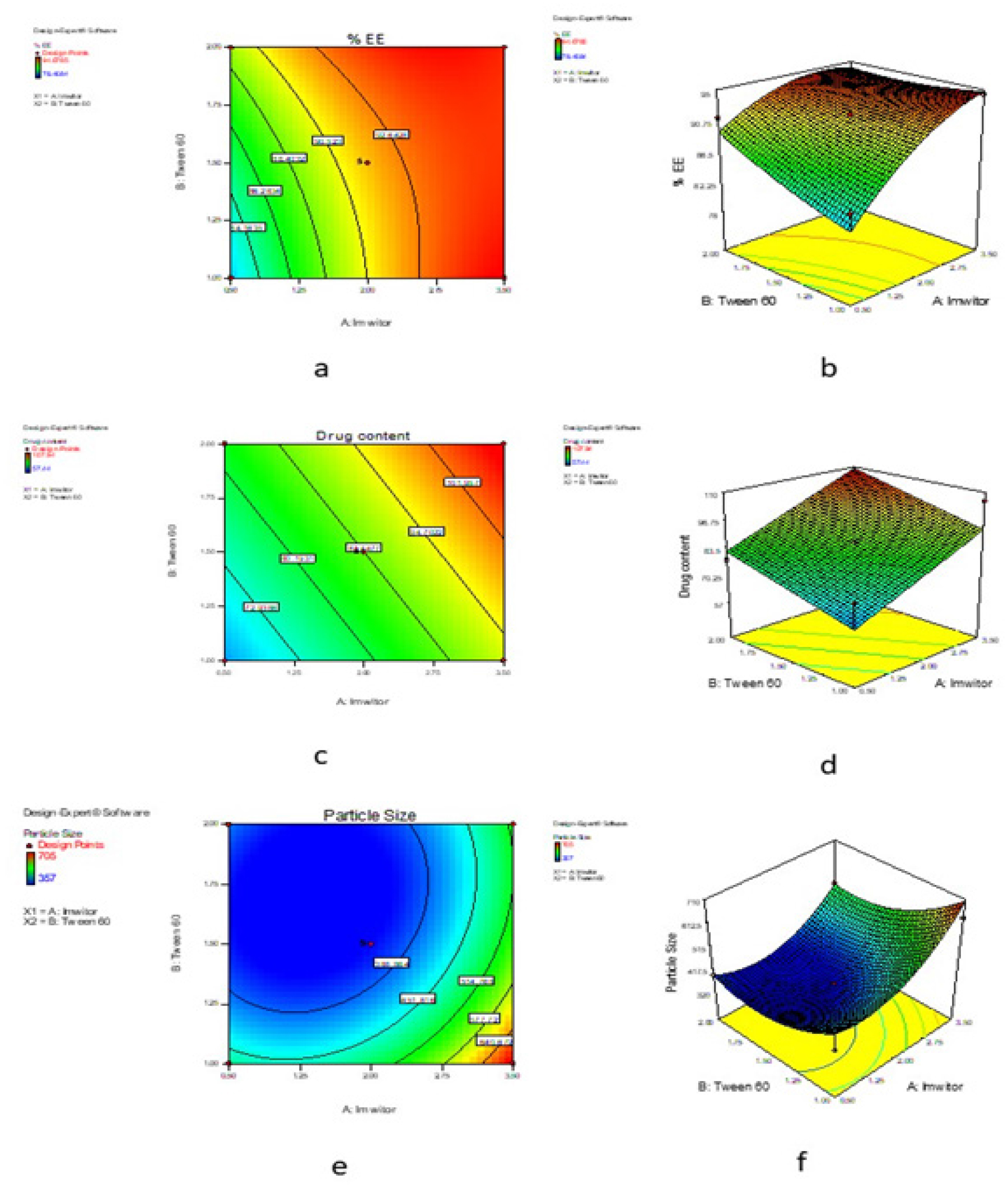
Figure 2:
Contour plot for %EE of SLN (a), Response surface plot for %EE of SLN (b), Contour plot for drug content of SLN (c), Response surface plot for drug content of SLN (d), Contour plot for particle size of SLN (e), Response surface plot for particle size of SLN (f).
