ABSTRACT
Background
Solubility is the most vital parameter in the formulation of different drug delivery systems and one of the core concepts of the formulations. More than 50% of the new drug molecules are facing poor solubility issues. Poor solubility leads to limited bioavailability. Dabigatran Etexilate (DBE) is an anti-coagulant drug belonging to BCS class II drug having low solubility and high permeability and its oral bioavailability is 30%. Self-Nano-Emulsifying Drug Delivery System (SNEDDS) is used for the enhancement of solubility and bioavailability of the drug. However, SNEDDS has some major disadvantages such as less drug loading and precipitation of the drug in the GI. Therefore, a Supersaturated Self Nano Emulsifying Drug Delivery System (S-SNEDDS) is prepared.
Materials and Methods
A ternary phase diagram was constructed to determine the nano-emulsion region. Liquid S-SNEDDS was prepared by mixing castor oil, Transcutol HP and Cremophor EL with DBE including Sodium taurocholate as a precipitate inhibitor The Liquid S-SNEEDS was further converted to Solid S-SNEDDS by using Aerosil 200 as an adsorbent to form a more stable dosage form. A higher amount of Aerosil 200 was used which exhibits good flow properties and preserved the self-emulsifying properties of Liquid SNEDDS.
Results and Conclusion
The final formulation was characterized using DSC and SEM and evaluated for in vitro dissolution. All the evaluations demonstrated that S-SNEDDS had particle size less than 200 nm, PDI was less than 0.5 and zeta potential was between -25 and -45. In vitro dissolution demonstrated that higher drug release was found in capsules incorporated with S-SNEDDS as compared to SNEDDS capsules.
INTRODUCTION
Approximately 70% of the new drug entities are lipophilic by nature so they are poorly soluble in water. Drugs that do not dissolve or do not absorb well may be enhanced via lipid-based formulations that increase a drugs solubility as well as absorption (Chai et al., 2016). One of the most appealing technologies among this lipophilic formulation has been self-nano-emulsifying drug delivery systems in recent years for improving the solubility and bioavailability of poorly water-soluble drugs (Villar et al., 2012).
Self-Nano-Emulsifying Drug Delivery Systems (SNEDDS) are self-emulsifying nanoparticles that are phospholipid-like nanoparticles that are being developed for the delivery of oral drugs because they have the property of enhancing the solubility of the drug and improving the rate and extent of absorption of poorly hydrophilic drugs. (Kuruvila et al., 2017; Subramanian et al., 2020). This form of drug delivery is a colloidal mixture of lipophilic drugs, oils, surfactants and co-surfactants which forms a clear nano-emulsion after dilution. SNEDDS are pigmented emulsions with a globule size less than 200 nm. SNEDDS consist of oil, surfactant and co-surfactant. It get emulsified when SNEDDS come in contact with an aqueous solution in the GIT under mild agitation. Self-emulsification is one of many ways SNEDDS improves oral absorption of poorly soluble drugs by using different dosage forms such as capsules, tablets, beads, nanospheres, etc (Nazlıet al., 2021; Parket al., 2020; Michaelsenet al., 2019). The main advantage of this is that the pre-dissolving compound overcomes the initial rate-limiting step of dissolution in the aqueous environment within the GI tract (Thomaset al., 2012).
Dabigatran etexilate is a BCS class II drug with challenging biopharmaceutical properties such as poor solubility and slow dissolution rate. These properties have lain the ground for the development and optimization of the lipid-based formulation of DBE (Singh and Pai, 2015; Bandyopadhyay et al., 2014). The solubilizing capacity of SNEDDS diminishes in vivo as the drugs oil content decreases during absorption, resulting in the precipitation of drug. Drugs contained in SNEDDS are less soluble than their equilibrium solubility (i.e. drugs that should be given at a higher dose) (Kumar and Sureshkumar, 2020). As precipitates form on a drug nucleus, precipitate inhibitor slows down the process. Supersaturated self-nano emulsifying drug delivery systems consist of a Polymeric Precipitation Inhibitor (PPI) which is blended into the formulation of SNEDDS. During the process of crystallization, it inhibits the process of nucleation by absorbing onto the hydrophobic surface of the crystal (ElKasabgy, 2014; Patelet al., 2016; Singh et al., 2021). Hydroxypropyl Methylcellulose (HPMC) is the most common PPI used in S-SNEDDS. Other common PPI which is used are 4- Bromo benzene boronic acid, polyvinyl pyrrolidone (Savjani et al., 2012).
MATERIALS AND METHODS
Materials
Dabigatran etexilate was purchased from Yarrow Chem. Castor oil, Transcutol HP and Cremophor EL was obtained from lobachemie. Aerosil 200 and sodium taurocolate was purchased from CDH. All other reagents were of analytical grade and used without further purification.
Determination of DBE solubility in the oil, surfactant and co-surfactant
The Solubility tests were carried out on various oils, surfactants and co-surfactants. One mL of each oil, surfactant and co-surfactant was added in glass vial containing excess amount of DBE. These vials are then sonicated for 15 min and then kept in water bath shaker for 24 hr. After 24 hr these vials are centrifuged at 15,000 rpm for 15 min (Jindal, 2017; Lalwaniet al., 2013). After centrifugation at 4000 rpm for 25 min the supernatant liquid was diluted using methanol and was analyzed in UV-spectrometer at 223 nm (Dashet al., 2015). The materials used in the study included olive oil, castor oil, arachis oil, Cremophor EL, Tween 80, Cremophor RH40, Transcutol HP, 1-2, propendiol. Results from each experiment were expressed as values in μg/mL.
Construction of Ternary Phase Diagram
For Construction of Ternary Phase Diagram oil, surfactant and co-surfactant (Smix) were mixed in different ratios (1:1, 1:2 and 1:3) with water at room temperature. The liquid mixtures were then titrated with dropwise addition of water in the magnetic stirrer (200-400 rpm) and the solution was monitored visually until the solution turned clear. In order to determine the percent transmittance of the clear solution, we measured it using a UV spectrophotometer at 638.2 nm and distilled water as a blank (Alothaidet al., 2021). If the solution formed is clear and transparent emulsion, then the emulsion is considered as good emulsion (% transmittance >85) and if the solution is turbid or formed coalescence then this emulsion is considered as bad emulsion (% transmittance <85). The values are then plotted in Chemix software to find the emulsification region (Parmaret al., 2015; Abo Enin and Abdel-Bar, 2016).
