ABSTRACT
Background
Agad Tantra deals with poison and its manifestation, investigation, and treatment. It also deals with medicine having poisonous ingredients which always needs to be evaluated for its toxicity. In India the drug regulatory authorities, drug inspectors, drug testing laboratory, state level committees are functioning a big role for the purpose of drug safety and standardization. In light of this, a study on Dhatrayadi lepa has been taken. It contains Snuhi i.e. Euphorbia nerifolia Linn., that is a poisonous plant, corrosive and irritant in nature, it is categorised under Schedule E1 drug in herbal poison group in the Drugs and Cosmetics Rules 1945. Dhatrayadi lepa is mentioned in Vangsen Samhita which is said to be therapeutically efficacious as “Kachchhudadruharolepah”.
Aim
For above purpose 2 studies were carried out on Dhatrayadi lepa, to evaluate (i) Sub-acute toxicity study as per OECD guideline 410 and (ii) In vitro Antifungal activity through cup plate diffusion method.
Materials and Methods
Before the study, collection, authentication of ingredients and preparation and physico-chemical analysis of Dhatrayadi lepa were performed. 24 Wistar albino rats were divided in four groups taken for sub-acute dermal toxicity study. Dhatrayadi lepa was apply over the skin of rats for 28 days. The antifungal activity was carried out with the fungus “Trichophyton rubrum” which causes several skin diseases to humans. Three different extracts i.e. Tushodak extract of Dhatrayadi lepa, ethanol extract of Dhatrayadi lepa and methanol extract of Dhatrayadi lepa were taken to evaluate this activity, taking a standard antifungal drug “Terbinafine”.
Results and Conclusion
During Sub-acute dermal toxicity study, experimental rats did not showed any significant toxic effect on physical, hematological, biochemical and histological parameters. Therefore, it can be concluded that Dhatrayadi lepa is safe. Activity revealed that all the extract of Dhatrayadi lepa in higher concentration have antifungal efficacy. Among the three extracts, Tushodak extract of Dhatrayadi lepa is found to be most significant, thereafter ethanol extract and methanol extract. Standard drug terbin a fine has observed maximum ZOI (Zone of Inhibition) 47.6±0.66 mm at 1000 µg/mL. Dhatrayadi lepa (Tushodak) extract was shown to have a maximum zone of inhibition 26.3±0.33 mm at 1000 µg/mL concentration level.
INTRODUCTION
Ayurveda is a life science. It resumes health without any side effects on body. Our sages have given us marvelous thoughts on human civilization that attends everyone’s lives. In the present era, lives are in danger due lack of knowledge of proper scientific evidence of drugs. It needs to be evaluated and examined in every aspect of Ayurvedic theories and technique of manufacturing them etc.
In the current global scenario, the world has looking at Ayurveda with a special eye since the COVID-19 pandemic. The market for Ayurveda is constantly growing. At the same time, adequate action needs to be taken on the availability of safe and standardized drugs. The use of poisonous drugs in Ayurvedic medicine has increased the necessity of their safe and standardized use.
For above purpose two studies were carried out on Dhatrayadi lepa, (i) Sub-acute toxicity study as per OECD guideline 410 and (ii) In vitro Antifungal activity through cup plate diffusion method. Before the study, collection, authentication of ingredients and preparation and physico-chemical analysis of Dhatrayadi lepa were performed.
Dhatrayadi lepa is a formulation described in Vangsen Samhita1 which contains Snuhi i.e. Euphorbia nerifolia Linn., that is included in Schedule E (1) drug, list of poisonous substance under Ayurvedic system of medicine in sub-heading Drugs of vegetable origin in Drugs and Cosmetic rules, 1945. The other four ingredients of this formulation are Amalaki (Emblica officinal Gaerth.), Chakramard (Cassia tora Linn.), Sarjras (Vateria idica Linn.) and Tushodak (fermented formulation that is prepare from fermentation of rind of black gram and Yava i.e. Hordeum vulgare) Snuhi (Euphorbia nerifolia Linn.) is a poisonous plant described as Upavish (subpoisonous) in Ayurveda classics. It is corrosive, caustic in nature, and harmful to skin. So, its dermal safety is evaluated in this research work, according to OECD Guideline 410.