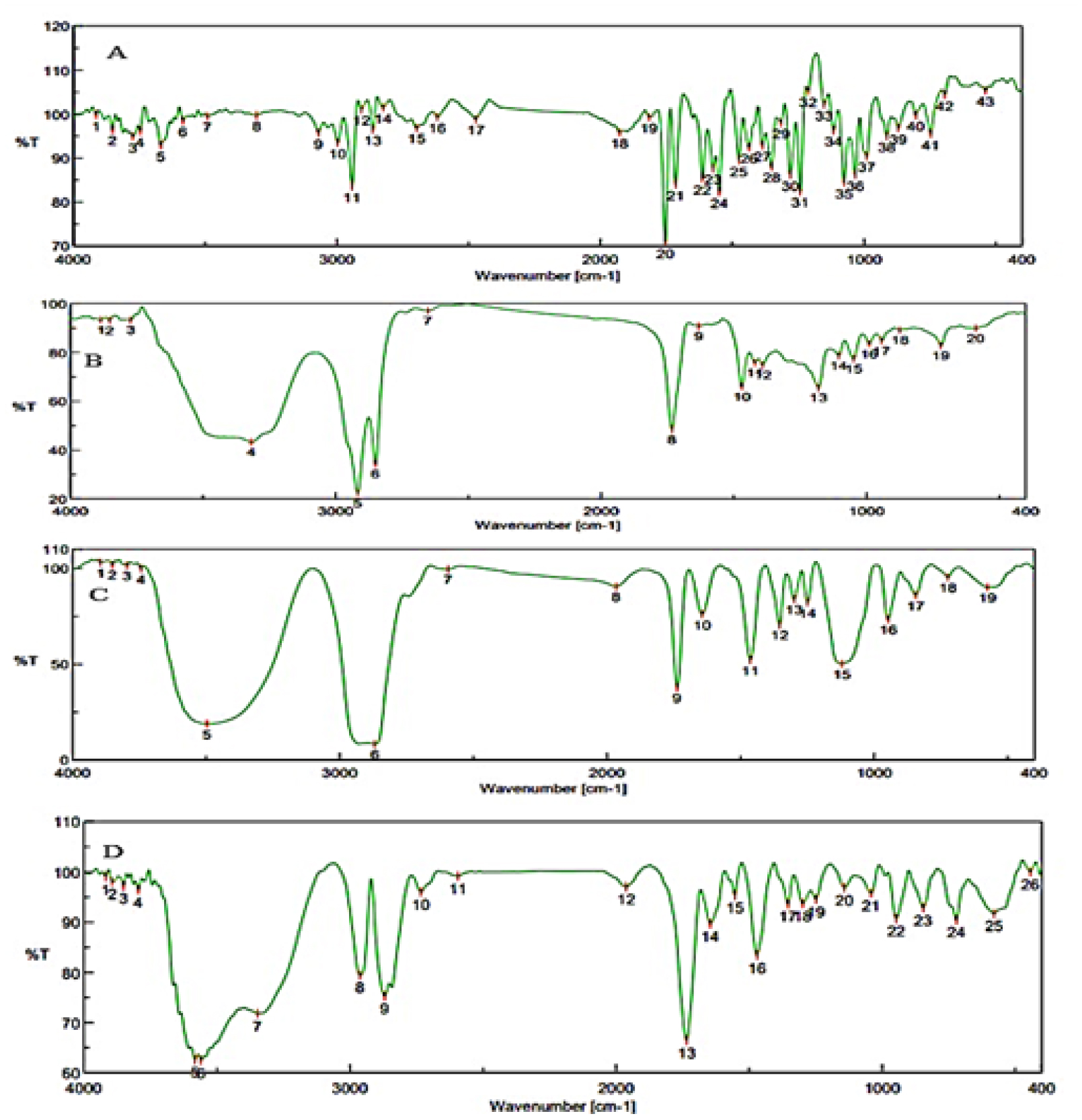
Figure 3:
FTIR images of (A) Candesartan cilexetil, (B) Imwitor, (C) Tween 60, (D) Drug-excipient mixture.