Characterization of prepared Super-saturated SNEDDS
In terms of globule size analysis, the self-emulsifying system with the larger nano-emulsion region (oil phase-castor oil, surfactant-Cremophor EL, co-surfactant-Transcutol HP) was chosen for further evaluation as seen in Table 1.

Figure 1:
Solubility data in different concentration of PPI.
| Sl. No. | Oil (Castor oil) %v/v | Surfactant (Cremophor EL) %v/v | Co-surfactant (Transcutol HP) %v/v | Remarks |
|---|---|---|---|---|
| 1. | 50 | 25 | 25 | Turbid |
| 2. | 45 | 25 | 30 | Clear |
| 3. | 40 | 30 | 30 | Clear |
| 4. | 35 | 30 | 35 | Clear |
| 5. | 30 | 35 | 35 | Clear |
| 6. | 25 | 35 | 40 | Clear |
| 7. | 20 | 40 | 40 | Clear |
| 8. | 15 | 40 | 45 | Clear |
| 9. | 10 | 45 | 45 | Clear |
| 10. | 60 | 20 | 20 | Turbid |
| 11. | 65 | 20 | 15 | Turbid |
| 12. | 70 | 15 | 15 | Turbid |
| 13. | 75 | 15 | 10 | Bluish |
| 14. | 80 | 10 | 10 | Turbid |
| 15. | 85 | 10 | 5 | Turbid |
| 16. | 90 | 5 | 5 | Turbid |
| Sodium taurocholate (PI) | S% |
|---|---|
| 0 | 50 |
| 0.5 | 50 |
| 1 | 50 |
| 2 | 100 |
| 5 | 100 |
Saturated solubility of Liquid supersaturated SNEDDS
An excess amount of the drug was added to 1 mL of the formulation (Data from the overlay plot) in the selected system (Castor oil/Cremophor EL/Transcutol HP) and put in a water bath shaker for 12 hr (Kimet al., 2018). Then they are centrifuged at 5000 rpm for 15 min. The supernatant was diluted with methanol and filtered and was determined spectrophotometrically at 223 nm (Thomaset al., 2012).
Preparation of Super-saturated SNEDDS
The liquid super-saturated SNEDDS was prepared in two continuous steps. First, an amount of drug equivalent to its saturated solubility was added to a mixture of oil, surfactant and co-surfactant by using a magnetic stirrer at a speed of 200-400 rpm at room temperature to produce a clear, isotropic solution (Thomaset al., 2013; Singh and Pai, 2016). Then the precipitation inhibitor was added to the above solution in different concentrations, i.e., 0.5%, 1%, 2% and 5%, to increase their equilibrium solubility as seen in Table 2 and (Figure 1). The super-saturated liquid SNEDDS will be mixed with aerosol 200 adsorbents until the adsorbents form a free-flowing powder (Dashet al., 2015). The prepared powder will be sieved to get a uniform size and stored in a desiccator for further evaluation. The solid granules or powder of Solid Super-saturated SNEDDS will be filled into the hard-shell gelatin capsules (Radhaet al., 2019). Characterization of the prepared S-SNEDDS formulation.
Determination of percentage transmittance
1 mL of the formulation was taken to this 0.1 mL SNEDDS formulation and was titrated with 100 mL of distilled water. The percentage transmittance was measured using distilled water as the blank in a UV spectrophotometer at 638.2 nm (Albaidhani and Hussein, 2019).
Visual observation
Self-emulsification time
To determine the emulsification time, 1 mL of liquid SNEDDS was titrated with 100 mL of distilled water at 37ºC using a magnetic stirrer at 100 rpm. The time required for the formation of clear or milky emulsion was noted visually (Nazlıet al., 2021).
| Components | Observed Solubility (mg/mL) |
|---|---|
| Water | 1.72±0.013 |
| OILS | |
| Castor oil | 75.6±1.048 |
| Olive oil | 16.19±0.0854 |
| Arachis oil | 3.92±1.608 |
| SURFACTANTS | |
| Cremophor EL | 93.98±0.0347 |
| Tween 80 | 58.92±0.184 |
| CO-SURFACTANTS | |
| 1-2, propendiol | 261.68±0.2406 |
| Transcutol HP | 368.96±0.235 |
| PEG 400 | 152.03±0.122 |
Droplet size and zeta potential
1 mL of the formulation was taken, to this 0.1 mL SNEDDS and supersaturated SNEDDS formulation was titrated with 100 mL of distilled water and sonicated for 10 min. The resulting nano-emulsion was checked for droplet size and zeta potential in a particle size analyzer (Malvern zetasizer) (Caiet al., 2016; Faltas, 2010). The Zeta and droplet size values were determined.
Drug Content
Characterization of prepared SS-SNEDDS formulation
Angle of repose (θ)
The angle of repose was done using funnel method. The accurate weigh sample was pour in a funnel at a fixed height, onto the base. The powder was allowed to flow through funnel freely onto the surface (Nantsupawatet al., 2018). The powder cone’s diameter was measured, and angle of repose was calculated by using below equation:
Where, h=height of heap, r=radius of heap.