Figure 1 coded from an Ayurvedic text Vangsen Samhita, chapter 25, verse 56, explained that Amalaki (Emblica officinal Gaerth.), Snuhi (Euphorbia nerifolia Linn.), Chakramard (Cassia tora Linn.), Sarjras (Vateria idica Linn.) and Tushodak (fermented formulation) in form of paste called Dhatrayadi lepa were used to treat skin diseases like tinea infections, scabies etc.
As Vangsen Samhita mentions “Kachchhudadruharolepah” as the effect of Dhatrayadi lepa, so in vitro antifungal study was carried out on a fungus (Trichophyton rubrum) that is responsible for various skin disorders, had been undertaken to find out it’s antifungal activity. Research on the component of Dhatrayadi lepa has found antifungal in previous studies. Studied on Amalaki (Hossain et al., 2012), Snuhi,2 Chakramard (Pawar Harshal et al., 2011), and Sarjras (M. Ali Mirzaet al., 2023) indicate that these drugs contain phytochemical that inhibit fungal growth. With modern correlation, Dadru is considered as Tinea infection (fungal infection or Ring worm) that has three types of genera i.e. Trichophyton, Microsporum, and Epidermophyton.3 Fungal infection cases are 1-12 per 1000 patients in India4 and 70% cases are of Trichophyton rubrum. It is the global cause of ringworm, jock itch, fungal nail infection, and athlete’s foot.
MATERIALS AND METHODS
Collection and authentication of drug
- Preparation of Dhatrayadi lepa.
- Analytical study of Dhatrayadi lepa.
Experimental study
- Sub-acute dermal toxicity study.
- In vitro antifungal study.
Collection and Authentication of Drugs
For the preparation of Dhatrayadi lepa, Amalaki (Emblica officinal Gaerth.), dry fruits, Sarjras (Vateria idica Linn.) exudate, Chakramard (Cassia tora Linn.), seeds, Yava (Hordeum vulgare Linn.) fruits and rind of black gram were purchased from market. Snuhi Ksheer (Euphorbia nerifolia Linn. latex) was collected from the herbal garden of Govt. Ayurvedic College Raipur (10-20 mL per day and dried in the indirect sunlight). 116 g of Snuhi Ksheer (SK) was collected in about two and a half months. All the ingredients of the Dhatrayadi lepa were identified and authenticated by Dravya Guna Vibhag, Govt. Ayurvedic College Raipur. Moreover, Amalaki, Chakramard and Yava the ingredients of formulation were also sent to CSIR-NIScPR, New Delhi for its identification and authentication.
Preparation of Dhatrayadi lepa
As Snuhi (Euphorbia nerifolia) is poisonous plant in Dhatrayadi lepa, its purification had been done according to an Ayurvedic text Rasatarangini5 and Ayurvedic pharmacopeia of India.6 For this, fresh leaves of Chincha (Tamarind indica) were collected from field and washed properly and then Swaras (Juice) was procured by Swedan (steam). After obtaining Chincha Patra Swaras (Tamarind indica leaves juice), it was mixed with Ashodhit (impure) SK powder on a steel plate. Then it was kept in sunlight to evaporate its water contents and dried until it became hard. It took nearly 3 days. After proper drying, it was grinded into a fine powder and stored in an airtight jar.
After Shodhan (purification) of SK, the Tushodak7 (fermented formulation) was prepared by Yava and roasted rind of black gram. Both drugs were crushed into a coarse powder and poured in vessel for the preparation of decoction. After proper boiling, decoction was filtered with a clean cloth and poured in a glass container and kept for 10 days. A matchstick test was done to check the fermentation. Then all the four dried drugs were used for the preparation of Dhatrayadi lepa Churna. Tushodak (fermented formulation) was added gradually to the Churna (powder) and mixed well, until the Churna became slightly wet, and then kept covered. The Lepa (paste) was neither too unctuous, nor too dry. The Lepa was neither thick nor thin in consistency and was of moderate in nature.