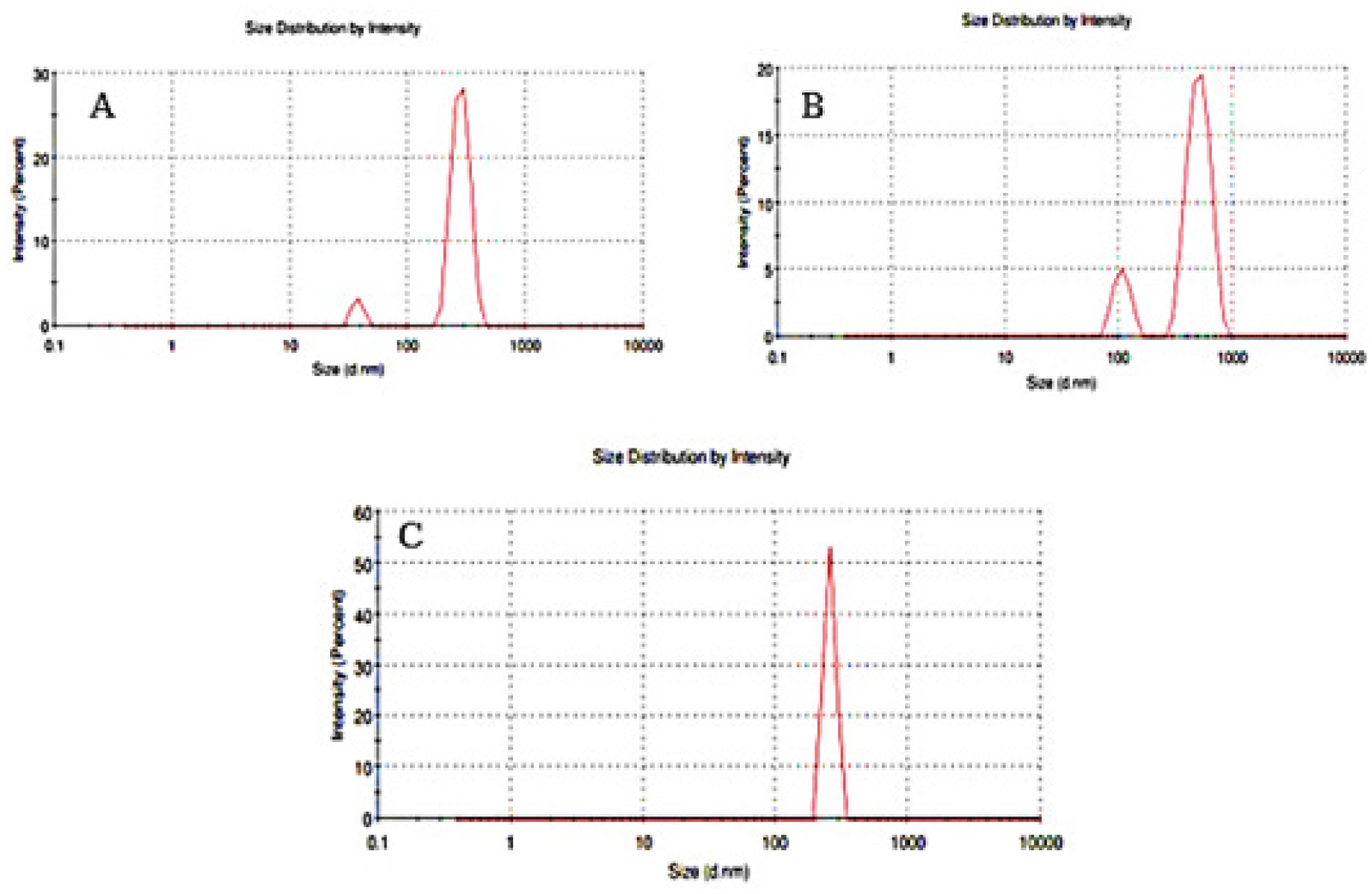
Figure 4:
(A) Particle size low (C-SLN-12), (B) Particle size medium (C-SLN-5), (C) Particle size high (C-SLN-8).