Bulk density
Tapped density
Several taps of the same powder were made from a height of 2.5 cm at 2 sec intervals. The tapping was continued until no further change in volume was noted (Sarah, 2013). Tapped density was calculated using equation:
Compressibility index
Hausner’s ratio
It is the ratio of tapped density to bulk density. It is used to indicated powder flow properties and can be calculated as:
Drug entrapment efficiency
The entrapment efficiency of formulated S-SNEDDS DBE was determined by checking the amount of unentrapped drug by centrifugation method. The samples were centrifuged at 1000 rpm for 15 min and the supernatant was collected, diluted with methanol and analyzed in UV spectroscopy at 223 nm (Deshmaneet al., 2024; Mundada and Sawant, 2018). % EE was calculated by following equation:
Differential Scanning Calorimetry (DSC)
In pharmaceutics, it is important to deliver a drug in the amorphous form, to check the drug temperatures below which crystallization occur. So Differential Scanning Calorimetry (DSC) is used for the identification of various physical properties and thermal transitions. Drug loaded-SDs are examined using differential scanning calorimeter equipped with auto sampler (Abed and Hussein, 2019; Choet al., 2017).
Scanning electron microscopy
SEM pictures were captured at 15 KeV accelerating voltage to observe the morphology and size of the prepared SNEDDS. Samples were attached on a brass stub using double sided adhesive tape and made electrically conductive by covering with a thin layer of gold (Nagadeepet al., 2019).
| Components | Quantity (1:1) mg | First week | Second week | Third week | Fourth week |
|---|---|---|---|---|---|
| DBE-Castor oil (A) | 100:100 | Yellowish light colour | No change | No change | No change |
| DBE-Cremophor EL (B) | 100:100 | Yellowish to slight orange colour | No change | No change | No change |
| DBE-Transcutol HP (C) | 100:100 | Whitish fine light colour | No change | No change | No change |
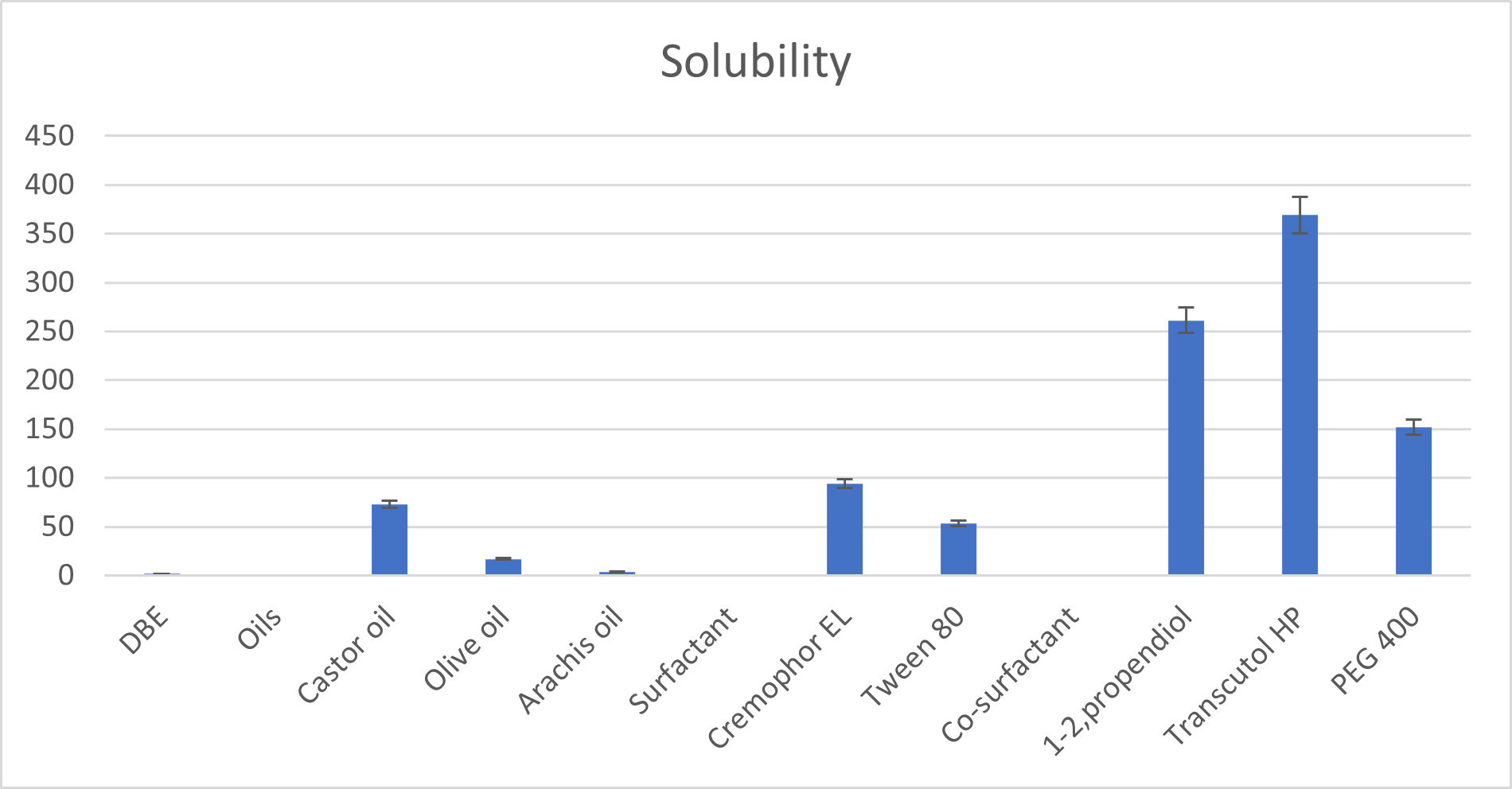
Figure 2:
Solubility of drug, oil, surfactant and co-surfactant.
In vitro dissolution test
In vitro dissolution studies were carried out using USP apparatus II electrolab, rotating paddle. Pure drug, SNEDDS and S-SNEDDS were filled in the three different capsules. Then, these capsules were placed in 750 mL of SGF at 37±0.5ºC in different baskets for 2 hr followed by exposure to SIF by the addition of 250 mL of a sodium phosphate solution (0.2 mol/L). An aliquot of 5mL was withdrawn at predetermined time intervals with the replacement of an equal volume and filtered through Whatman filter paper. The concentration of DBE was measured by UV spectrophotometry (Scioliet al., 2020; Pooraniet al., 2016).