Analytical Study of Dhatrayadi lepa
Analytical study of Dhatrayadi lepa had been done at the Drugs Testing Laboratory Evum Anusandhan Kendra Raipur C.G.
Table 1 showing the physicochemical results for different parameters of Dhatrayadi lepa are not mentioned in the API.
Sub-Acute Dermal Toxicity Study
Animals
A total of 24 Wistar albino rats of male and female weighing between 200 to 300 g were taken. The animals were marked with picric acid on tail, trunk and neck. Rats were kept in the cages at least 7 days before dosing, for acclimatization to the laboratory environment.

Figure 1:
Ayurvedic text for Dhatrayadi lepa.
| Name of Test | Result | Values |
|---|---|---|
| Loss on drying | 60.5% | Not mentioned |
| Total ash | 1% | Not mentioned |
| Acid insoluble ash | 2% | Not mentioned |
| Water soluble extractive | 5.6% | Not mentioned |
| Alcohol soluble extractive | 9.4% | Not mentioned |
Study center
Columbia Institute of Pharmacy, Tekari, Raipur (CG.).
Approval of Institutional Animal Ethical Committee
Ethical clearance had been obtained from the Institutional Animal Ethics Committee of the institute, where the research had been carried out, and the approval number is CIP/IAEC/2022/195.
Number of animals and dose levels
In sub-acute dermal Toxicity experiment, total 24 albino rats had been divided into four groups.
- 06 animals (3 male and 3 female rats) had been used for each group.
- Group A (Controlled group) had applied-Only Vehicle (Tushodak).
- Group B had applied-1000 mg/kg of Dhatrayadi lepa.
- Group C had applied-800 mg/kg of Dhatrayadi lepa.
- Group D had applied-600 mg/kg of Dhatrayadi lepa.
Inclusive criteria was healthy, young, adult Wistar Albino rats for both sexes weighing between 200 g to 300 g and exclusive criteria was unhealthy, parous, pregnant and was not within ±20% of the mean initial weight.
Before applying the Dhatrayadi lepa, about 10% of body surface area of rats was cleaned. The testing drug was applied on skin in graduated dose to all the group of animals in one dose per group for 28 days. Each animal was observed twice daily, on weekdays and once daily on weekends. Signs of ill health, reaction to the treatment, or mortality were recorded. The body weights of the rats in all groups were recorded once before the start of dosing, once weekly during the treatment period and finally on the day of sacrifice. The weight of organs like the heart, brain, liver, and kidneys was recorded after sacrifice. Behavioral changes and other parameters such as urinations, food intake, respiration, drowsiness, temperature, changes in eye and skin colors, diarrhea, etc. were noted. At the end of the experiment (on the 29th day), blood samples were collected from the rats after an overnight fast (but with drinking water allowed) for hematological, biochemical, and histopathological studies, respectively. The observed results were recorded as a sign of toxicity or the number of animals studied. Signs of toxicity and mortality were observed daily for 28 days, and were monitored weekly for changes in body weight.
In vitro Antifungal Study
Study Center
The research had been done at Chhattisgarh Council of Science and Technology, Vigyan Bhawan, Vidhyan Sabha Road, Daldal Seoni, Raipur, Chhattisgarh, and approval no. of this research is 76/CGCOST/2024, Raipur dated 19/04/2024.
Fungus Obtained From
Trichophyton rubrum fungus was ordered online from MTCC Chandigarh. Its MTCC number is 296, and order no. is 21910.