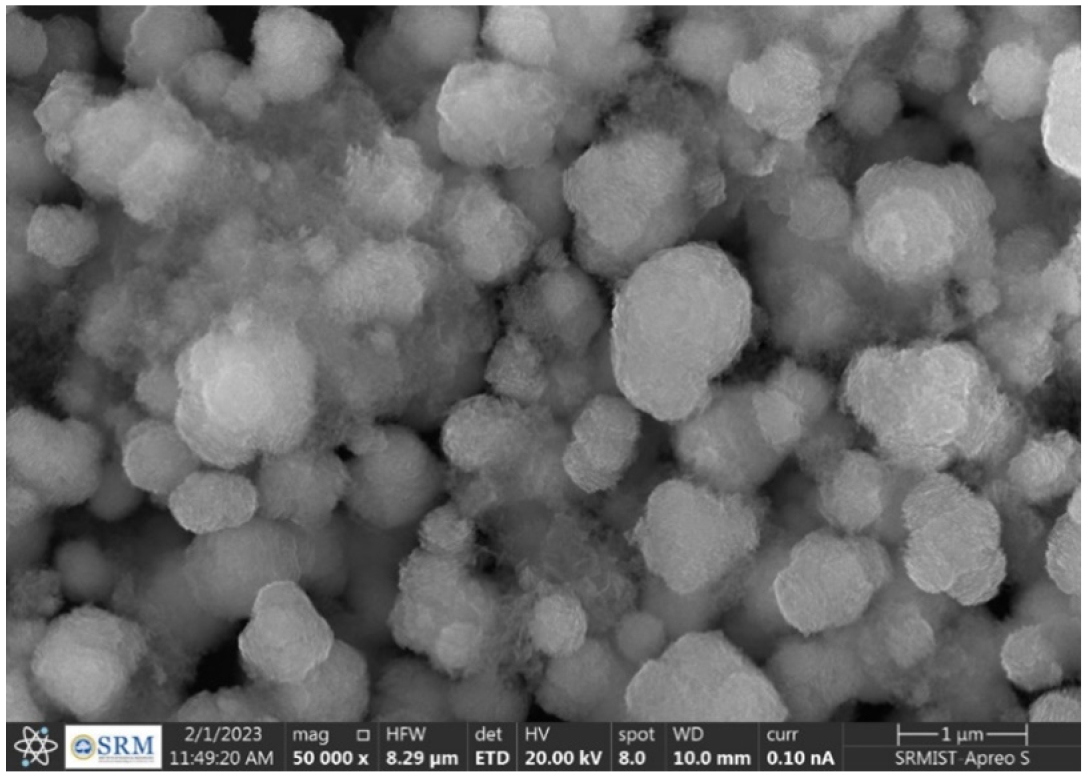
Figure 5:
SEM image of formulation C-SLN-8.