RESULTS
Determination of Drug Solubility in water, Oils, Surfactants and Co-surfactants
The method of determination of solubility of DBE in various oils is mentioned in Table 3. The equilibrium solubility of DBE in oils, surfactants and co-surfactants is shown in Figure 2.
Firstly, DBE solubility was tested in various vegetable oils. Vegetable oils are the most common lipid phases used in lipid-based delivery systems to solubilize lipophilic/hydrophobic drugs owing to their safety, digestibility and absorption. Among the selected oils, castor oil exhibited the highest and good solubility for DBE compared to all other oils, it was selected as the oil phase for S-SNEDDS formulation. Hence, castor oil was chosen as an oil phase for formulating DBE-loaded SNEDDS. In several studies, castor oil was used as an oil phase in micro/nano-emulsion formulations of quercetin (Kaziazi et al., 2020). The highest solubility of DBE at 75.6±1.048 mg/mL was found in castor oil. Among the selected surfactants, cremophor EL exhibited the highest and best solubility for DBE compared to all other surfactants. Cremophor RH 40 has less solubilization capacity, but it shows good emulsification ability (transmittance above 90%) as compared to Cremophor RH 40 and Tween 80. Hence, Cremophor EL was used as a surfactant in the present study. Cremophor EL was studied for its emulsification ability with Castor oil. Co-surfactants screened for improving the nano-emulsification ability of the selected surfactant were found to increase the spontaneity of nano-emulsion formation which was monitored by several inversions required to produce nano-emulsion. Among the selected co-surfactants, transcutol HP exhibited the highest and best solubility for DBE compared to all other co-surfactants.
Drug-Excipient compatibility study
The study was conducted at room temperature as described in Table 4 and (Figure 3). It was observed that the physical interaction was seen after one month of storage at room temperature. Before any excipients are used in the preparation of formulation, the compatibility between the drug and excipients must be ensured. Therefore, it is essential to conduct drug excipient interaction studies during pre-formulation.
The FTIR spectra of castor oil with drug (A) showed characteristic peaks of castor oil and drug at 3418.82, 2854.27, 2727.56 and 1746.23 cm-1 respectively. It indicates that there was no interaction between the drug and castor oil and they both were compatible with each other.
The FTIR spectra of Cremophor EL with drug (B) showed characteristic peaks at 3449.05, 2921.00, 1967.25 and 1735.02 cm-1 which confirms the compatibility between Cremophor EL and drug.
The FTIR spectra of the physical mixture of Transcutol HP and drug (C) showed characteristic peaks at 3452.51, 2928.43, 2346.06 and 1733.31 cm-1 which confirms the compatibility between Transcutol HP and drug. No change in peaks showed there was no physical interaction between the drug and Transcutol HP.
| Parameters | Range | Remarks |
|---|---|---|
| Bulk density | 0.52 | Within limits |
| Tapped density | 0.62 | Within limits |
| Hausner’s ratio | 1.2 | Excellent |
| Angle of repose | 28.34 | Excellent |
| Compressibility index | 15.8 | Excellent |
Construction of Ternary Phase Diagram
A ternary phase diagram was used to identify the self-emulsifying regions and to optimize the percentages of different liquid SNEDDS components as shown in Figure 4. Based on the data obtained from solubility studies, Castor oil was used as the oil phase and Smix (Cremophor EL: Transcutol HP) was used as a surfactant and co-surfactant to construct the ternary phase diagram. Different ratios of surfactant/co-surfactant were used to prepare different ternary systems. The results suggested that the emulsification ability was good when the surfactant/co-surfactant concentration increased demonstrated by the wide clear nano-emulsification region and the emulsification ability was bad/turbid as shown when the oil concentration increased. The maximum concentration of oil that was solubilized by this ratio in the phase diagram was found to be 22% w/w by incorporating Smix around 52% w/w, itself showed the domination of surfactant (Cremophor-EL) along with co-surfactant in nanosizing. The data indicated that the larger the shaded area, the more the self-emulsification ability. All experiments were done in triplicates.
Characterization of the prepared SNEDDS
Determination of percentage transmittance
The formulation selected was subjected to percent transmittance. Percentage transmittance is a crucial factor in determining if a system is isotropic. By utilizing a visible spectrophotometer with a wavelength of less than 650 nm to measure the turbidity of an emulsion produced from SNEDDS in aqueous media, the percentage of transmittance was determined. The value of the formulation was found to be 99.5%. The value above 90% shows that the particles are nano size. It showed that the formulation was clear and transparent. The formulation’s percent transmittance showed that it was transparent. Because the droplets in the dispersed phase were no larger than 1/4 of the wavelength of visible light, the system was transparent (Chenhen et al., 2015). The nano-emulsion appears translucent or transparent because it scatters light. On increasing the concentration of oil, the percentage transmittance was more turbid due to the presence of the lyophilic group.

Figure 3:
FTIR spectra of DBE with components.

Figure 4:
Ternary phase diagram showing the effect of oil (castor oil), surfactant (Cremophor EL) and co-surfactant (Transcutol HP). Nano-emulsion regions are shaded.

Figure 5:
DSC curve of a) Dabigatran etexilate b) S-SNEDDS Formulation.
Phase Separation
The formulation was visually observed for any phase separation, creaming and cracking. There was no phase separation of the formulation checked for 24 hr. Phase separation was not observed in formulations with oil concentrations less than 30% and surfactant concentrations greater than 60%.
Self-emulsification time
The time at which the formulation emulsifies is an important parameter to check the efficiency of self-emulsification because SNEDDS should dissolve quickly and completely when subjected to dilution under mild agitation. When diluted with gentle agitation, the ideal SNEDDS formulation should be able to disperse instantly and fully. The rate and depth of water penetration into the various phases (liquid crystalline or gel phase) generated on the surface of the droplet are connected to the rate and depth of emulsification. When the energy needed to expand the surface area between the oil and aqueous phases of the dispersion is larger than the entropy changes favoring dispersion, self-emulsification occurs. When the oil phase is added to the aqueous phase of a self-emulsifying system with gentle stirring, the aqueous phase will permeate through the oil phase’s interface until the two phases’ interfaces are broken. As a result, oil droplets are created, which causes emulsification (Basaliouset al., 2010). The time of emulsification for the formulation was found to be 42±2.5 sec.