Procedure
Antifungal activity was carried out on Dhatrayadi lepa (Tushodak) extract, methanol extract, ethanol extract, and the standard drug ‘Terbinafine extract’. A fine powder 20 g of Dhatrayadi lepa was soaked with 200 mL of Tushodak, methanol and ethanol in succession, and stirring for 48 hr. Subsequently, a Watman filter paper was used for filtration, and the residue obtained after filtering was collected. The filtered residue was kept at a temperature of 40ºC until complete evaporation of the water, after which the dry extract obtained. Thus, 7.326 g of Tushodak extract, 3.44 g of methanol extract, and 2.986 g ethanol extract were obtained.
To prepare extract of different concentrations, a stock solution was made by dissolving 100 mg (100000 µg) of extract in 100 mL of distilled water, which is, 1000 µg/mL.
- For 50 µg/mL concentration, added 5 mL of stock solution into 95 mL of distilled water
- For 100 µg/mL concentration, added 10 mL of stock solution into 90 mL of distilled water.
- For 250 µg/mL concentration, added 25 mL of stock solution into 75 mL distilled water.
- For 500 µg/mL concentration, added 50 mL of stock solution into 50 mL distilled water.
- For 750 µg/mL concentration, added 75 mL of stock solution into 25 mL distilled water.
After that, a fungal culture medium was prepared for the growth of fungus. All the media was sterilized before culture. Fungal strain Trichophyton rubrum was inoculated for culture. The prepared fungal culture was ready for test. The antifungal activity of these extracts against Trichophyton rubrum was performed by the cup-plate diffusion method, comparing with standard antifungal drug ‘Terbinafine’.
Cup-plate diffusion method
In cup plate diffusion method, the antifungal substance diffused from the solidified agar layer carrying cultured fungus in a plate. Then, with the help of a sterile borer, 6 mm-diameter cavities (cup like) were made, which were later filled with the described concentrations (50, 100, 250, 500, 750, and 1000 µg/mL) of the extracts and standard antifungal drug extract. Then plates were incubated for 48 hr at 27ºC. After incubation, circular area around the extracts, called the zone of inhibition (in mL) were measured with a scale or a zone reader. Three observations were taken for antifungal activity (Figure 2).
Statistical Analysis
All the observed values were expressed after statistics as mean±standard error of the mean. ANOVA test for toxicity study and, ANOVA and post hoc test for antifungal activity were considered for statistical analysis. A level of p<0.05 was considered for statistical significance in the both studies.
RESULTS
In this study, Dhatrayadi lepa was applied for 28 days on the skin of rats in all the groups A, B, C and D. After application appearance, general behavioral patterns, morbidity and mortality were observed for twice daily and once daily on weekend for 28 days. The animals were observed as normal and did not show any significant change in skin, eye, mucous membrane, salivation, lethargy, sleep, behavior pattern, breathing, impairment in food intake, water consumption and postural abnormalities.
Various Parameters were used to access the toxicity potential of the Ayurvedic formulation, such as hematological, biochemical, ponderal, and histopathological parameters. The statements on those parameters for sub-acute dermal toxicity are summarized below:
Parameters were used to access the toxicity potential of the Ayurvedic formulation, such as body weight, hematological, biochemical, and histopathological parameters. Effects on body weight of rats were observed for four weeks (Table 2). All the rats have taken between 200 g to 300 g and find no significant change. All the nineteen parameters of hematology were covered in subacute dermal toxicity study (Table 3). No significant changes were observed in HB, RBC, WBC, Platelet, PCV, MCV, Mean Corpuscular Hb Conc., Red cell distribution. Dhatrayadi lepa (Ayurvedic formulation) did not produced significant effect on any of the parameters studied at any dose level. This indicates that Dhatrayadi lepa does not affect size, shape, quality of red blood cells, the amount of hemoglobin in red blood cells, the percentage of red blood cells.
All the above parameters of biochemical were observed and no significant changes were found (Table 4). Dhatrayadi lepa (Ayurvedic formulation) did not produce significant effect on any of the parameters studied at any dose level. This indicates that Dhatrayadi lepa does not affect chemical constituents like calcium, uric acid, urea, serum creatinine, phosphate in the rats.