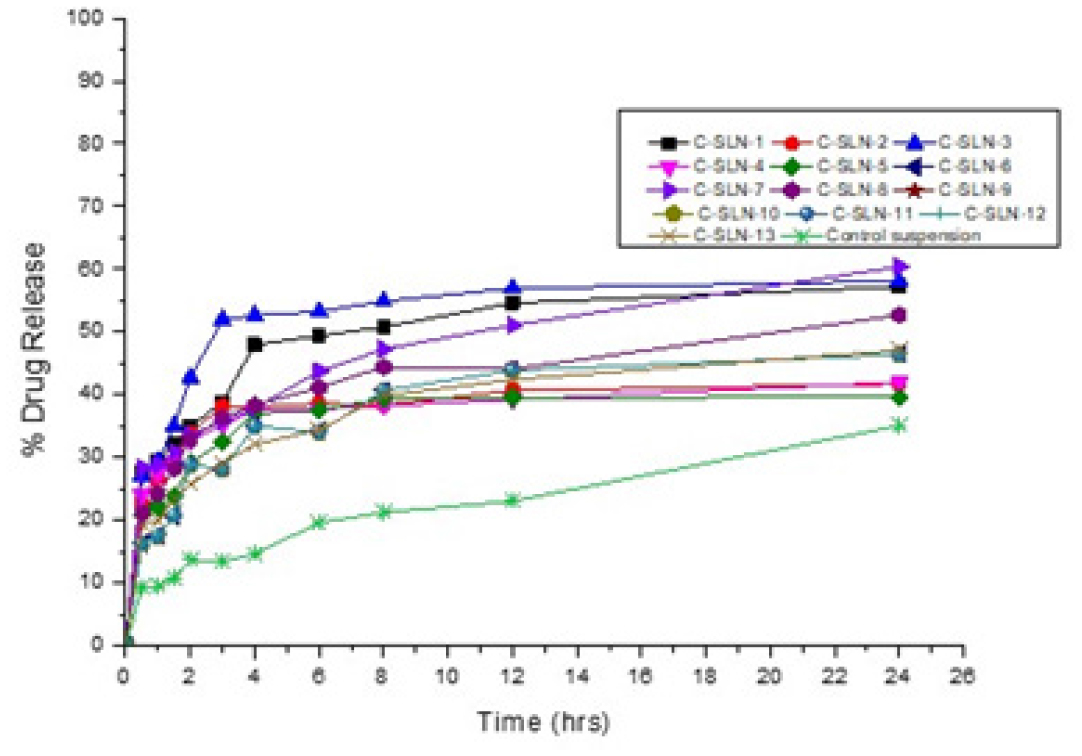
Figure 6:
Dissolution profile of candesartan cilexetil SLN.
CC in vitro release (Table 6) showed a maximum of 60.39% drug release from the C-SLN-7 and 46.4% released from C-SLN-12, and control suspension shows that 35.05% of drug release within 24 hr. The higher drug release from formulation may be attributed to a higher concentration of the Imwitor present in the SLN as well as the optimum sonication when compared to other formulations and the suspension. Moreover, this possesses a great entrapment efficiency of 94.34% when compared with other developed SLN formulations (Figure 6). Formulations C-SLN1, 3&8 shows good in vitro release profile due to the presence of a higher amount of lipid to accommodate more amount of drug. Formulations C-SLN 2, 4, 5, 6, 9-13 shows moderate release, which makes it less attractive due to the variable concentration of Imwitor and Tween 60 (Table 2).
| % Drug Released | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time in hrs. | C-SLN-1 | C-SLN-2 | C-SLN-3 | C-SLN-4 | C-SLN-5 | C-SLN-6 | C-SLN-7 | C-SLN-8 | C-SLN-9 | C-SLN-10 | C-SLN-11 | C-SLN-12 | C-SLN-13 | Control Suspension |
| 0.5 | 27.55 | 22.81 | 27.07 | 23.91 | 20.72 | 16.25 | 28.38 | 21.13 | 16.25 | 16.25 | 16.25 | 16.25 | 19.32 | 9.29 |
| 1 | 29.55 | 26.71 | 29.45 | 27.38 | 22.10 | 17.46 | 28.79 | 24.23 | 17.46 | 17.46 | 17.46 | 17.46 | 19.95 | 9.52 |
| 1.5 | 32.19 | 29.37 | 34.98 | 29.15 | 23.85 | 20.70 | 30.83 | 28.26 | 20.70 | 20.70 | 20.70 | 20.70 | 23.59 | 10.80 |
| 2 | 34.90 | 33.94 | 42.76 | 32.75 | 29.11 | 28.85 | 33.58 | 32.84 | 28.85 | 28.85 | 28.85 | 28.85 | 25.68 | 13.66 |
| 3 | 38.90 | 38.21 | 51.89 | 35.11 | 32.50 | 28.16 | 35.72 | 36.35 | 28.16 | 28.16 | 28.16 | 28.16 | 29.16 | 13.44 |
| 4 | 47.97 | 38.01 | 52.63 | 37.51 | 37.03 | 34.98 | 37.89 | 38.33 | 34.98 | 34.98 | 34.98 | 34.98 | 32.09 | 14.60 |
| 6 | 49.38 | 38.65 | 53.28 | 37.68 | 37.54 | 34.07 | 43.79 | 41.14 | 34.07 | 34.07 | 34.07 | 34.07 | 34.43 | 19.58 |
| 8 | 50.76 | 38.32 | 54.90 | 38.24 | 39.39 | 40.65 | 47.22 | 44.43 | 40.65 | 40.65 | 40.65 | 40.65 | 39.84 | 21.22 |
| 12 | 54.58 | 40.65 | 56.99 | 39.21 | 39.53 | 43.88 | 51.07 | 44.12 | 43.88 | 43.88 | 43.88 | 43.88 | 42.44 | 23.03 |
| 24 | 57.25 | 41.66 | 58.07 | 41.97 | 39.59 | 46.40 | 60.39 | 52.71 | 46.40 | 46.40 | 46.40 | 46.40 | 47.09 | 35.05 |