Figure 6:
SEM of DBE and SEM of Solid S-SNEDDS.
Zeta potential and PDI
The size of droplets after nano-emulsion is the most important parameter as it affects the absorption of the drug as well as drug release. Small droplets have more surface area, hence enhancing the absorption. Zeta potential is an indicator of the physical stability of the emulsion. From the ternary phase diagram, it is estimated that when increasing the concentration of oil resulted in turbidity while increasing the Smix. concentration resulting in clear formulation. From the ternary phase diagram, the droplet size, PDI and Zeta potential were found to be 114.9, 0.463 and -55.7 which was similar to the optimized formulation. Smaller particles were recorded with high zeta potential conferring to the higher stability of the product (Singhet al., 2011). It was also reported that an increase in surfactant concentration to 60% increased mean droplet size (Alghananimet al., 2020).
Drug content
The drug content of the prepared S-SNEDDS was calculated. The drug content of the formulation was found to be 94.3±0.5%.
Characterization of the prepared Solid supersaturated-SNEDDS formulation
Flow properties of solid S-SNEDDS powder were determined from bulk density, tapped density, Hausner’s ratio, Carr’s index and angle of repose was mentioned in Table 5. The bulk density of the powder was determined which shows the density as well as arrangement of the powder particles. Tapped density shows that the void spaces decrease when the powder is tapped many times.
This study shows that the bulk density was found to be 0.52±0.05 and the tapped density was found to be 0.62±0.09. The powder has good flow when the Hausner’s ratio is lower than 1.2 and if it is more than 1.2 it is considered as bad. From the above bulk and tapped density Hausner’s ratio and Carr’s index were determined and were found to be 1.2±0.12 and 15.8±0.699. The angle of repose was found to be 28.34±0.02. The flow property of powder was in the range and was considered excellent.
Differential Scanning Colorimetry
The thermal behavior and physical state of a drug within a formulation can be determined by DSC studies. DSC of the drug (DBE) and supersaturated SNEDDS formulation of DBE are shown in Figure 5. Pure DBE exhibited a sharp endothermic peak corresponding to its melting point of 159.8ºC, indicating that the drug is present in a crystalline form. The supersaturated SNEDDS formulation did not show an obvious endothermic peak corresponding to the melting point of crystalline DBE. Therefore, the formulation probably contains an amorphous component of the drug in the formulation. Amorphous substances require less energy for their dissolution, resulting in increased dissolution rates and thus, higher apparent solubility (Kimim et al., 2021). This might explain the reason for the higher solubility of the SNEDDS observed in this study.
Scanning Electron Microscopy
The surface morphology and globule size of the formulated SNEDDS were also determined microscopically using SEM. The SEM image shows rough and spherical shapes with a size smaller than 50 nm. It is clear that the globules were well dispersed, and no aggregation occurred. The image of the solid SNEDDS of powdered medication and the formulation containing DBE shows that the particles had the same exterior macroscopic morphology, which consisted of well-separated spherical particles with moderately deep dents and similar sizes (Figure 6).

Figure 7:
In vitro drug release profile comparison between marketed formulation, the capsule containing SNEDDS and the capsule containing S-SNEDDS in 0.1N HCl and pH 6.8 phosphate buffer.

Figure 8:
Percentage CDR release with respect to time (min).
In vitro Drug Release of SNEDDS
In vitro drug release studies of marketed formulation and capsule containing SNEDDS formulation without and with the incorporation of PI were carried out in 0.1N HCl and 6.8 pH phosphate buffer and were done using “USP type II apparatus”. The dissolution was carried out in 0.1N HCl and in pH 6.8 phosphate buffer (Figure 7). The marketed formulation of DBE, SNEDDS and S-SNEEDS shows greater drug release of 90% in SGF in about 60 min and there was approximately 67% dissolution of DBE when switching to SIF. This is because SNEDDS can rapidly form oil-in-water nano-emulsions when subjected to aqueous dilution under gentle agitation. The marketed formulation of DBE was highly dissolved in SGF (93%) but precipitated immediately in SIF (3%); the precipitation in the small intestine delays the oral absorption and the bioavailability of poorly water-soluble drugs. For this S-SNEDDS was formulated and inhibited the precipitation in SIF and showed drug release of about 82% in 6 hrs. A capsule containing S-SNEDDS shows a greater drug release of 96% in 60 min as compared to a capsule containing SNEDDS which shows a drug release of 92% in 0.1N HCl. Whereas 6.8 pH buffer capsules containing S-SNEDDS showed faster drug dissolution (82%) as compared to SNEDDS (64%) which is due to low surface free energy (Figure 8).
DISCUSSION
Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) are advanced formulations designed to improve the bioavailability of poorly water-soluble drugs. These systems offer a unique solution for delivering lipophilic drugs that struggle with low solubility and poor absorption in the Gastrointestinal (GI) tract. SNEDDS are isotropic mixtures of oils, surfactants and co-surfactants or solvents that spontaneously form oil-in-water nano-emulsions upon contact with aqueous environments such as the GI fluids, without the need for external energy like mechanical stirring.
SNEDDS was prepared using oil, surfactant and co-surfactant. Castor oil (triglyceride) is a Ricinoleic acid, a C18:1 carbon fatty acid with a hydroxyl group. Cremophor EL is used as a medium-chain triglyceride. Transcutol HP is used as a co-surfactant. To formulate supersaturated SNEDDS; Castor oil, Cremophor EL and Transcutol HP was used in the most appropriate ratio to produce a stable nano-emulsion. Sixteen SNEDDS formulations of these components were prepared using different amounts of oil, surfactant and co-surfactant. Then from the Ternary phase diagram formulation is selected. From the formulation Dabigatran Etexilate (150 mg) and Sodium taurocholate (2%) were incorporated and Liquid supersaturated SNEDDS formulation was prepared.