Histopathology of skin, heart, kidney, liver, brain had been observed normal. Dhatrayadi lepa (Ayurvedic formulation) did not produce significant effect on skin, heart, kidney, liver, brain histology at any dose level (Figure 3).
In the antifungal study, the result showed remarkable inhibition of fungal growth on the all extracts of Dhatrayadi lepa against Trichophyton rubrum (Table 5). The antifungal potential of the all extracts of Dhatrayadi lepa, depend upon the concentration of test drug. The minimum zone of inhibition was observed in the 50 µg/mL concentration group, whereas the maximum zone of inhibition was observed in the 1000 µg/mL concentration group. Comparing to the standard drug Terbinafine extract, the Dhatrayadi lepa (Tushodak) extract also shows the highly significant zone of inhibition. Ethanol extract has observed less zone of inhibition compared to methanol extract, and methanol extract has observed less zone of inhibition compared to Dhatrayadi lepa (Tushodak) extract. When the activity of the all extract was compared with the standard drug Terbinafine extract, it is found to be more significant, though significant results are also found in all the extracts of Dhatrayadi lepa.

Figure 2:
Culture plates for antifungal activity.
| Week/Group | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| 1st week | 287.5±3.37 | 259 ±20.97 | 285.5±19.89 | 227.6667±14.22 |
| 2nd week | 289.1667±3.56 | 265.1667±22.84 | 291±20.81 | 229.5±14.21 |
| 3rd week | 285.8333±2.89 | 266.5±22.7 | 292.5±20.86 | 231±14.12 |
| 4th week | 283±1.36 | 268.3333±22.49 | 293.5±20.7 | 232.5±14.02 |
| Hematological Values/Group | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| HB | 11.75± 0.25 | 12.75±1.25 | 12.25±0.25 | 12.2±0.60 |
| PCV | 32.25±0.15 | 36.15±2.25 | 33.3±0.8 | 32.85±0.05 |
| RBC | 6.71±0.36 | 7.54±0.86 | 6.86±0.01 | 6.85±0.31 |
| MCV | 48.23±2.4 | 48.23±2.5 | 48.545±1.23 | 48±2.25 |
| MCH | 17.56±0.58 | 16.95±0.27 | 17.86±0.39 | 17.89±1.68 |
| MCHC | 36.44±0.60 | 35.19±1.27 | 36.79±0.13 | 37.14±177 |
| RDWCV | 14.15±0.05 | 13.65±0.15 | 13.7±1.10 | 13.6±1.10 |
| WBC | 8500±1200.0 | 7450±10500 | 12650±38500 | 6000±500.0 |
| Neutrophils | 57±17.0 | 34±6.0 | 40±10.0 | 36.5±1.5 |
| Lymphocytes | 35.5±17.5 | 58.5±6.5 | 54±10.0 | 57±3.0 |
| Eosinophils | 6±1.0 | 6.5±0.5 | 4.5±0.5 | 5±1.0 |
| Monocytes | 1.5±0.5 | 1±.00 | 1.5±0.5 | 1.5±0.5 |
| Neutro-lympho_ratio | 2.43±1.68 | 0.43± | 1.14± | 0.64±0.06 |
| Platelet-count | 505500±30500.0 | 249003.775±248996.225 | 337001.59±336998.41 | 781000±46000.0 |
| MPV | 7.6±0.2 | 7.35±0.25 | 7.05±0.55 | 7.15±0.15 |
| PDW | 15.2±0.20 | 15.6± | 14.9± | 15.2±0.10 |
| PCT | 0.405±0.05 | 0.39± | 0.46± | 0.585±0.05 |
| ESR | 17±1.0 | 11±1.0 | 17±1.0 | 17±3.0 |
DISCUSSION
Ayurveda, with its scientific foundation and emphasis on safety and efficacy, is being revived as a safer alternative to conventional medicines. Toxicology plays a role in assessing the effects of chemicals, including those used in Ayurvedic preparations, and ensuring their safe utilization. Continued research and standardization efforts can further enhance the understanding and acceptance of Ayurveda in the broader medical community.