DISCUSSION
The BCS class II low soluble CC is formulated as SLN and the calibration curve shows a straight line with R2 value of 0.9978 which represents the standard curve of drug. From the design expert, the formulations are optimized as per independent factors concentration of Imwitor 900K and concentration of tween 60 and dependent factors %EE, drug content, particle size. The FTIR graphs representing there is no interaction between drug and excipients. From the optimized 13 formulation, the %EE was found between 78.47 to 94.61 with drug content ranges from 57 to 107% and the particle size was found to be 357 to 705 nm. The SEM analysis shows that the particles are near to spherical and some are coagulated which justifies the particle size and dispersion. The in vitro drug release showed a maximum release of 60.39% drug release from the C-SLN-7 and minimum release of 46.4% from C-SLN-12 within 24 hr.
CONCLUSION
The present work aimed to carry out to improve the therapeutic efficacy of Candesartan cilexetil by formulating as SLNs. C-SLN was prepared by 32 factorial designs, and the effect of dependent variables was studied. The formulations were studied for entrapment efficiency, drug content, and particle size. Based on the statistical optimization technique, three formulations were selected. Based on the study results, it may be concluded that the developed C-SLNs formulation could be able to improve the in vitro drug release and further sustainable delivery of CC with the use of selected formulation compositions. The optimized formulation shows very less particle size, tremendous drug content, and enhanced entrapment efficiency altogether that may help to improve the in vitro drug release. This lipid nanocarriers could provide a remarkable lowering of blood pressure once better absorption of the drug takes place. In this connection, our developed optimized C-SLNs may possess the better quality than the other developed formulations.
Cite this article
Venkatesan K, Dutta G, Narayanaswamy D, Sugumaran A. Development and Optimization of Candesartan Cilexetil Loaded Solid Lipid Nanoparticles. Int. J. Pharm. Investigation. 2023;13(4):754-62.
ACKNOWLEDGEMENT
The authors are thankful to the management of SRM College of Pharmacy, SRMIST, Kattankulathur, for their valuable support.