The FTIR spectra of castor oil with the drug showed characteristic peaks of castor oil and drug at 3418.82, 2854.27, 2727.56 and 1746.23 cm-11. The FTIR spectra of Cremophor EL with the drug showed characteristic peaks at 3449.05, 2921.00, 1967.25 and 1735.02 cm-1 which confirms the compatibility between Cremophor EL and the drug. The FTIR spectra of the physical mixture of Transcutol HP and the drug showed characteristic peaks at 3452.51, 2928.43, 2346.06 and 1733.31 cm-1 which confirms the compatibility between Transcutol HP and the drug. PDI, Zeta and globule size of Liquid supersaturated SNEDDS were found to be 114.9, 0.463 and -45.7. The drug content of the formulation was found to be 94.3±0.5%.
supersaturated SNEDDS formulation is achieved by adsorbing liquid supersaturated SNEDDS in aerosil 200 to fill the solid granules in capsules. Pure DBE exhibited a sharp endothermic peak, that corresponds to its melting point 159.8ºC and this indicates that the drug is present in a crystalline form. The SEM image shows rough and spherical shapes with a size smaller than 50 nm. S-SNEDDS was formulated and inhibit the precipitation in SIF and show drug release of about 82% in 6 hr. In vitro drug release studies showed greater drug release in supersaturated SNEDDS as compared to marketed formulation and SNEDDS formulation.
CONCLUSION
In this study supersaturated SNEDDS formulation was prepared. To formulate supersaturated SNEDDS; Castor oil, Cremophor EL and Transcutol HP was used in the most appropriate ratio to produce a stable nano-emulsion. From the ternary phase diagram sixth formulation was selected that shows significant zeta potential and PDI index. In this preparation Sodium taurocholate as a precipitation inhibitor at different concentrations was added to the best formulation whose zeta potential and PDI were determined. The Zeta potential and PDI of the formulation were found to be -45.7 and 0.46. The drug content of the formulation was found to be 94.3±0.5% with increased oral bioavailability. Overall, this study showed that employing HPMC as a precipitation inhibitor might stabilize and enhance the in vitro performance of Super-saturated SNEDDS of Dabigatran etexilate.
Cite this article:
Mittal H, Kaushik S, Bhatt B, Babbar A. Super-Saturated SNEDDS with Vegetable Oils: Formulation and Evaluation. Int. J. Pharm. Investigation. 2025;15(2):10-8.
REFERENCES
- Abed, H. N., & Hussein, A. A. (2019). Ex vivo absorption study of a novel dabigatran etexilate loaded nanostructured lipid carrier using non-everted intestinal SAC model. Iraqi Journal of Pharmaceutical Sciences, 28(2), 37–45. https://doi.org/10.31351/vol28iss2pp37-45
- Abo Enin, H. A., & Abdel-Bar, H. M. (2016, November 1). Solid super saturated self-nanoemulsifying drug delivery system (sat-SNEDDS) as a promising alternative to conventional SNEDDS for improvement rosuvastatin calcium oral bioavailability. Expert Opinion on Drug Delivery, 13(11), 1513–1521. https://doi.org/10.1080/17425247.2016.1224845
- Albaidhani, S. F., & Hussein, A. A. (2019, March 1). Preparation and evaluation of solid supersaturable self-nanoemulsifying drug delivery system of candesartan cilexetil. Journal of Pharmaceutical Sciences and Research, 11(3), 859–868.
- Alghananim, A., Özalp, Y., Mesut, B., Serakinci, N., Özsoy, Y., & Güngör, S. (2020). A solid ultra fine self-nanoemulsifying drug delivery system (S-SNEDDS) of deferasirox for improved solubility: Optimization, characterization and in vitro cytotoxicity studies. Pharmaceuticals, 13(8), 162. https://doi.org/10.3390/ph13080162
- Alothaid, H., Aldughaim, M. S., Yusuf, A. O., Yezdani, U., Alhazmi, A., Habibullah, M. M., & Khan, M. G. (2021, January 1). A comprehensive study of the basic formulation of supersaturated self-nanoemulsifying drug delivery systems (SNEDDS) of albendazolum. Drug Delivery, 28(1), 2119–2126. https://doi.org/10.1080/10717544.2021.1986601
- Bandyopadhyay, S., Katare, O. P., & Singh, B. (2014, April 1). Development of optimized supersaturable self-nanoemulsifying systems of ezetimibe: Effect of polymers and efflux transporters. Expert Opinion on Drug Delivery, 11(4), 479–492. https://doi.org/10.1517/17425247.2014.877885
- Basalious, E. B., Shawky, N., & Badr-Eldin, S. M. (2010). SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: development and optimization. International Journal of Pharmaceutics, 391(1–2), 203–211. https://doi.org/10.1016/j.ijpharm.2010.03.008
- Cai, Z.-Q., Hou, X., Kong, D.-L., Hou, L., & Hu, Z.-Q. (2016, August). Synthesis, crystal structural and spectral characterisation of dabigatran etexilate tetrahydrate. Journal of Chemical Research, 40(8), 461–466. https://doi.org/10.3184/174751916X14664340623037