The field of Ayurveda recognizes Agada Tantra8 as one of its eight clinical specialties, which focuses on toxicology and the management of the toxic effects. It emphasizes the importance of proper purification and dosage monitoring for formulations to prevent potential toxicity.
In the context of Ayurvedic formulations, it is essential to conduct dermal toxicity studies, for the formulations which contain Schedule E-1, drug of Drugs and Cosmetic rules, 1945, to assess the safety and potential adverse effects of topical formulations. Similarly, studies should be conducted for its specific activity to evaluate its efficacy, if the topical formulations possess. Such studies help researchers and practitioners to understand the appropriate dosage levels and potential clinical outcomes associated with the formulations under investigation.
Purification of several toxic drugs explained in Ayurvedic text many years ago. Purification of Snuhi latex (Euphorbia nerifolia Linn.) has been explained in Rasatarangini by Tamarind indica leaf juice. This might be the reason that, Dhatrayadi lepa which contains purified Snuhi (Euphorbia nerifolia Linn.) did not produce any corrosive and irritant effect on the skin. In toxicity study Dhatrayadi lepa did not produce any significant effect on body weight of Wistar albino rats in control group (group A) and test groups i.e. group B, C and D. Similarly, Dhatrayadi lepa did not produce any statistically significant effect on hematological and biochemical parameters between control group A and test group i.e. group B, C and D. Skin of Wistar albino rats histologically did not showed any morphological change after applying Dhatrayadi lepa.
| Biochemical parameters/Group | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| Bilirubin_total | 0.825±0.155 | 0.905±0.035 | 0.745±0.95 | 0.92±0.06 |
| Bilurubin_direct | 0.17±0.04 | 0.18±0.03 | 0.165±0.01 | 0.185±0.015 |
| Bilurubin_indirect | 0.655±0.11 | 0.725±0.065 | 0.58±0.11 | 0.735±0.075 |
| Total_protien | 5.3±0.40 | 6.25±0.25 | 5.45±0.25 | 5.75±0.15 |
| Albumin | 1.85±0.25 | 2.05±0.35 | 2.15±0.25 | 2.3±0.00 |
| Globulin | 3.45±0.15 | 4.2±0.10 | 3.3±0.50 | 3.45±0.15 |
| A/G_ratio | 0.53±0.05 | 0.495±0.095 | 0.68±0.18 | 0.67±0.03 |
| SGOT | 17±3.00 | 89.8±5.20 | 81.45±30.55 | 78.7±29.30 |
| SGPT | 257.3±188.90 | 73.25±3.05 | 64.15±13.15 | 75.1±9.1 |
| Alkaline_phosphate | 154±4.00 | 193.55±13.15 | 142.85±34.15 | 140.1±8.9 |
| Blood_Glucose_Random | 70±60.00 | 47.5±16.50 | 66.05±14.05 | 65.05±5.05 |
| Blood_Urea | 45.7±3.60 | 38.65±1.45 | 41.25±8.85 | 33±0.60 |
| Serum_creatinine | 0.4±0.00 | 0.5±0.10 | 0.3±0.00 | 0.25±0.05 |
| Serum_uric_acid | 2.55±0.65 | 2.3±0.20 | 1.65±0.05 | 1.75±0.15 |
| Serum_calcium | 8.5±0.20 | 8.5±0.40 | 8.15±0.25 | 9±1.40 |
| Material Use | Zone of Inhibition in millimetre (Mean±SE) | |||||