ABBREVIATIONS
| SLN | Solid Lipid Nanoparticles |
|---|---|
| CC | Candesartan cilexetil |
| EE | Entrapment efficiency |
| BCS | Biopharmaceuticals Classification System |
| ACE | Angiotensin Converting Enzyme |
| DoE | Design of Expert |
| ANOVA | Analysis of Variance |
| FTIR | Fourier Transform InfraRed |
| KBr | Potassium Bromide |
| DLS | Dynamic Light Scattering |
| SEM | Scanning Electron Microscopy |
| NaOH | Sodium Hydroxide |
| UV | Ultraviolet Visible spectroscopy |
| C-SLNs | Candesartan loaded Solid Lipid Nanoparticles |
| RPM | Rotation Per Minute |
References
- Garud A, Singh D, Garud N. Solid Lipid Nanoparticles (SLN): method, characterization and applications. Int Curr Pharm J. 2012;1(11):384-93. [CrossRef] | [Google Scholar]
- Priyanka K, Sathali AA. Preparation and evaluation of montelukast sodium loaded solid lipid nanoparticles. J Young Pharm. 2012;4(3):129-37. [PubMed] | [CrossRef] | [Google Scholar]
- Mahajan A, Kaur S, Kaur S. Design, formulation, and characterization of stearic acid-based solid lipid nanoparticles of candesartan cilexetil to augment its oral bioavailability. Asian J Pharm Clin Res. 2018;11(4):344-50. [CrossRef] | [Google Scholar]
- Hao J, Fang X, Zhou Y, Wang J, Guo F, Li F, et al. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box-Behnken design. Int J Nanomedicine. 2011;6:683-92. [PubMed] | [CrossRef] | [Google Scholar]
- Soma D, Attari Z, Reddy MS, Damodaram A, Koteshwara KBG. Solid lipid nanoparticles of irbesartan: preparation, characterization, optimization and pharmacokinetic studies. Braz J Pharm Sci. 2017;53(1):1-10. [CrossRef] | [Google Scholar]
- Dhoranwala KA, Shah P, Shah S. Formulation optimization of rosuvastatin calcium-loaded solid lipid nanoparticles by 32 full-factorial design. Nanoworld J. 2016;1(4):112-21. [CrossRef] | [Google Scholar]
- Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127(2):97-109. [PubMed] | [CrossRef] | [Google Scholar]
- Lee G-S. Preparation and characterization of bis-ethylhexyloxyphenolmethoxy-phenyltriazine (BEMT) loaded Solid Lipid Nanoparticles (SLN). J Ind Eng Chem. 2007;13:1180-7. [PubMed] | [CrossRef] | [Google Scholar]
- Das S, Ng WK, Kanaujia P, Kim S, Tan RBH. Formulation design, preparation and physicochemical characterizations of solid lipid nanoparticles containing a hydrophobic drug: effects of process variables. Colloids Surf B Biointerfaces. 2011;88(1):483-9. [PubMed] | [CrossRef] | [Google Scholar]
- Chalikwar SS, Belgamwar VS, Talele VR, Surana SJ, Patil MU. Formulation and evaluation of nimodipine-loaded solid lipid nanoparticles delivered via lymphatic transport system. Colloids Surf B Biointerfaces. 2012;97:109-16. [PubMed] | [CrossRef] | [Google Scholar]
- Charman WN, Porter CJH. Lipophilic prodrugs designed for intestinal lymphatic transport. Adv Drug Deliv Rev. 1996;19(2):149-69. [CrossRef] | [Google Scholar]
- Yasir M, Sara UVS. Preparation and optimization of haloperidol loaded solid lipid nanoparticles by Box–Behnken design. J Pharm Res. 2013;7(6):551-8. [CrossRef] | [Google Scholar]
- Husain A, Sabir AMd, Mitra M, Bhasin PS. A review on candesartan: pharmacological and pharmaceutical profile. J Appl Pharm Sci. 2011;1:12-7. [CrossRef] | [Google Scholar]
- Dudhipala N, Veerabrahma K. Candesartan cilexetil loaded solid lipid nanoparticles for oral delivery: characterization, pharmacokinetic and pharmacodynamic evaluation. Drug Deliv. 2016;23(2):395-404. [PubMed] | [CrossRef] | [Google Scholar]
- Zhang Z, Gao F, Bu H, Xiao J, Li Y. Solid lipid nanoparticles loading candesartan cilexetil enhance oral bioavailability: in vitro characteristics and absorption mechanism in rats. Nanomedicine. 2012;8(5):740-7. [PubMed] | [CrossRef] | [Google Scholar]
- Kamble SS, Gambhire MS, Gujar KN. Optimization and development of candesartan cilexetil loaded solid lipid nanoparticle for the treatment of hypertension. 2015;3:53-64. [PubMed] | [CrossRef] | [Google Scholar]