- Chai, F., Sun, L., Ding, Y., Liu, X., Zhang, Y., Webster, T. J., & Zheng, C. (2016, July). A solid self-nanoemulsifying system of the BCS class IIb drug dabigatran etexilate to improve oral bioavailability. Nanomedicine, 11(14), 1801–1816. https://doi.org/10.2217/nnm-2016-0138
- Chen, J., Mosquera-Giraldo, L. I., Ormes, J. D., Higgins, J. D., & Taylor, L. S. (2015). Bile salts as crystallization inhibitors of supersaturated solutions of poorly water-soluble compounds. Crystal Growth and Design, 15(6), 2593–2597. https://doi.org/10.1021/acs.cgd.5b00392
- Chen, X.-L., Liang, X.-L., Zhao, G.-W., Zeng, Q.-Y., Dong, W., Ou, L.-Q., Zhang, H.-N., Jiang, Q.-Y., & Liao, Z.-G. (2021, May 1). Improvement of the bioavailability of curcumin by a supersaturatable self nanoemulsifying drug delivery system with incorporation of a hydrophilic polymer: In vitro and in vivo characterisation. The Journal of Pharmacy and Pharmacology, 73(5), 641–652. https://doi.org/10.1093/jpp/rgaa073
- Cho, J. H., Kim, J. C., Kim, H.-S., Kim, D. S., Kim, K. S., Kim, Y. I., Yong, C. S., Kim, J. O., Youn, Y. S., Oh, K. T., Woo, J. S., & Choi, H.-G. (2017, June 15). Novel dabigatran etexilate hemisuccinate-loaded polycap: Physicochemical characterisation and in vivo evaluation in beagle dogs. International Journal of Pharmaceutics, 525(1), 60–70. https://doi.org/10.1016/j.ijpharm.2017.04.028
- Dash, R. N., Mohammed, H., Humaira, T., & Reddy, A. V. (2015, August 1). Solid supersaturatable self-nanoemulsifying drug delivery systems for improved dissolution, absorption and pharmacodynamic effects of glipizide. Journal of Drug Delivery Science and Technology, 28, 28–36. https://doi.org/10.1016/j.jddst.2015.05.004
- Deshmane, S., Kendre, K., Deshmane, S., Jain, S., Sawant, A., & Solanki, H. (2024, July 16). Utilizing phospholipid as an amphiphilic carrier for solid dispersion of ambrisentan: Formulation and evaluation. Journal of Dispersion Science and Technology, 1–9. https://doi.org/10.1080/01932691.2024.2381020
- ElKasabgy, N. A. (2014, January 2). Ocular supersaturated self-nanoemulsifying drug delivery systems (S-SNEDDS) to enhance econazole nitrate bioavailability. International Journal of Pharmaceutics, 460(1–2), 33–44. https://doi.org/10.1016/j.ijpharm.2013.10.044
- Faltas, B. (2010, October 1). A new anticoagulant for a new era: Review of recent data on dabigatran etexilate. Clinical Advances in Hematology and Oncology: H&O, 8(10), 697–702.
- Ge, L., He, X., Zhang, Y., Zhang, Y., Chai, F., Jiang, L., Webster, T. J., & Zheng, C. (2018, June 1). A dabigatran etexilate phospholipid complex nanoemulsion system for further oral bioavailability by reducing drug-leakage in the gastrointestinal tract. Nanomedicine: Nanotechnology, Biology and Medicine, 14(4), 1455–1464.
- Jindal, K. (2017). Review on solubility: A mandatory tool for pharmaceuticals. International Research Journal of Pharmacy, 8(11), 11–15. https://doi.org/10.7897/2230-8407.0811210
- Kazi, M., Shahba, A. A., Alrashoud, S., Alwadei, M., Sherif, A. Y., & Alanazi, F. K. (2020). Bioactive self-nanoemulsifying drug delivery systems (Bio-SNEDDS) for combined oral delivery of curcumin and piperine. Molecules, 25(7), 1703. https://doi.org/10.3390/molecules25071703
- Kim, D. H., Kim, J. Y., Kim, R. M., Maharjan, P., Ji, Y.-G., Jang, D.-J., Min, K. A., Koo, T.-S., & Cho, K. H. (2018, November 5). Orlistat-loaded solid SNEDDS for the enhanced solubility, dissolution and in vivo performance. International Journal of Nanomedicine, 13, 7095–7106. https://doi.org/10.2147/IJN.S181175
- Kim, J. S., Choi, Y. J., Woo, M. R., Cheon, S., Ji, S. H., Im, D., Ud Din, F., Kim, J. O., Youn, Y. S., Oh, K. T., Lim, S.-J., Jin, S. G., & Choi, H.-G. (2021). New potential application of hydroxypropyl-β-cyclodextrin in solid self-nanoemulsifying drug delivery system and solid dispersion. Carbohydrate Polymers, 271, Article 118433. https://doi.org/10.1016/j.carbpol.2021.118433
- Kumar, R. S., & Sureshkumar, R. (2020). A review on solid supersaturable SNEDDS. Research Journal of Pharmacy and Technology, 13(7), 3530–3535. https://doi.org/10.5958/0974-360X.2020.00625.3
- Kuruvila, F. S., Mathew, F., & Kuppuswamy, S. (2017, March 1). Solid self nanoemulsifying drug delivery system (SNEDDS) devolopment, applications and future perspective: A review. Indo American Journal of Pharmaceutical Sciences, 4(3), 651–669.
- Lalwani, J. T., Thakkar, V. T., & Patel, H. V. (2013). Enhancement of solubility and oral bioavailability of ezetimibe by a novel solid self nano-emulsifying drug delivery system (SNEDDS). International Journal of Pharmacy and Pharmaceutical Sciences, 5(3), 513–522.