|---|---|---|---|---|---|---|
| 50 µg/mL | 100 µg/mL | 250 µg/mL | 500 µg/mL | 750 µg/mL | 1000 µg/mL | |
| Methanolex. of D. lepa | 6.66±0.57 | 7.6± 0.33 | 10.3±0.33 | 15 ±0.57 | 18±0.57 | 22±0.57 |
| Ethanolex. of D. lepa | 6±0.57 | 6.6±0.66 | 9±0.57 | 14±0.57 | 16.3±0.88 | 20±0.57 |
| Dhatrayadi lepa extract (Tushodak) | 8.3±0.32 | 10.3±0.33 | 13.6±0.32 | 18±0.32 | 22.6±0.93 | 26.3±0.33 |
| Terbinafine extract | 22.3±0.32 | 26±0.40 | 30.6±0.66 | 36.3±0.71 | 41.3±0.33 | 47.6±0.66 |
The components of Dhatrayadi lepa have shown antifungal activity. The methonolic extract of fruit of Amalaki i.e. Embilica officinalis, exhibited antifungal activity against fungal strain; Neurospora crassa, Aspergillus brasileinsis and Cladosporium oxysporoa.9 Snuhi i.e. Euphorbia nerifolia, the latex milk with chitosan at 60 µL dose was reduced the percentage of spore germination in Aspergillus flavus, and mucor,10 Chakramard i.e. Cassia tora, alcoholic extract of seeds were showed dose dependant inhibition of dermatophytes collected from skin sample of patient,11 Sarjras i.e. Vateria idica, the resinous preparation was found to be free from fungal up to 14 days.12
The results of the antifungal activity of Dhatrayadi lepa (Tushodak) extract, ethanol extract, methanol extract, and the standard drug Terbinafine were carried out by the Cup plate diffusion method. The different concentrations of Dhatrayadi lepa (Tushodak) extracts, ethanol extract, methanol extract, and standard drug terbinafine (50 µg/mL, 100 µg/mL, 250 µg/mL, 500 µg/mL, 750 µg/mL, and 1000 µg/mL) were used against Trichophyton rubrum. The results for the antifungal activity of Dhatrayadi lepa (Tushodak) extracts, ethanol extract, methanol extract, and the standard drug Terbinafine showed a clear zone of inhibition against Trichophyton rubrum. The result also showed remarkable inhibition of fungal growth by the extract of Dhatrayadi lepa (Tushodak) against Trichophyton rubrum. The antifungal potential of the extracts was found to be dose-dependent. The minimum ZOI observed in the 50 µg/mL concentration group in all extracts and the maximum zone of inhibition in the 1000 µg/mL concentration. As compared to standard drug Terbinafine extract, Dhatrayadi lepa (Tushodak) extract shows a highly significant zone of inhibition. Ethanol extract has observed less zone of inhibition compared to methanol extract, and methanol extract has observed less zone of inhibition compared to extract of Dhatrayadi lepa (Tushodak). When the activity of the extract was compared with the standard drug Terbinafine, it was found to be significant, and this confirms that the selected formulation, Dhatrayadi lepa, has antifungal potential.
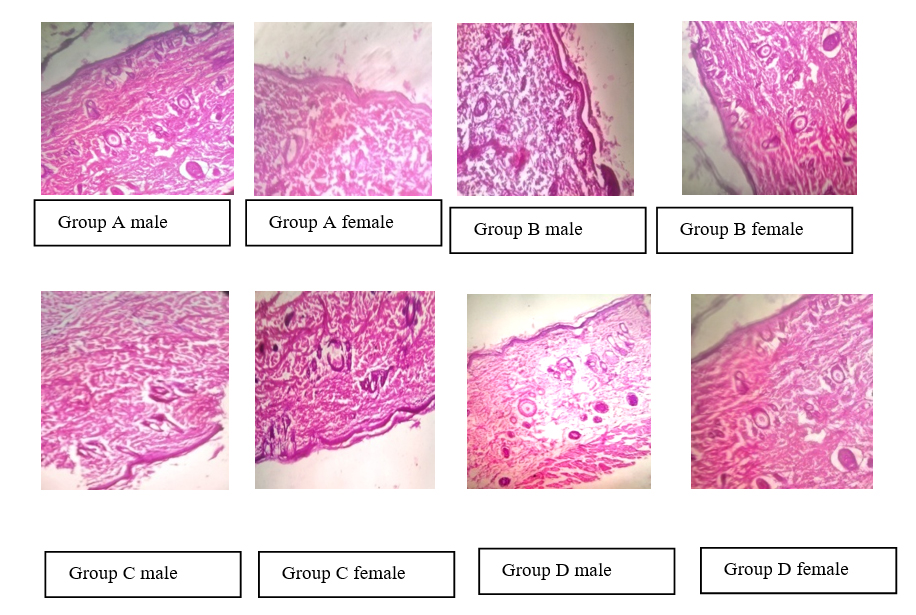
Figure 3:
Histopathology of skin of rats.
CONCLUSION
In modern medicine, antifungal treatments have side effects and are expensive, whereas on the comfortably, easily available, and are not very expensive. Dhatrayadi lepa contains poisonous drug Snuhi, therefore animal trial has carried out for study. Based on the findings of animal trial, it can be concluded that Dhatrayadi lepa is not toxic, at dose level 600 mg/kg, 800 mg/kg and 1000 mg/kg in Wistar albino rats during sub-acute dermal toxicity study. The result of antifungal study shows that the fungus Trichophyton rubrum was found to be highly susceptible to the all the extracts of Dhatrayadi lepa. Standard drug Terbinafine has observed maximum ZOI (Zone of Inhibition) 47.6±0.66 mm at 1000 µg/mL. Dhatrayadi lepa (Tushodak) extract was shown to have a maximum zone of inhibition 26.3±0.33 mm at 1000 µg/mL concentration level in comparison to ethanol and methanol extract.
Cite this article:
Dewangan SK, Bhagat S, Inchulkar SR, Satapathy T, Chauhan NS. Dermal Safety Study and Antifungal Activity of Dhatrayadi lepa. Int. J. Pharm. Investigation. 2025;15(2):10-8.
REFERENCES
- Tripathi HP. Chapter 25. Verse 56. Vang Sen Samhita. In: Chaukhambha Sanshkrit series office. 2016;332.
- Sumathi H, Dharani, Sivaprabha, Malathy, Radha, Padma. Isolation and characterization of chitin from prawn shell waste and incorporation into medical textiles. International Journal of Recent Scientific Research. 2012;3(8):676-80.
- Golwallia AF, Golwallia SA. Chapter 14. Medicine for students. Jaypee Brothers Medical Publishers. Pvt. Ltd., 2022;12:874.
- Shenoy M. Ringworm Outbreak of India: the despicable dermatological disease. Forbes, India. 2021.
- Sharma S. Rasatarangini commentary by Shashtri H, Shashtri D. In: Motilal Banarasidas SK, editor. 2012;744:chapter 24:Verse517-8.
- Department of Ayush, Ministry of Health and Family Welfare, Govt. of India. The ayurvedic pharmacopoeia of India, Part I vol. I. New Delhi, page no 200.
- Sharma RP, Mina M, Vigyan BK. Jagdish Sanskrit Pustkalaya. 2018;7:231.
- Chapter SS. Maharshi Sushrut. Shushrut Samhita commentary by Ambikadutta. Chaukhambha Sanskrit Sansthan. 2012;1:5-7:1/7-16.
- AI-Samman AM, Siddique NA. Gas Cromatography-Mass Spextrometry (GC-MS/MS) Analysis, Ultrasonic Assisted Extraction, antibacterial; and antifungal Activity of E. officinalis Fruit Extract. Pharmacogn J. 2019;11(2):315-23.
- Pawar PV, Shaikh MA, Sali TP, Salunke LN, Salunke DV, Jayswal N. A short review on Euphorbia nerifolia. Asian J Pharm Res. 2023;13(2):109-3.
- Y.R. SK, Mahajon B, NS, acharya R. Cassia tora Linn.-a pharmacological review. Int J Curr Sci Res Rev. 2022;05(05):1774-7.
- Mirza MA, Hasan M, Ramesh S, Rafiq M, Siddiqui H, Khan M, et al. Vateria indica Linn. resin based ointment for the topical treatment of Radiation-Induced burns in cancer patients. J King Saud Univ Sci. 2023;35(5):102659.