- Michaelsen, M. H., Siqueira Jørgensen, S. D., Abdi, I. M., Wasan, K. M., Rades, T., & Müllertz, A. (2019, September 1). Fenofibrate oral absorption from SNEDDS and super-SNEDDS is not significantly affected by lipase inhibition in rats. European Journal of Pharmaceutics and Biopharmaceutics, 142, 258–264. https://doi.org/10.1016/j.ejpb.2019.07.002
- Mundada, V. P., & Sawant, K. K. (2018). Enhanced oral bioavailability and anticoagulant activity of dabigatran etexilate by self-micro emulsifying drug delivery system: Systematic development, in vitro, ex vivo and in vivo evaluation. Journal of Nanomedicine and Nanotechnology, 9(1), 1–3. https://doi.org/10.4172/2157-7439.1000480
- Nagadeep, J., Kamaraj, P., & Arthanareeswari, M. (2019, December 1). Gradient RP-HPLC method for the determination of potential impurities in dabigatran etexilate in bulk drug and capsule formulations. Arabian Journal of Chemistry, 12(8), 3431–3443. https://doi.org/10.1016/j.arabjc.2015.09.006
- Nantsupawat, T., Soontrapa, S., Nantsupawat, N., Sotello, D., Klomjit, S., Adabag, S., & Perez‐Verdia, A. (2018, February). Risk factors and prevention of dabigatran‐related gastrointestinal bleeding in patients with atrial fibrillation. Journal of Arrhythmia, 34(1), 30–35. https://doi.org/10.1002/joa3.12015
- Nazlı, H., Mesut, B., & Özsoy, Y. (2021, October 27). In vitro evaluation of a solid supersaturated self nanoemulsifying drug delivery system (Super-SNEDDS) of aprepitant for enhanced solubility. Pharmaceuticals, 14(11), 1089. https://doi.org/10.3390/ph14111089
- Park, H., Ha, E.-S., & Kim, M.-S. (2020, April 16). Current status of supersaturable self-emulsifying drug delivery systems. Pharmaceutics, 12(4), 365. https://doi.org/10.3390/pharmaceutics12040365
- Parmar, K., Patel, J., & Sheth, N. (2015, October 1). Self nano-emulsifying drug delivery system for embelin: Design, characterization and in vitro studies. Asian Journal of Pharmaceutical Sciences, 10(5), 396–404. https://doi.org/10.1016/j.ajps.2015.04.006
- Patel, G., Shelat, P., & Lalwani, A. (2016, October 12). Statistical modeling, optimization and characterization of solid self-nanoemulsifying drug delivery system of lopinavir using design of experiment. Drug Delivery, 23(8), 3027–3042. https://doi.org/10.3109/10717544.2016.1141260
- Poorani, G., Uppuluri, S., & Uppuluri, K. B. (2016, August 17). Formulation, characterization, in vitro and in vivo evaluation of castor oil based self-nano emulsifying levosulpiride delivery systems. Journal of Microencapsulation, 33(6), 535–543. https://doi.org/10.1080/02652048.2016.1223199
- Radha, G. V., Sastri, K. T., Burada, S., & Rajkumar, J. (2019, January 7). A systematic review on self-micro emulsifying drug delivery systems: A potential strategy for drugs with poor oral bioavailability. International Journal of Applied Pharmaceutics, 11(1), 23–33. https://doi.org/10.22159/ijap.2019v11i1.29978
- Redondo, S., Martínez, M.-P., Ramajo, M., Navarro-Dorado, J., Barez, A., & Tejerina, T. (2011, December). Pharmacological basis and clinical evidence of dabigatran therapy. Journal of Hematology and Oncology, 4, 53. https://doi.org/10.1186/1756-8722-4-53
- Sarah, S. (2013, January). The pharmacology and therapeutic use of dabigatran etexilate. Journal of Clinical Pharmacology, 53(1), 1–13. https://doi.org/10.1177/0091270011432169
- Savjani, K. T., Gajjar, A. K., & Savjani, J. K. (2012). Drug solubility: Importance and enhancement techniques. ISRN Pharmaceutics, 2012(1), Article 195727. https://doi.org/10.5402/2012/195727
- Scioli Montoto, S., Muraca, G., & Ruiz, M. E. (2020, October 30). Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Frontiers in Molecular Biosciences, 7, Article 587997. https://doi.org/10.3389/fmolb.2020.587997
- Sharif, S. K., Ramudu, B. S., Nunna, R., & Ramachandran, D. (2017, June 1). An improved process for preparation of dabigatran etexilate mesylate. Asian Journal of Chemistry, 29(6), 1253–1257. https://doi.org/10.14233/ajchem.2017.20448
- Singh, B., Khurana, L., Bandyopadhyay, S., Kapil, R., & Katare, O. O. (2011). Development of optimized self-nano-emulsifying drug delivery systems (SNEDDS) of carvedilol with enhanced bioavailability potential. Drug Delivery, 18(8), 599–612. https://doi.org/10.3109/10717544.2011.604686
- Singh, G., & Pai, R. S. (2015, May 19). Trans-resveratrol self-nano-emulsifying drug delivery system (SNEDDS) with enhanced bioavailability potential: Optimization, pharmacokinetics and in situ single pass intestinal perfusion (SPIP) studies. Drug Delivery, 22(4), 522–530. https://doi.org/10.3109/10717544.2014.885616
- Singh, G., & Pai, R. S. (2016, February 17). In vitro and in vivo performance of supersaturable self-nanoemulsifying system of trans-resveratrol. Artificial Cells, Nanomedicine, and Biotechnology, 44(2), 510–516. https://doi.org/10.3109/21691401.2014.966192
- Singh, N., Singh, A. P., & Singh, A. P. (2021, January). Solubility: An overview. International Journal of Pharmaceutical Chemistry and Analysis, 7(4), 166–171. https://doi.org/10.18231/j.ijpca.2020.027
- Subramanian, P., Rajnikanth, P. S., Kumar, M., & Chidambram, K. (2020, January 1). In vitro and in-vivo evaluation of supersaturable self-nanoemulsifying drug delivery system (SNEDDS) of dutasteride. Current Drug Delivery, 17(1), 74–86. https://doi.org/10.2174/1567201816666191112111610
- Thomas, N., Holm, R., Garmer, M., Karlsson, J. J., Müllertz, A., & Rades, T. (2013, January). Supersaturated self-nanoemulsifying drug delivery systems (Super-SNEDDS) enhance the bioavailability of the poorly water-soluble drug simvastatin in dogs. The AAPS Journal, 15(1), 219–227. https://doi.org/10.1208/s12248-012-9433-7
- Thomas, N., Holm, R., Müllertz, A., & Rades, T. (2012, May 30). In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). Journal of Controlled Release, 160(1), 25–32. https://doi.org/10.1016/j.jconrel.2012.02.027
- Villar, A. M. S., Naveros, B. C., Campmany, A. C. C., Trenchs, M. A., Rocabert, C. B., & Bellowa, L. H. (2012, July 15). Design and optimization of self-nanoemulsifying drug delivery systems (SNEDDS) for enhanced dissolution of gemfibrozil. International Journal of Pharmaceutics, 431(1–2), 161–175. https://doi.org/10.1016/j.ijpharm.2012.04.001
